
Research Article
Austin J Biomed Eng. 2015; 2(1): 1030.
Biodegradable Polyurethane Materials of Different Origin Based on Natural Components
Savelyev YuV1, Travinskaya TV1*, Robota LP1, Markovskaya LA1, Akhranovich ER1, Brykova AN1, Savelyeva OA1, Furmanov YuA2 and Savitskaya IM2
1Department of Chemistry of Hetero chain Polymers and Interpenetrating Networks, Institute of Macromolecular Chemistry, Ukraine
2Department of Experimental Surgery, National Institute of Surgery and Transplantation named after A. Shalimov, Ukraine
*Corresponding author: Travinskaya TV, Department of Chemistry of Hetero chain Polymers and Interpenetrating Networks, Institute of Macromolecular Chemistry, NAS of Ukraine, Kharkovskoe shosse, 48, 02160, Kiev, Ukraine
Received: November 15, 2015; Accepted: December 30, 2015; Published: December 31, 2015
Abstract
The “sustainable development” of environmentally efficient and harmless polymer materials is one of the most important tasks of the modern polymer chemistry. With an aim to develop environmentally friendly materials of wide range of applications, including (bio) medicine, we synthesized biodegradable polyurethanes of different nature: Ionic Polyurethanes (IPU) and Polyurethane Foams (PUF) based on renewable components of biotechnological exopolysaccharide Xanthan (Xa) and vegetable Castor Oil (CO). The comprehensive study of the “structure - properties - ability to (bio) degradation” relationship of structurally modified polymeric materials allows to make the conclusion, that due to incorporation of natural components into the polymer chain, the material acquires the property of biodegradation under environmental factors. The structure of the synthesized polymers has been proved by wideand small-angle X-rays scattering, infrared- and pyrolytic mass spectrometry. The biodegradability of synthesized polyurethane materials was confirmed by studies of the adhesion of microorganisms to their surface, by degree of hydrolysis in acid and alkali media and composting into a soil. Histological study of developed laboratory sample of composite material based on Xa containing PUF carried out on white mice has shown the complete resorption of the polyurethane component in the absence of injury and inflammation of the surrounding soft tissues.
Keywords: Ionic polyurethane; Polyurethane foam; Biodegradation; Natural components; Histological study
Abbreviations
IPU: Ionic Polyurethane; PUF: Polyurethane Foam; Xa: Xanthan; CO: Castor Oil; POPG: Polyoxypropylene Glycol; ???G: Oligooxytetramethyleneglycol; P-503: Polyester, the condensation product of diethylene glycol, adipic acid and glycerol; TDI: Toluene Diisocyanate; HMDI: Hexamethylenediisocyanate; DMPA: Dimethylolpropionic Acid; TEA: Triethylamine; TO: Tin Octoate; UP-606/2: Tris-(dimethylamino-methyl)phenol; KEP-2: Blockcopolymer of polydimethylsiloxane and alkylene oxides; VO: Vaseline Oil; PP: Polypropylene; PTFE: Polytetrafluoroethylene; SAXS: Small- Angle X-ray Diffraction Scattering; WAXS: Wide- Angle X-ray Diffraction Scattering; PMS: Pyrolytic Mass Spectrometry; FTIR: Fourier Transform Infrared Spectroscopy; BS: Bacillus subtilis; MO: Microorganisms
Introduction
One of the most important tasks of the modern international scientific community is the “sustainable development” of production of environmentally efficient and harmless materials today for the maintenance of “sustainable and green” tomorrow. One of the ways of this problem’s solution is the employment of renewable resources instead of fossil fuels, the use of clean technologies and the degradation of the materials after the end of their operation life. The concept revolves around the focal point of “green chemistry” or “chemistry of eco-friendly substances.” The materials used in biomedical purposes must be biocompatible and products of their degradation should not have a toxic effect on human body. This idea has already made its world debut in the form of environmentally friendly technologies, precluding the use of organic solvents, the use of water-based monomers/polymers, obtained from renewable resources. Vegetable oils, in particular, constitute the most rich, cost-effective, non-toxic biological resource of the nature [1,2]. For several years, they have traditionally been used as starting materials in the production of environmentally friendly biodegradable polymeric materials [3]. Today, the successes of biotechnology have provided widespread use of microbial polysaccharides (exopolysaccharides), which are often referred to as biopolymers. They are widely used in various fields of human activity. Some microbial polysaccharides are similar or even identical to plant or animal polysaccharides, but most of them have a unique structure, specific only for this type [4]. The most popularity has accrued the bacterial cellulose and Xanthan based products, extracellular polysaccharide of Xanthomonas Campestris bacterium. Microorganisms are cultivated in special bioreactors, which provide for them all the necessary conditions (nutrient medium, aeration or anaerobic conditions), temperature, pH, removal of metabolic products). The main chain of Xanthan is constructed similarly to cellulose (1-4-β-glycopyranosa) and in the branches there is trisaccharide consisting of β-D-mannose, β-D-glucuronic acid and a-D-mannose. Residues of glucuronic acid and acid pyruvic groups provide the xanthan molecules an anionic nature. Xanthan molecules are susceptible to self–association in aqueous solutions, and with an increase in ionic strength of solution or polysaccharide concentration the gel is formed which is a three-dimensional network formed from Xanthan double helices associated with intermolecular hydrogen bonds [5]. One of the areas of exopolysaccharides and vegetable oils use in the macromolecular chemistry is the development of (bio) degradable polymer materials of biomedical application on the basis of known synthetic polymers. Previously we have created polymers on the basis of the ionomeric polyurethanes and natural (poly) saccharides (alginate, starch, lactose, glucose, etc.), that degrade under the environmental factors, and comprehensively studied their structure and the effect of the natural component on the properties and propensity to (bio) degradation of polyurethane [6-9]. It has been proved that it is a chemical bond between synthetic and natural components plays a crucial role in giving to polymeric materials the ability to (bio) degradation in whole, unlike the mechanical mixtures, where with the lapse of time the only a natural component has degraded. The purpose of this work is the development of methods of synthesis and comprehensive study of the “structure - properties - ability to (bio) degradation” relationship of structurally modified polymeric materials based on different type of polyurethanes and renewable components of biotechnological (Xanthan) and vegetable (castor oil) nature and ability of their (bio) medical application.
