
Research Article
Austin J Biomed Eng. 2015; 2(1): 1032.
Gastrogastric Fistula Post-Roux-En-Y Gastric Bypass Surgery
Quadri P¹*, Sanchez-Johnsen L1,2, Gonzalez- Heredia R¹ and Elli EF¹
¹Department of Surgery, University of Illinois at Chicago, USA
²Department of Psychiatry, University of Illinois at Chicago, USA
*Corresponding author: Quadri P, Department of Surgery, Division of General, Minimally Invasive and Robotic Surgery, University of Illinois at Chicago, 840 South Wood Street, Room 435E, Chicago, IL 60601, USA
Received: December 01, 2015; Accepted: December 30, 2015; Published: December 31, 2015
Abstract
Introduction: Obesity has reached epidemic proportions. Roux-en-Y gastric bypass (RYGB) is the second most prevalent bariatric procedure in the United States. The rate of gastrogastric fistulas (GGFs) has dramatically declined since the advent of divided RYGB and is now a relatively rare complication.The aim of this paper is to describe the clinical presentation of a patient with a GGF post- RYGB surgery as well as the surgical management and technique to repair a GGF using a minimally invasive robotic approach. Recommendations regarding the management of GGFs in clinical practice are also presented.
Materials and Methods: This is a case report of a patient with a GGF post-RYGB surgery who underwent a minimally invasive robotic surgical repair. Information about the patient was obtained from the electronic medical record and the surgical procedure and technique were described in detail.
Results: This patient was a 47-year-old female. She underwent an open RYGB in 2003. She began to experience severe epigastric pain, acid reflux and bloating ten years after the RYGB. An esophagogastroduodenoscopy (EGD) confirmed a 3 cm GGF. She then underwent a minimally invasive robotic-assisted fistula repair. The procedure started with the lyses of multiple adhesions and the dissection of the fistula using the robotic platform. An intra-operative endoscopy confirmed the anatomy. The fistula was completely dissected and transected using a stapler. An additional endoscopy was performed to assess the repair. The patient recovered uneventfully.
Conclusion: Initially, GGFs can be conservatively managed. Persistently symptomatic patients require endoscopic or surgical interventions. Endoscopy can be attempted in small fistulas (less than 10 mm) but the recurrence rate is high. There is no standardized surgical treatment for GGFs. The use of the robotic platform and intra-operative endoscopy are useful tools that can assist in complex cases.
Keywords: Gastric bypass complications; Roux-en-Y gastric bypass complications; gastrogastric fistula; management of gastrogastric fistula; bariatric revisional surgery
Abbreviations
(SG): Sleeve Gastrectomy; (RYGB): Roux -En- Y Gastric Bypass; (GGF): Gastrogastric Fistula; (EMR): Electronic Medical Record; (BMI): Body Mass Index; (GERD): Gastroesophageal Reflux Disease; (EGD): Esophagogastroduodenoscopy
Introduction
Obesity (BMI = 30) has reached epidemic proportions [1-3] and surgical interventions to treat obesity currently provide the best method to achieve significant weight loss and improvement in medical comorbidities in morbidly obese patients [1,2,4]. The number of bariatric surgeries as well as revisional bariatric surgical procedures is increasing worldwide [5]. Several bariatric procedures have been utilized with a wide spectrum of success and complications [1]. During 2014, approximately 193,000 bariatric surgeries were performed in accredited bariatric surgery centers in the United States. After sleeve gastrectomy (SG), Roux-en-Y gastric bypass (RYGB) is the second most prevalent bariatric procedure in the United States, accounting for 26.8% of all bariatric surgeries [6].
