
Case Report
Austin J Biomed Eng. 2015; 2(1): 1031.
Motor Evoked Potential Monitoring During Robot- Assisted Transaxillary Thyroid Surgery: A Report of Feasibility in Two Cases
Gilles Boccara¹*, Helen Pickburn¹, Patrick Aidan¹, M Bernard Ollat² and M Pascal Pagneux²
¹Department of Anesthesia and Intensive Care, American Hospital of Paris, Neuilly S/Seine, France
²Biomedical Department of American Hospital of Paris, France
*Corresponding author: Gilles Boccara, Department of Anesthesia and Intensive Care, American Hospital of Paris, Neuilly S/Seine, France
Received: October 12, 2015; Accepted: December 30, 2015; Published: December 31, 2015
Abstract
Gasless Transaxillary robotic thyroid surgery has recently been proposed and developed in many countries. We report the feasibility of continuous intraoperative neuromonitoring of Transcranial Electric Motor Evoked Potentials (tceMEP) in two cases in order to monitor the risk of brachial plexopathy due to ipsilateral arm positioning during the axillary approach. The anesthetic implications are also described, specifically the use of intraoperative monitoring.
Keywords: Motor evoked potential; Anesthesia; Thyroid; Robot; Axillary
Introduction
The transaxillary robotic approach to thyroid surgery was first proposed in 2007 in Korea and has since been performed in North America [1-4]. During the initial experience at our clinical centre with this procedure one case of transient ipsilateral brachial plexopathy occurred [5].
The incidence of ipsilateral brachial plexopathy during the transaxillary robotic approach to thyroid surgery is assessed to be near to 0.3% [6], but if systematically searched for using neuromonitoring this figure would be likely be higher. There are two techniques currently proposed to prevent brachial plexopathy during the Transaxillary robotic approach. Firstly, owing to the constraints of surgical duration and the learning curve of this method the risk of plexopathy could be prevented by using the modified arm position as proposed by Kuppersmith et al. [7]. Secondarily, intraoperative multimodality neuromonitoring to aid the detection of evolving positional brachial plexus injuries could be utilized [8,9]. We report in two cases the feasibility of continuous intraoperative neuromonitoring of Transcranial Electric Motor Evoked Potentials (tceMEP) during the transaxillary robotic approach to thyroid surgery and the ensuing anesthetic implications.
Case Report
This report describes two patients who underwent thyroid lobectomy using the robotic axillary approach (da Vinci Si HD Surgical System, Intuitive Surgical, Sunnyvale, CA). The patients were enrolled and clearly informed about the advantages and risks of conventional open versus robot-assisted transaxillary thyroid surgery and gave preoperative written consent.
After oral premedication with hydroxyzine (1 mg.kg-1) and gabapentin (5 mg.kg-1) general anesthesia was initiated with a Target Controlled Infusion (TCI) of propofol and remifentanil. A single dose of intravenous atracurium (0.5 mg.kg-1) was used to facilitate endotracheal intubation. Endotracheal intubation was performed using a tube equipped with electrodes to allow for nerve stimulation and to aid in the detection of injury to the recurrent nerve by stimulated electromyography of the vocal cords (Xomed NIM II EMG endotracheal tube II, Medtronic Inc., Jacksonville, FL). Propofol and remifentanil administration using TCI were adjusted according to the depth of sedation, assessed by EEG bispectral analysis with an objective range 40 to 55 (BIS, Aspect Medical Systems, Newton, MA), and guided by blood pressure and heart rate monitoring. Mechanical ventilation on volume control with mixed oxygen/air was titrated an oxygen saturation (SpO2) > 97% and end-tidal carbon dioxide between 30 and 36 mmHg. A forced-air warming blanket was positioned before induction and used throughout the procedure.
According to the guidelines relating to remifentanil use, intravenous acetaminophen (1 g), ketoprofen (100 mg) and nefopam (20 mg) were infused 60 minutes prior to skin closure and TCI interruption to prevent post-operative analgesia. Upon waking, and after endotracheal extubation, intravenous morphine was titrated in the recovery room using the Visual Analog Scale (VAS) (a score greater than 3/10 indicating the need for opiate analgesia). Injury to the recurrent laryngeal nerve was assessed intraoperatively using nerve stimulation and post-operatively by the detection of voice changes.