Materials and Methods
Polyether
Polyoxypropylene glycol POPG 5003 (MM 5000) - polyether on the basis of polyatomic alcohols and a copolymer of propylene and ethylene oxide. Hydroxyl number is about 32.0–36.0mg KOH/g. Acid number is not more than 0.10mg KOH/g (“Macro Oligooxytetramethyleneglycol; mer Ltd”, Russia). Oligooxytetramethyleneglycol ?? 1030 (???G-1000) (“Macromer Ltd”, Russia).
Polyester: P-503 (MM 500) is the condensation product of diethylene glycol, adipic acid and glycerol. Hydroxyl number is 280.0–330.0mg K??/g. The acid number is not more than 2.0 mg K??/g (“Corundum Ltd”, Russia).
Diisocyanate: Toluene Diisocyanate (TDI) is the mixture of 2,4 and 2,6-isomers (“Okakhim”, Russia), cleaned by vacuum distillation. Hexamethylenediisocyanate (HMDI) (“Okakhim”, Russia).
Dimethylolpropionic acid (DMPA) and Triethylamine (TEA) were purchased from Aldrich and used as received; Acetone (Fluka).
Catalysts: Tin Octoate (TO) (“Baltic manufacture”, Russia); tris-(dimethylamino-methyl) phenol (UP-606/2) (“Khimeks Ltd”, Russia).
Foam stabilizers: KEP-2 block-copolymer of polydimethylsiloxane and alkylene oxides (“Orgsintez”, Russia). Vaseline Oil (VO) (“Medkhim”, Russia).
Xanthan: (Xa) dry powder (Sigma, Xanthomonas camprestris pv camprestris (MM 2000000 - 50000000)).
Castor Oil: (CO) – triglyceride of ricinoleic acid (90%), linoleic and oleic acid (10%), hydroxyl number is about 150, Fluka, India.
Synthesis of polyurethane foam, sample of comparison (PUF-matrix)
Distilled water (0.42–3.00 wt.%), TO (0.53–2.07 wt.%), VO (0.11–0.4 wt.%), KEP-2 (0.73–1.53 wt.%), and UP-606/2 (0.37–1.53 wt.%) were charged at room temperature into the wide-necked flask equipped with a mechanical stirrer and all ingredients were mixed until a homogeneous mixture was formed. Then POPG-5003 (18.75– 52.65 wt.%) and ?-503 (4.18–19.1 wt.%) were added to the reaction mass under the stirring. After mixing and obtaining the homogeneous product (component I) the TDI (14.62–39.5) (component II) was added to the reaction mixture. The content of the flask was stirred until foam appearance (2–3 min), thereafter it was poured into the molds. Foam forming has occurred due to the release of carbon dioxide during the decomposition of unstable carbamic acid- the product of interaction of isocyanate groups with water.
Synthesis of CO/Xa based polyurethane foam
PUF, containing Xa / (50 % wt), PUF/Xa50; PUF, containing Xa (50 % wt), and CO (50 % wt), PUF/CO50/Xa50, where Xa was used in the native state (ns) and as a 15 % water gel (gel) have been prepared according to [10-12].
Polypropylene (PP) based mesh materials for hernioplasty with increased Xa content (70 % wt) PP/PUF/Xa70 were prepared as follows: PUF was obtained by mixing at room temperature (up to foaming - 60-100 sec.) of polyether component that is a mixture of POPG 5003 and P-503, catalysts TO and UP-606/2, foam stabilizers KEP-2 and VO and Xa – in native state with the isocyanate component TDI. The reaction mixture was poured on the PTFE form, rolled out in a thin layer and rolled on with polypropylene mesh.
Synthesis of ionic water dispersion, sample of comparison (IPU-matrix)
Preparation of IPU-matrix in the form of aqueous dispersion was achieved by the reaction of OTMG and HMDI based isocyanate precursor (component ratio 1:2, reaction time 2hours) with TEA neutralized DMPA, in acetone solution, followed by extension of anionic oligourethane prepolymer with water, dispersion and acetone removal.