Minimally invasive RYGB has contributed to easier recovery, lower morbidity, less disability, less pain, shorter length of hospitalization and better cosmetic outcomes than open surgery [1,2]. However, minimally invasive RYGB is not without its set of complications. The complications of minimally invasive RYGB can be divided into early and late complications. Early complications include anastomotic or staple line leaks (0-5.2%) [7,8], postoperative hemorrhage (1.9- 4.4%) and small bowel obstruction (internal hernia:1-9%) [8]. Late complications include gastrojejunostomy anastomotic stricture (2.9- 23%), marginal ulceration (1-16%) [8], gastrogastric fistula (1.2-6%) [2,4,8-10], weight regain and nutritional deficiencies [8]. Although complication rates of minimally invasive RYGB are relatively low [1], surgeons must still learn how to effectively deal with these complications. A frequent complication that was noted in nondivided gastric restrictive procedures was gastrogastric fistula (GGF), which is defined as the communication of the gastric pouch and the gastric remnant [11]. GGFs have been noted to occur in more than 50% of non-divided gastric restrictive procedures [7,10]. However, since the advent of divided RYGB with complete transection of the stomach, the rate of GGFs have significantly decreased and it is now a rare complication, with an incidence across studies ranging from 1.2% to 6% of RYGB surgeries [2,4,8-10].
The aim of this paper is to describe the clinical presentation of a patient with a GGF post-RYGB surgery as well as describe the surgical management and technique to repair the GGF using a minimally invasive robotic approach. Recommendations regarding the management of GGFs in clinical practice are also presented.
Materials and Methods
This is a case report on the clinical presentation and surgical management of a GGF post-RYGB surgery using a minimally invasive robotic approach. The data was obtained from the electronic medical records (EMRs) at the University of Illinois Hospital and Health Sciences System. Information on age, sex, pre-surgical BMI, time between RYGB and the fistula repair, past medical history, clinical presentation, comorbidities, diagnosis, intraoperative outcomes (operative time, blood loss, surgical technique and intraoperative complications), length of hospitalization and postoperative complications was collected. Information about the surgical procedure and technique to repair the GGF was described in detail and also obtained from the EMRs.
Results
This patient was a 47-year-old female. She reported a history of mild gastroesophageal reflux disease (GERD), hypertension, anxiety, depression and fibromyalgia. She underwent an open RYGB in 2003 at a facility outside of the University of Illinois Hospital and Health Sciences System. A review of her previous medical history revealed that her post-surgical recovery was good, but two years prior to her first visit at our clinic, she began to experience severe epigastric pain, acid reflux and bloating. The patient was then referred to our bariatric surgery program for an evaluation. A review of her prior medical history also revealed that her esophagogram was normal and she did not have GERD. In addition, her medical record revealed that an esophagogastroduodenoscopy (EGD) was conducted and confirmed a 3 cm gastrogastric fistula with no marginal ulcer, in communication with the gastric remnant. Gastro-jejunostomy was patent. She then underwent a minimally invasive robotic-assisted fistula repair. Preoperative BMI was 34.2kg/m².
Surgical technique
The procedure started with a diagnostic laparoscopy that showed a subacute inflammatory process with multiple adhesions. The liver was densely adhered to the small bowel and gastric remnant, and the gastric pouch was adhered to the spleen. All adhesions were lysed using blunt and sharp dissection, and a monopolar hook. A medium-sized hiatal hernia was identified. The gastroesophageal membrane was opened, and the distal esophagus was mobilized in the mediastinum using a monopolar hook. The repair of the hernia was performed using interrupted non absorbable 2.0 sutures. The gastro-jejunostomy was identified and the alimentary limb was followed distally. Once the dissection of the fistula was completed (Figure 1), an intra-operative endoscopy was performed to confirm the anatomy (Figure 2). The EGD showed the GGF and a patent gastro-jejunostomy. The gastric pouch and remnant were completely dissected and were only connected by the fistula. At this point, a posterior window was created and the fistula was transected using two stapler loads (Figure 3). The gastric pouch was finally excluded from the remnant (Figure 4). An additional endoscopy was performed to confirm a hermetic closure, a patent gastro-jejunostomy, and to rule out any leaking or bleeding (Figure 5). The gastric pouch suture line was then over sewn.