During surgery the patients were positioned supine on the operating table with a soft triangular pillow under the shoulders and the contralateral upper limb alongside the body. Continuous intraoperative neuromonitoring of transcranial electric motor evoked potentials (tceMEP) was performed using the NIM-Eclipse (Medtronic, Medtronic Inc., Jacksonville, FL). To perform the tceMEP needle electrodes were placed in biceps, triceps, extensor carpi radialis and flexor carpi ulnaris. Electrical stimulation with brief, high-voltage (300–1,000 V) anodal pulse trains (pulse width ¼ 50 ls, N pulses ¼ 3–7, interpulse interval ¼ 1–5) was induced. The stimulus was delivered between two subdermal needle electrodes inserted subcutaneously over motor cortex regions C1 and C2. In order to check the effective response and disappearance of muscle relaxation the extensor carpi radialis in the contralateral arm was also monitored. The ipsilateral upper limb was placed in the modified position suggested by Kuppersmith et al. [10], with right angle flexion of the elbow and the forearm positioned before the head (Figure 1) so the axillary space could be freely accessed [5,11]. Stimulation was performed at the following times: in neutral arm position, in modified arm position before incision, during dissection of the working space, after retractor placement, during robotic working time (Figure 2), after removal of robotic instruments, after skin closure and after infiltration by 10-15 ml of 0.75% ropivacaine and placement of the ipsilateral arm alongside the body.
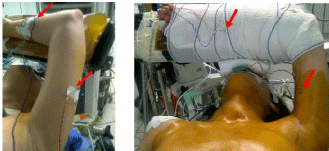
Figure 1: Proposed positioning of the ipsilateral upper limb for the axillary
approach to the thyroid, according Kuppersmith RB. The red arrows show
some electrical needles of TceMEP.
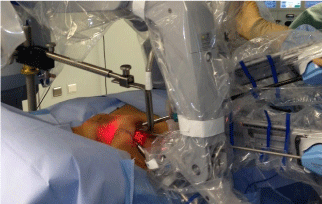
Figure 2: Placement of the retractor and Da Vinci SiHD robot arms in position
during console working time.
The first case was a 39-year-old female (77 kgs; 168 cm) who presented with a right-sided cold thyroid nodule (3cm in size). Fineneedle aspiration demonstrated follicular cells and final pathology established it as an adenomatoid nodule. The working space time, console time and total duration of surgery were respectively 55, 50 and 140 minutes. The second case was a 24-year-old female (60 kgs; 169 cm) who presented with a left-sided cold thyroid nodule (3.5cm in size). Final pathology demonstrated it to be a follicular adenoma. The working space time, console time and total duration of surgery were respectively 35, 55 and 120 min.
Endotracheal extubation was performed at the end of surgery and neither patient required morphine titration in the recovery room or experienced nausea or vomiting. Postoperative bleeding was less than 100 ml in both cases. The patients were discharged from hospital on day 3 post-operative without complications.
When measured intraoperatively the tceMEPs at each time of measurement were similar to the initial recording undertaken 30 min after anesthesia induction (Figure 3). Muscle relaxation by single dose of atracurium did not modify the results when measured after 30 minutes. Neither patient developed clinical paresis of the ipsilateral upper limb nor no other neurologic deficits occurred. There were no recurrent laryngeal nerve injuries.
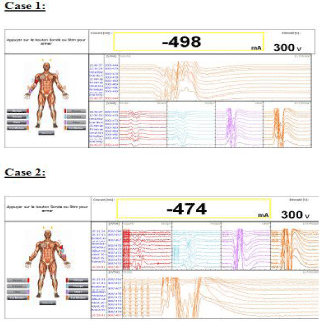
Figure 3: Response to stimuli through needles of TceMEP in the two cases
(case 1 and 2).
Discussion
For the two cases described above we have standardized our general anesthesia technique; choosing to monitor the depth of anesthesia with limited access to the head and to use an or tracheal intubation tube equipped with electrodes to monitor the vocal cords and allow for stimulation of the recurrent laryngeal nerve during surgery [12].
Lee et al. have recently reported experience with 1043 patients in Korea [6] and described temporary brachial plexus stretch injury in 0.3% of these cases using clinical diagnosis, however if systematically searched for using neuromonitoring this figure would likely be higher. In terms of the risk of injury to the brachial plexus the first concern is the longer duration of this procedure in comparison to open or endoscopic methods. The surgery takes longer because it occurs in two stages: initially an open axillary dissection to create the operative space (working space time), and a second robotic stage to resect the thyroid or parathyroid glands (console time). In addition to this a second concern is the learning curve for surgeons leading to extended operative times in their early experiences with the procedure.
Luginbuhl et al. suggested that the proximity of movable bony structures such as the clavicle, first rib, and humeral head to the brachial plexus predisposes the neural elements to stretch or compression injury when the arm and shoulder are placed in extreme abduction [8]. Hyperextension of the neck with the arm fully abducted could also be involved in the mechanism of brachial plexopathy. The initial positioning of the ipsilateral arm proposed by Chang et al. involved the ipsilateral limb being placed on an arm support in full extension of the elbow with 180-degree abduction of the arm at the shoulder, as is the South Korean practice [1]. However, following one case of reversible brachial paresis potentially related to stretching of the brachial plexus we have modified the ipsilateral arm position according to Kuppersmith et al. [10] and the patient is comfortably positioned, without point compression or stretching of the neurovascular plexus of the ipsilateral upper limb. Since we adopted this positioning no further cases of nerve injury were observed in this small sample size [11].