Xa based ionomeric polyurethane dispersion (IPU/Xa) was produced analogously to IPU-matrix, except for Xa introduced into a precursor in the form of a dry powder in a stage of chain extension (content of Xanthan amounts 20% calculated with reference on dry substance) [13].
CO based ionomeric polyurethane dispersion (IPU/CO) was prepared by reaction of isocyanate precursor based on a mixture of OTMG, CO (wherein CO was added in an amount 20%) and HMDI in a molar ratio of 1:2, respectively (reaction time - 2hours, temperature – 80o C) with DMPA.
CO/Xa based ionomeric polyurethane dispersion (IPU/CO/ Xa)
In this case Xa was added as a dry powder to polyurethane precursor based on CO on the stage of chain extension sequentially after DMPA adding. Neutralization of the carboxyl groups of DMPA fragments was performed using TEA.
The general scheme of obtaining is as follows:
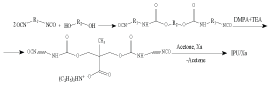
scheme:
where: R1 – [-(CH2)6-]; R2 – - (CH2CH2CH2CH2O)n,/-; n = 14; DMPA – ??3?(??2??)2????; ??? – N(C2H5)3.
The compositions of synthesized IPU PUF and their structuremodified analogues are performed in (Table1).
Composition of PU material
Matrix composition
Content of modifier, %(wt.)
IPU-matrix
OTMG-1000, HMDI, DMPA(?E?), ?2?
-
IPU /Xa5
- « -
5% Xa
IPU /Xa20
- « -
20% Xa
IPU /CO20
- « -
20% CO*
IPU / CO20/Xa20
- « -
20% Xa + 20%CO*
PUF-matrix
POPG-5003, P-503, H2O, KEP-2, UP-606/2, VO, TO , TDI
-
PUF /Xa50(ns)
- « -
50%Xa(ns)
PUF /Xa50(el)
- « -
50%Xa(gel 15%)
PUF /CO50/Xa50(ns)
- « -
50% CO*, 50%Xa(ns)
PUF /Co50/Xa50(gel)
- « -
50%CO*
50%Xa(gel 15%)
*CO content in hydroxyl comprising component
Table 1: Compositions of IPU, PUF, and their natural components based structural-modified analogues.
Colloid-chemical characteristics
Colloid-chemical characteristics of aqueous IPU dispersions: pH value was determined using pH-meter “pH-150 M” (Russia) according to [14]; Particle size measurements were determined from the turbidity spectrum using FEK-56M, according to Ref [15].
Mechanical properties
Mechanical properties were tested using a RM-30-1 test machine (a device constructed in Ivanovo Measure-works, Russia). The number of samples used in each measurement was three. Samples were prepared in a form of strips (width - 4mm, operating length - 2mm). Measurements were carried out in accordance with [16]; allowed error - 3%.
Density was tested according to [17].
Water vapor permeability and moisture absorption were tested according to [18].
Water absorption
Water absorption was tested according to [19] Preliminary weighed dry films were immersed in water for 1 h and 24 h, whereupon the excess water was removed with filter paper and samples were weighed. Water absorption (WH2O, wt. %) was calculated according to: WH2O (%) = [(Ww-Wd)/WD] ?100%, where Ww and Wd –weight of the films in a wet and dry state, correspondingly.
FTIR measurements
FTIR measurements were performed on Bruker “Tensor-37” Fourier transform infrared spectrometer.
The Wide-Angle X-Ray Diffraction Scattering (WAXS)
The Wide-Angle X-Ray Diffraction Scattering (WAXS) were recorded with X-ray diffractometer DRON-4-07 with roentgen schema made according to DebyeSherer method (transmission).
The Small-Angle X-ray Diffraction Scattering (SAXS)
The small-angle X-ray Diffraction Scattering (SAXS) were recorded using small-angle camera KRM-1 with flat-filled collimator, made according to Kratki method [20]. All X-ray measurements were carried out in CuKa radiation, monochromatic with Ni-filter at T = 22 +- 2°C.
Degree of hydrolysis in acid and alkali medium
Degree of hydrolysis in acid and alkali medium was determined by weighing the samples before and after hydrolysis. Pre-weighed samples were immersed in 0.1N solution of KOH and HCl and kept in a thermostat for 30 days at T = 37°C, afterwards the samples were dried to constant weight with following control weighing.
Pyrolytic Mass-Spectrometry (PMS)
The device consisting of mass spectrometer MX-1321 with allows to define the components of gas mixtures in the range of mass numbers of 1-4000 and cell for linear programmable pyrolysis at temperatures of 25-400°C has been used for study of polymer samples by PMS method. The sample was placed in a cell, which was evacuated (1.33x10-4 Pa) for 30 minutes at 25°C. The same pressure was maintained during the experiment. The heating rate was (6 ± 1) °C/min. The accuracy of determining the sample temperature was ± 1°C. The ionization energy in the chamber of the mass spectrometer was 70eV.
