Editorial
Austin J Biomed Eng. 2014;1(3): 1014.
Analogue Front-end ICs for Low-cost Healthcare are Ready for Volume Production
Lei Wang* and Jinyong Zhang
Shenzhen Institutes of Advanced Technology, Chinese Academy of Sciences, China
*Corresponding author: :Lei Wang, Shenzhen Institutes of Advanced Technology, Chinese Academy of Sciences, Shenzhen University Town, Shenzhen, 518055, China.
Received: May 15, 2014; Accepted: July 04, 2014; Published: July 08, 2014
It is reported from Chinese Academy of Sciences (CAS), China that a set of analogue front-end (AFE) IC chips is on the final phase of the preparation for the volume production. The research and development team with Shenzhen Institutes of Advanced Technology, CAS (CAS-SIAT), has worked on the medical-application specified IC design arena since 2008. Their efforts were not wasted - after several prototyping Multiple-Project-Wafers (MPW) some AFE IC chips will come to the market as earlier as middle of year 2014.
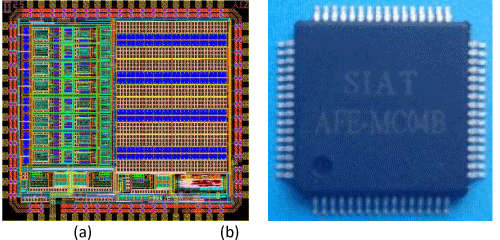
The AFE IC chips were designated for the acquisition of multiple-channel physiological signals such as electrocardiogram (ECG), electromyogram and photoplethysmographic signals, both as differential and single-ended inputs. Figure 1.a illustrated the microphotograph of one IC chip among them - the MC04, which is comprised of eight low-noise pre-amplifiers (Pre-Amps), eight bands pass filters (BPFs) and programmable gain amplifiers (PGAs), and a multiple-voltage power management unit (PMU), along with an on-chip RC oscillator, varies voltage references and band gaps, and an SPI-compatible digital interface. The monolithic chip was fabricated using the mainstream SMIC 0.18 um CMOS 1P6M technology and the chipset comes in a 9mm by 9mm, 64-pin, Low-profile Quad Flat Package (LQFP), as illustrated in Figure 1.b.
Figure 1: The microphotograph of the MC04 (a), and the packaged samples (b).
The MC04 is highly integrated. It could be employed to significantly reduce the Bill of Material (BoM) for developing many portable/wearable medical electronics devices. For example, a conventional twelve-lead ECG acquisition system could use up to 50 off-the-shelf components, such as various amplifiers, regulators and passive components, by using the MC04 the BoM could be reduced down to this single chip plus <10 discrete components. The reduction of the BoM could benefit the manufacturing cost and the miniaturization of the complete medical device, accordingly.
Aside from the system-level integration, the MC04 was configured to optimize the noise performance and power consumption. The design philosophe here could be concluded as three ‘L’s - low frequency, low noise and low power. The brief specification of the MC04 is listed in Table I, comparing with an off-the-shelf product (also listed in Table I), the MC04 outperformed in input-referred noise, power dissipation per channel, as well as the versatile programmable bandwidth.
Parameter
MC04 from CAS
One off-the-shelf product
Channels
Eight, differentia or single-ended
Eight
Supply Voltage
Analog: 1.8V
Digital: 1.8V
Analog: 2.7-5.25V
Digital: 1.65-3.6V
Input-Referred Noise
~1.0 uVpp
(with 150Hz BW/Gain=300)
~4 uVpp
(with 150Hz BW/Gain=6)
Power Dissipation
80 uW/Channel
750 uW/Channel
CMRR
>100dB
115dB
Programmable Gain
100 * (2, 3, 15, 22)
1, 2, 3, 4, 6, 8, 12
Programmable Bandwidth
Low-pass filter: 45Hz, 150Hz, 215Hz, 300Hz and 350Hz;
High-pass filter: 380mHz ~ 14Hz
N/A
Temperature
~-35 ~ +75?
~-40 ~+85?
Table 1: Performance comparison between the MC04 and one off-the-shelf product.
The combination of monolithic integration and superior characteristics makes the chipset suitable for a wider range of mobile healthcare, ambulatory monitoring and as clinical systems, for both conventional and emerging applications. Particularly, it is extremely important for the low-cost healthcare, which means to use state-of-the-art technologies to provide affordable healthcare for under development countries and regions. The current retail price for the counterpart chip (which is listed in the right column in Table I) is approximately twenty five USDs, while the MC04 from CAS is targeted to be sold less than two USDs after the volume production.
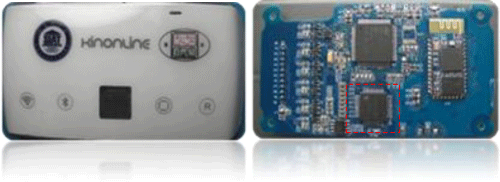
Meanwhile, CAS provide several turn-key solutions, such as the configurations of a miniature Holter device (Figure 2), a portable health information acquisition module and an Intensive Care Unit (ICU) vital sign monitor, based upon the chipset. The motivation here is to accelerate the translation from the research laboratory to the market. Several local medical device / healthcare service vendors already developed some new products using the chipset. The responses from the market for these new products are quite promising; therefore the vendors are quite keen to get the volume-produced chipset A.S.A.P.
Figure 2: One turn-key solution using the AFE chipset (red box in the right figure), this configuration is for a Holter device and the experimental results suggested the approval of industry standard, regulated by the CFDA (China Food and Drug Administration, equivalent to the FDA in USA).
Further work included the incorporation of an active electrode IP and an ARM7TDMI-based microprocessor core. The attempt for using System-in-Package (SiP) technology to boost the performance was also started. It is envisaged that the medical-application specified IC design is a significant milestone towards the affordable healthcare in anytime, anywhere and for anybody.