Luginbuhl et al. also proposed the use of intraoperative and continuous transcranial electric motor evoked potentials and somatosensory evoked potential monitoring [8]. In the first case of pediatric robotic thyroid surgery this specific neuromonitoring was also proposed [9]. The use of continuous intraoperative neuromonitoring of Transcranial Electric Motor Evoked Potentials (tceMEP) in the cases described above suggests that the technique is viable and effective in the monitoring and prevention of brachial plexopathy, although evidently should be assessed in large clinical trials to confirm its reliability.
In reference to the anesthetics ramifications of the use of intraoperative neuromonitoring the use of volatile anesthetic agents should be avoided [13-15]. The authors choose a total intravenous anesthesia regime using TCI of propofol and remifentanil. Under BIS monitoring the patients were anesthetized without disturbances of the neuromonitoring. Based on the potential effects of general anesthesia and the elimination of the initial muscle relaxant we recommend that the contralateral arm, carefully positioned and padded, is used as a control.
In conclusion, the intraoperative neuromonitoring of Transcranial Electric Motor Evoked Potentials (tceMEP) can be proposed for use during Robotic Thyroid Surgery in clinical practice. It is easy to use and can effectively monitor the risk of brachial plexopathy according to the specific intraoperative ipsilateral arm position. The anesthetic implication for this monitoring is to avoid volatile anesthetics and the authors suggest TCI using propofol and remifentanil. Further studies are required to assess the frequency of nerve injury under this specific monitoring and the intraoperative implications for the surgeon in cases of TceMEP alteration.
References
- Chang EH, Lobe TE, Wright SK. Our initial experience of the transaxillary totally endoscopic approach for hemithyroidectomy. Otolaryngol Head Neck Surg. 2009; 141: 335-339.
- Kang SW, Lee SC, Lee SH, Lee KY, Jeong JJ, Lee YS, et al. Robotic thyroid surgery using a gasless, transaxillary approach and the da Vinci S system: the operative outcomes of 338 consecutive patients. Surgery 2009; 146: 1048-1055.
- Holsinger FC, Terris DJ, Kuppersmith RB. Robotic thyroidectomy: operative technique using a transaxillary endoscopic approach without CO2 insufflation. Otolaryngol Clin North Am 2010; 43: 381-388.
- Kang SW, Jeong JJ, Nam KH, Chang HS, Chung WY, Park CS. Robotassisted endoscopic thyroidectomy for thyroid malignancies using a gasless transaxillary approach. J Am Coll Surg. 2009; 209: 1-7.
- Boccara G, Guenoun T, Cohen B, Aidan P. Perianaesthetic concerns for the new robot-assisted transaxillary thyroid surgery: a report of seven first cases. Ann Fr Anesth Reanim. 2011; 30: 600-603.
- Lee J, Yun JH, Nam KH, Choi UJ, Chung WY, Soh EY. Perioperative clinical outcomes after robotic thyroidectomy for thyroid carcinoma: a multicenter study. Surg Endosc. 2011; 25: 906-912.
- Kuppersmith RB, Salem A, Holsinger FC. Advanced approaches for thyroid surgery. Otolaryngol Head Neck Surg. 2009; 141: 340-342.
- Luginbuhl A, Schwartz DM, Sestokas AK, Cognetti D, Pribitkin E. Detection of evolving injury to the brachial plexus during transaxillary robotic thyroidectomy. Laryngoscope. 2012; 122: 110-115.
- Davis SF, Abdel Khalek M, Giles J, Fox C, Lirette L, Kandil E. Detection and prevention of impending brachial plexus injury secondary to arm positioning using ulnar nerve somatosensory evoked potentials during transaxillary approach for thyroid lobectomy. Am J Electroneurodiagnostic Technol 2011; 51: 274-279.
- Kuppersmith RB, Holsinger FC. Robotic thyroid surgery: an initial experience with North American patients. Laryngoscope. 2011; 121: 521-526.
- Boccara G, Guenoun T, Aidan P. Anesthetic implications for robot-assisted transaxillary thyroid and parathyroid surgery: a report of twenty cases. J Clin Anesth. 2013; 25: 508-512.
- Donnellan KA, Pitman KT, Cannon CR, Replogle WH, Simmons JD. Intraoperative laryngeal nerve monitoring during thyroidectomy. Arch Otolaryngol Head Neck Surg. 2009; 135: 1196-1198.
- Rampil IJ, King BS. Volatile anesthetics depress spinal motor neurons. Anesthesiology. 1996; 85: 129-134.
- Scheufler KM, Reinacher PC, Blumrich W, Zentner J, Priebe HJ. The modifying effects of stimulation pattern and propofol plasma concentration on motor-evoked potentials. Anesth Analg. 2005; 100: 440-447.
- Hargreaves SJ, Watt JW. Intravenous anaesthesia and repetitive transcranial magnetic stimulation monitoring in spinal column surgery. Br J Anaesth. 2005; 94: 70-73.