Histological study
Histological study of developed laboratory sample of composite material PP/PUF/Xa70 assigned for hernioplasty has been carried out on white mice. An incision of 2cm was carried out on animals under an anesthesia after treatment of the surgical field on the white line of the abdomen. The skin on either side of the incision was peeled with medical instrument for the formation of a subcutaneous pocket of the size 2 by 2cm. Aponeurosis was dissected by 1.5cm, followed by the imposition in a top o of the cut of mesh implant, the edges of which were placed in a subcutaneous pocket. Mesh implant was fixed to the abdominal wall with the locking stitch around the perimeter. After the ending of experiment in terms of 10,30,60 and 90 days, the divisions of abdominal wall in the implant region were removed and fixed in formaldehyde solution with a volume fraction of 10% for at least 24 hours. The material was then dehydrated in alcohols of increasing concentration series, clarified in chloroform and embedded in paraffin. Then sections with a thick of 7 μm were colored and studied by light microscope Leica ICC50 HD.
Adhesion of microorganisms on the film surfaces
The active strain Bacillus Subtilis (IMB B-7023), used for microorganisms’ adhesion study is highly widespread in nature and possesses a high hydrolytic activity relative to polymer compounds, and may serve as a model of cells-destructors [21]. Adhesion of B. subtilis strain to the film surfaces was tested in accordance with [22]. Initial suspension of bacillus cells contains (1.0 ± 0.07)•109 cel/ml. Object plates covered with IPU/Xa were immersed into the liquid nutrient medium by Menkina with preliminary grown microorganisms strain and kept for 5hours. Clean object plate and the plate covered with IPU without Xa were used as references. The count up method with the use of polarizing-interference microscope “Biolar” (Polland) was applied. Calculation of microorganisms’ quantity was carried out in 50 fields of vision; each contained no less than 15 bacteria cells. Experiments were repeated 3 times for each sample.
The degradation rate of IPU and PUF in model environments [23] has been studied by weight loss during the time of samples’ incubation in containers with a soil of medium biological activity at pH 7.3, relative humidity 60% and temperature 12-25°C. Study of the soil microflora has shown the presence of the following fungal genera Rhizopus, Aspergillus, Penicillium.
Results and Discussion
FTIR analysis
The presence of fragments of Xa and CO in the structure of IPU macromolecules caused both by chemical and physical interactions of macro chain components has been confirmed by IR studies. There are all characteristic bands of block Polyurethane (PU) observed in IPU matrix (Figure1 (1)): ? (NH) - 3325 cm-1, ? (C = O) of urethane group - 1720cm-1, d (NH) and ? ( CO-N) - 1540cm-1, ?assym (COC) - 1244cm- 1 and ?sym (COC) - 1168cm-1, ? (NH)assoc - 3314cm-1. A comparative analysis of IR - spectra of IPU/CO20/Xa20 and IPU-matrix (Figure 1 (2)) shows both the intermolecular hydrogen bonds between polar groups of PU and OH groups of Xa (CO) and presence of free OH groups. The absorption region 1700 - 1800cm-1 is characterized by redistribution of the intensities of the bands of associated (1704 - 1710cm-1) and non-associated (1720-1730cm-1) C = O – groups; associated NH - group (region 3325 -3360cm- 1) (Figure 1(4)). A broad band of 3564cm-1 IPU/CO20 (Figure 1(3)) is a result of OH groups of CO.
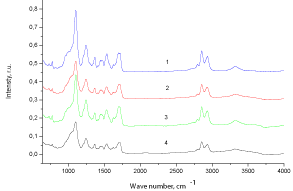
Figure 1: FTIR – spectra: 1 - IPU, 2 - IPU/CO20/Xa20, 3 - IPU/CO20, 4 -IPU/
Xa20.
FTIR-spectra of Xa(ns) and PUF/CO50/Xa50(ns.) are performed in (Figure 2). FTIR-spectrum of Xa(ns) (Figure 2 (2)) contains the characteristic bands: 3200-3600?m-1 – hydrogen bonds; 2896 and 2921 ?m-1 – ? (?-?)-bonds.
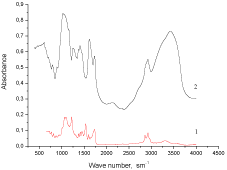
Figure 2: FTIR-spectra of 1 - PUF/CO50/Xa50(ns), 2 - Xa(ns).
The presence in the FTIR-spectrum of PUF/CO50/Xa50(ns.) (Figure 2 (1)) of bands (3318-3286) ?m-1 – ? ( N?), (1727-1725) ?m-1 – ?(?=?) of urethane groups, as well as the band (1640-1598)?m-1 – ? (?=?) of urea groups has confirmed the availability of Xa and CO fragments in PUF/CO50/Xa50(ns.) composition and redistribution of hydrogen bonds system in the region of 3200-3600?m-1.
X-ray analysis
X-ray scattering results of IPU/Xa, IPU/CO, and IPU/CO20/Xa20 are performed on (Figure 3 and 4). The broad diffraction hupm at 2? = 20,0°C could be observed for IPU/CO20, indicating the amorphous nature of the film (Figure 3), curve 1). In accordance with the Bragg equation d=? (2sin?)-1, where ? – the wavelength of the characteristic radiation (for ?uKa radiation ?=0,154 nm), the middle period amounts d=0.443nm. CO in IPU/CO20 composition, also has an amorphous nature, as indicated by WAXS data at 2? = 6,1°C - 13,3°C. Structural-chemical modification of IPU-matrix with Xa is confirmed by comparing the experimental X-ray diffraction pattern and calculated on the assumption of additive contributions of IPU and Xa to the diffraction pattern of their mixture (Figure 3, curves 2 and 2’), which is manifested in the differences of amorphous-crystalline structure of Xa. In its turn IPU/CO20 and IPU/ CO20/Xa20 (Figure 4, curves 1,3) according to X-ray data are characterized by amorphous structure [24]. The homogeneity of the IPU/Xa20 can be explained by the high level of intermolecular physical interactions between macro chain components compared with IPU/CO20/Xa20.
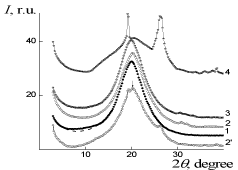
Figure 3: WAXS patterns of: 1 – IPU/CO20, 2, 2'– IPU/Xa20 (2' - additive
diffractogram), 3 – IPU/CO20/Xa20, 4 – Xa.
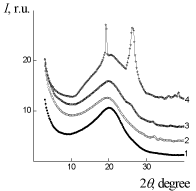
Figure 1: SAXS patterns: of 1 – IPU/CO20, 2 – IPU/Xa20, 3 – IPU/CO20/Xa20.
WAXS diffraction patterns of PUF, PUF/Xa50(ns) and PUF/Xa50(gel) (Figure 5) show a higher level of macromolecular interactions in the PUF/Xa50(ns) as compared to the PUF/Xa50(gel), in which the crystalline structure of Xa is more pronounced. PUF matrix as well as PUF/ Xa50(ns) and PUF/Xa50(gel) is characterized by a heterogeneous structure (Figure 6), however, the range of the heterogeneity lp [24] directly connected with averaged diameter of micro regions of hetrogenity, depends on the physical state of Xa in PUF composition (Table 2 ). The relative level of heterogeneity, characterized by Porod invariant Q' [25] in the PUF matrix is in 1.6 and 3.4 times higher in comparison with PUF/Xa50(ns) and PUF/Xa50(gel), which is fully correlated with the results of X-ray diffraction (Table 2).
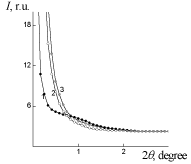
Figure 5: WAXS patterns of: 1 – PUF-matrix, 2 – PUF/Xa50(ns), 3 – PUF/
Xa50(gel), 4-Xa.
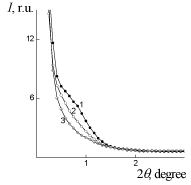
Figure 6: SAXS patterns: of: 1 – PUF-matrix, 2 – PUF/Xa50(ns), 3 – PUF/
Xa50(gel).
PUF composition
lp , nm
Q', r. u.
PUF-matrix
18
1,53
PUF/Xa50(ns)
15
0,97
PUF/Xa50(gel)
19
0,45
Table 2: Parameters of heterogeneous structure of PUF.
Pyrolytic mass spectrometry
The presence of fragments of structural modifiers in macromolecule structure has been proved by PMS method. The complete thermal degradation of Xa occurs in a narrow temperature range with maximum intensity at 243°C (Figure 7) whereas the twostage thermal degradation is characterized for PUF/Xa50(gel) at 220 and 317°C in the absence of the peak, which characterizes the Xa degradation.
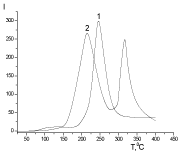
Figure 7: The temperature dependence of the intensity of the emission of
volatile thermal destruction products 1-Xa, 2-PUF/Xa50(gel).
Thus, the results of FTIR spectroscopy, X-ray scattering and PMS indicate the presence of Xa and CO fragments in the IPU and PUF structure, which confirms the structural-chemical modification of polyurethane matrices.
Colloid-chemical, mechanical properties and hydrolysis of the samples
?olloid-chemical properties of IPU dispersions and physicmechanical properties of the films are performed in (Table 3) The increase of the Xa amount in the IPU composition, as well as the replacement of the part of oligoether component of IPU-matrix on CO (20 wt %) in IPU/CO does not effect on stability of dispersions. The pH of the dispersions reduces, due to the presence of carboxyl groups and glucuronic and pyruvic fragments of Xa. Water absorption and film density of IPU/Xa and IPU/CO20/Xa20 increases with the increase of Xa content. Tensile strength of IPU/Xa5 compared with IPU-matrix is lower as the result of violation of hydrogen bonds system of IPU. The strength of IPU/Xa20 and IPU/CO20/Xa20 increases significantly and exceeds the value of IPU-matrix in 4-2 times, respectively, as a result of intermolecular physical interactions, which is accompanied by a decrease of elasticity. Evidently at Xa content 20% an optimal realization of intra- and intermolecular hydrogen bonds, leading to the improvement of mechanical properties occurs.
Properties of the dispersions
Properties of the films
IPU composition
Average particle size rav, nm
Tensile strength, MPa
??
Elongation, ε,
% Water absorption (%), 24 hours
. Density, ρ, g/cm³
Hydrolysis ???/??l
IPU-matrix
71
7,84
7,3
1470
2,6
1,054
0,1 /0,22
IPU/Xa5
176
7,17
2,3
742
22,9
1,075
1,8/1,68
IPU/Xa20
351
6,52
29,0
235
179
1,126
4,7 / 4,6
IPU/CO20
268
8,09
3,3
500
3,8
1,055
0,9 / 0,6
IPU/CO20/Xa20
198
7,70
14,9
-
1,1
1,134
9,6/6,2
Table 3: Properties of aqueous dispersions and based film materials.
The presence of Xa in the IPU chain determines the nature of hydrolysis of obtained materials - one of the key factors of the degradation under environmental conditions. The hydrolytic degradation of the materials in acidic and alkaline media was conducted under model conditions. The higher the content of Xa in the samples, the greater the weight loss (Table 3). The films IPU/ Xa are more susceptible to hydrolytic degradation as compared with IIPU matrix and CO based polymer IPU/CO. Moreover, all samples are more susceptible to alkaline hydrolysis.
The results of study of physical and mechanical properties of PUF/ CO/Xa are presented in Table 4. The tensile strength of structurallymodified PUFs increases by 13-14% compared to the matrix (except PUF/Xa50(gel), which correlates with the WAXS results) while a vapor permeabilty is reduced along with significant increase of water absorption in 46-73 times, which is factor contributing to the activity of microorganisms and thus, the process of PUF/CO/Xa degradation under the influence of aggressive environmental factors.
PUF composition
Tensile strength, ?P?
Vaporpermeability mg/?m²hour
Moisture absorption %
PUF-matrix
0, 230
4,35
0,027
PUF/Xa50(gel)
0,058
4,50
2,30
PUF/Xa50(ns)
0,285
6,00
1,54
PUF /CO50/Xa50(gel.)
0,300
3,52
1,77
PUF /CO50/Xa50(ns.)
0,330
2,90
1,98
Table 4: Physic-mechanical properties of PUF, vapor permeability and moisture absorption.
Histological study
The degradation of polymeric materials occurs as a result of exposure to aggressive abiotic and biotic environmental factors, including those produced by Microorganisms (MO) enzymes and metabolic products - organic acids (citric acid is a product, produced by mold fungi of the genus Aspergillus). The biotic factor which affects the implanted materials is the biological media of the living body. Problems of plasty of soft tissue including herniography (plasty of hernia with a mesh) are the acute inflammatory reactions with the formation of dense scar tissue, distorting the mesh; purulent inflammation of wounds, as well as the seromas formation (accumulation of serous fluid associated with the traumatization of the soft tissues).
These reasons stimulate the search for new synthetic materials for solving the abovementioned problems. The most promising direction is the creation of composite polymer materials for endoprosthesismeshs using degradable biologically inert polymeric materials.
A histological study (10-90 days) of developed laboratory samples of PP/PUF/Xa70 mesh material, assigned for hernioplasty of hernia has been carried out.
In 10 days a seroma was formed. The common capsule with the packed inner layer, heavily vascularized and infiltrated with lymphocytes had uneven thickness. Its thin loose outer layer contained a significant amount of tissue basophils. The masses of detritus were observed in a cavity of the capsule. The vascularized bands of newly formed tissue containing significant amounts of lymphocytes and macrophages were observed in some parts inside the capsule. (Figure 8a). In some areas along the periphery of these bands the fragments of homogeneous foreign body were visible.
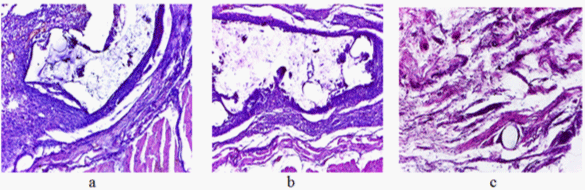
Figure 8: Zone of implantation in 10 days (a), 30 days (b), and 90 days (c) (colouring with haemotohyline and eosine; magnification in 100 times).
In 30 days around the mesh implant a common connective-tissued capsule of moderate thickness, richly vascularized and infiltrated by lymphocytes (Figure 8b) was formed. Its outer layers contained a significant amount of tissue basophils. Small accumulations of tissue’s detritus and a few cellular elements, mainly macrophages and lymphocytes, as well as isolated neutrophils were noted in PUF pores.
In 90 days there was no seroma. Single monofilaments of PP, surrounded by thin vascularized connective-tissued capsule (Figure 8c) were seen in the area of implantation. Furthermore, individual clusters of macrophages were observed. There were no signs of inflammation in the surrounding the implant tissue.
Thus, the obtained results have shown the complete resorption of the polyurethane component and the absence of injury and inflammation of the surrounding soft tissues.
Adhesion of microorganisms on the film surfaces
The determining factor of degradation of polymer materials under the influence of Microorganisms (MO) is their adhesion to the surfaces of materials [26] which was evaluated both as the quantity of adhered phosphatomobilizing cells of a strain of Bacillus subtilis (BS) (Figure 9), and by measuring of electrokinetic properties of IPU/Xa (Figure 10).
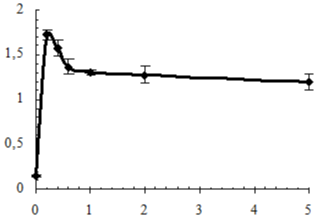
Figure 9: Adhesion of Bacillus subtilis to the surface of IPU/Xa films vs. Xa
content.
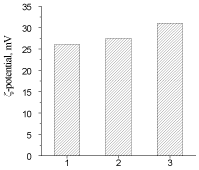
Figure 10: ?-potential of: 1-IPU-matrix, 2- IPU/Xa5, 3-IPU/Xa0,2
IPU/Xa materials in comparison with the IPU matrix possess more than 10 times greater susceptibility to MO attack, which, however, decreases with increasing of Xa content as the result of the chemical nature of Xa (presence of a large amount of glucuronic and pyruvic carboxyls imparting anionic character to the Xa molecules), which reduces the electrostatic interaction between the electronegative charge of the BS cell membrane with the IPU/Xa sample surface. This assumption is confirmed by the study of electrokinetic properties (?-potential) of IPU/Xa, the value of which (Figure 10) was calculated according to [27].
The presence of Xa in IPU/Xa samples leads to growth of ?-potential as compared to the IPU-matrix due to anionic nature of Xa. However with the increase of Xa this dependence is reversed. Possibly, at low Xa content (0.2 wt.%) the motion of chemicallybonded fragments of Xa and IPU occurs with sufficient rate and their charges are summarized, resulting in increase of ?- potential (Figure 9, sample 3), which leads to the maximum adhesion of BS to the surface of the sample (Table 5). Lowering of ?-potential in the IPU/Xa5, may be a result of increasing of steric sizes of IPU/Xa macromolecule, where Xa is largely bonded by covalent and hydrogen bonds [28].
Content of modifier(%wt)
Mass loss (%) during 1,2,4,6 months
?? of the soil after the test *
CO
Xa
1
2
4
6
1
2
4
6
-
-
0,3
1,0
1,3
3,0
7,28
7,15
7,17
7,06
-
5
6,2
6,3
10,2
11,3
7,33
6,88
6,53
6,51
-
20
18,3
20,7
24,0
38,8
7,63
7,59
7,56
7,53
20
-
0,4
0,85
1,5
1,7
7,60
7,56
7,37
7,22
20
20
14,3
18,2
28,8
68
7,61
7,60
7,49
7,21
* – ?? of the soil before the test amounted to 6,82
Table 5: Results of biodegradation (model conditions) of IPU structurally modified with CO and Xa.
Influence of composition of IPU and PUF containing structural modifiers on mass loss and pH of the soil has been studied under the model conditions [23] during 6 months (Table 5). Mass loss of incubated samples increases with the duration of the experiment, and in 6 months it amounts to 11,3-68%, which exceeds the content of renewable components in 2 times, and the specified characteristics of the matrix in 3.8 - 23 times.
Under the action of aggressive media on structurally modified IPU within 30 days at (25 ±1)°C (Table 5) the fragmentation of the film samples was observed, which increased with the increase of Xa content. The systematic reduction over time of pH of the soil is the result of organic acids formed due to vital functions of MO.
Thus, chemically bonded renewable components contribute to degradation processes of polyurethanes. The more the content of natural component, the higher the mass loss of the modified sample.
Comparative studies of the effect of foam composition on the degree of their degradation in the soil, and at exposure to aggressive environments (Table 6) have been carried out. More prone to the effects of these factors is the PUF/CO/Xa50(gel) compared with its analogue PUF/CO/Xa50(ns) due to a higher level of interaction between components in PUF/CO/Xa50(ns), which has been confirmed by X-ray diffraction and physic-mechanical studies.
PUF
Mass loss (%) after hydrolysis
(30 days, (20±1)o?
Mass loss (%) after incubation in the soil (month.)
0,1n. ???
0,1 n. ???
1
2
3
4
PUF- matrix
1,88
1,92
0,0
0,0
0,00
0,15
PUF/CO/Xa50(gel)
35,31
45,47
38,50
43,52
49,71
7,65
PUF/CO/Xa50(ns)
6,86
11,85
21,9
27,64
33,47
40,55
Table 6: Effect of PUF composition on the degree of their degradation in the soil and after hydrolysis.
Mass loss of PUF/CO/Xa50(gel) in acidic and alkaline media exceeds this characteristic of PUF/CO/Xa50(ns) in 5 and 3.8 times, correspondingly, and after 4 months of incubation in the soil - in 1.4 times.
Conclusion
New multi-purpose ecologically friendly (bio) degradable polymeric materials based on different type of polyurethanes and renewable components of biotechnological and vegetable nature have been obtained at the maintaining the inherent to polyurethanes service performance. It was shown that structural-chemical modification of ionic polyurethanes and foam polyurethanes with products of biotechnological and natural origin, due to the chemical interaction of their macro chain components, as well as the variation of their nature and ratio promote the initiation of the degradation processes under environment conditions (after the end of the term of exploitation), and allows to regulate their properties and life time
Structural studies have shown the presence of chemically bonded natural fragments in the macro chain of synthesized polyurethane materials that provide a degradation of the material as a whole, not just its natural component. This has been confirmed by comparative studies of incubated samples and modeling studies of aggressive environments’ effect.
New renewable resources based polyurethane materials in comparison with the polyurethane matrix possess more than 10 times greater susceptibility to microorganisms attack.
A histological study (in vivo) of developed laboratory samples of PP/PUF/Xa70 material has shown the complete resorption of the polyurethane component and the absence of injury and inflammation of the surrounding soft tissues that allows considering synthesized structurally-modified polyurethanes as potential biocompatible materials advanced for biomedical application.
References
- Manawwer A, Deewan A, Eram S, Fahmina Z, Sharif A. Vegetable oil based eco-friendly coating materials. Arabian Journal of Chemistry. 2014; 7: 469-479
- Miao S, Wang P, Su Z, Zhang S. Vegetable-oil-based polymers as future polymeric biomaterials. Acta Biomater. 2014; 10: 1692-1704.
- Eram S, Fahmina Z, Deewan A, Manawwer A, Sharif A. Recent advances in vegetable oils based environment friendly coatings. Industrial Crops and Products. 2015; 76: 215-229.
- Holst O, Müller-Loennies S. Microbial Polysaccharide Structures. Kamerling P, editor. In: Comprehensive Glycoscience. From Chemistry to Systems Biology. 2007; 1: 123-179.
- Moldovenau SC. Analytical pyrolysis of natural organic polymers. Brown & Williamson Tobacco Corp. Macon, GA, USA. 1998.
- Travinskaya TV, Savelyev YuV. Aqueous polyurethane-alginate compositions: Peculiarities of behavior and performance. European Polymer Journal. 2006; 42: 388-394.
- Travinskaya TV, Mishchuk ??, Perepelitsyna LN, Savelyev YuV. Preparation and properties of (bio) degradable materials based on ionic polyurethane and polysaccharide. Polymer Journal (in Russian). 2010; 32: 66-74.
- Travinskaya T, Savelyev Yu, Mishchuk E. Waterborne polyurethane based starch containing materials: preparation, properties and study of degradability. Polymer degradation & stability. 2014; 101: 102-108.
- Savelyev YuV, Mishchuk EA, Markovskaya LA, Travinskaya TV. Method of obtaining of polymer composition. Patent 51301. Ukraine. 2010.
- Savelyev Y, Veselov V, Markovskaya L, Savelyeva O, Akhranovich E, Galatenko N, et al. Preparation and characterization of new biologically active polyurethane foams. Mater Sci Eng C Mater Biol Appl. 2014; 45: 127-135.
- Savelyev YuV, Markovskaya LA, Akhranovich ER, Savelyeva A, Parkhomenko NI. Polyurethane foam material. Patent 92178 Ukraine. 2014.
- Savelyev YuV, Markovskaya LA. Patent 106843 Ukraine. A method of obtaining of polyurethane foams capable to degradation. 2014.
- Savelyev YuV, Travinskaya TV, Markovskaya LA, Brykova AN. Method of obtaining of biodegradable composition. Patent 93372 Ukraine. 2014.
- ISO 976: 1996 Rubber and plastics. Polymer dispersions and rubber latices. Determination of pH.
- Shegolev SYu, Klenin VI. Determination of parameters of complicated disperses polymer system from turbidity spectrum. Vysokomol Soed B. 1971; 13: 2809-2815.
- Standard 14236-81 Polymer films. Tensile strength test method.
- Standard 15139-69. Plastics. Methods for the determination of density.
- Standard 22900-78. Artificial leather and films. Methods for determination of water vapor permeability and moisture absorption.
- Standard 4650-80. Plastics. Methods for the determination of water absorption.
- Kratky O, Pilz I, Schmitz PJ. Absolute intensity measurement of small-angle x-ray scattering by means of a standard sample. J. Colloid Interface Sci. 1966; 21: 24-34.
- Semenov SA, Gumargalieva KZ, Zaikov GE. Biodeteriorations of materials and products (in Russian) Encyclopedia inzhenera-khimika. 2007; 4: 4-7.
- Roy AA, Kisten LG, Kurdish IK. Physiological activity of methanotrophs in mixed cultures with typical representatives of microfloras of coal mines. Microbiol J 1998; 60: 24-30.
- Ermolovich, Makarevich V, Goncharova P. Methods of assessment of biodegradability of polymer materials. Biotechnology. 2005; 4: 47-54.
- Perret R, Ruland W. Eine verbesserte Auswertungsmethode fur die Rönt-genkleinewinkelstreuung von Hochpolymeren. Kolloid Z. – Z. Polymere.1971; 247: 835-843.
- Porod G. General theory //Small-angle x-ray scattering. London: Acad. Press. Glatter O, Kratky O, editors. 1982; 17-51.
- Zviagintsev DG. Interaction of microorganisms with solid surfaces. Moscow: Ed. of Moscow University. 1973; 176.
- Mikheeva V, Pikula NP. Determination of zeta potential by electrophoresis. Tomsk: Ed of Tomsk polytechnic inst-t. 2000; 19.
- Travinskaya TV, Brykova N, Kurdish IK, Chevychalova AV, Savelyev YuV. Degradable ionomeric polyurethanes on the basis of exopolysachcaride xanthan. Reports of the NAS of Ukraine (in Russian). 2014; 7: 132-139.