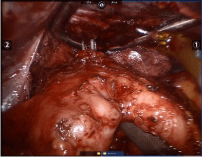
Figure 1: Complete dissection of the gastro gastric fistula.
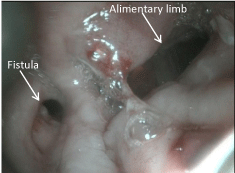
Figure 2: Intraoperative endoscopy.
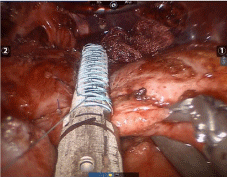
Figure 3: Stapler placement for transection.
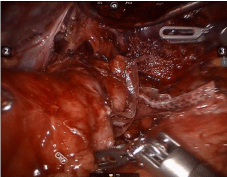
Figure 4: Complete transection of the gastro gastric fistula.
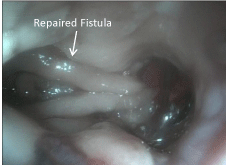
Figure 5: Postoperative endoscopy.
There were no intraoperative or postoperative complications. The estimated blood loss was 80 cc. The operative time was 178 minutes. Discharge was on the first postoperative day with normal oral tolerance. The patient’s symptoms of epigastric pain, acid reflux and bloating improved dramatically after surgery and during her followup visit.
Discussion
Gastrogastric fistulas are rare complications of divided RYGB with an incidence rate across studies ranging from 1.2% to 6% [2,4,8- 10]. However, the exact incidence is unknown as asymptomatic patients are not evaluated [10]. The incidence rate of GGFs have also declined with the advent of the complete gastric transection in RYGB [4]. Although the exact etiology of fistulas is unknown, many theories have been postulated such as the operative technique in non-divided gastric pouches, an inadvertent failure to completely divide the stomach during the surgical procedure, anastomotic leak, marginal ulcer, perforation of the gastric remnant, gastric tissue migration (capacity of the gastric tissue to migrate and reattach to the remnant in the absence of an inflammatory process) and erosions due to foreign bodies [7,10]. One of the most important risk factors for viscero-visceral fistulas is the anastomotic leak. In RYGB, the rate of gastrojejunostomy leak across studies ranges from 0% to 5.2% [7,8]. Moreover, Carrodeguas and colleagues have reported an incidence rate of GGF of 27% after gastro-jejunostomy leak [7].
The most common symptom of GGFs is abdominal pain, but they may also present with nausea, vomiting, hematemesis, hematoquezia, weight regain and refractory ulcers [1]. Upper gastrointestinal fluoroscopy and/or computed tomography usually show the flow of contrast into the gastric remnant [11]. An EGD is also a critical part of the assessment process in the management of GGFs. Findings from the EGD can vary from a clear fistula to a marginal ulcer with necrotic tissue and no clear communication between the pouch and remnant. Endoscopy is an additional valuable tool that can aid in the decisionmaking process regarding the adequate management of fistulas. EGD can provide information on the location, size and association with the marginal ulcer [7].
In general, the characteristics of the fistula will determine the approach to managing and treating GGFs. Initially, the management of GGFs may be through conservative medical approaches such as proton pump inhibitors, which reduce acid secretion [10]. When concomitant marginal ulcers are present, sucralfate should be added to create a protective coat, which helps to relieve pain and decreases exposure to acid secretion. NSAIDS should also be strictly avoided. This regimen can be prescribed for 6 to 8 weeks. If helicobacter pylori is present, it must be treated with antibiotics. If conservative treatment fails or a patient regains weight, then endoscopic or surgical interventions must be sought [7,10,12].
Endoscopic repair must be attempted in selected cases to avoid unnecessary interventions and fistula recurrence. This procedure is usually performed under general anesthesia. Endoscopy uses different devices such as endoscopic sutures, endoclips, argon plasma coagulation and fibrin glue for the fistula closure. The procedure includes the closure of the fistula with previous ablation of the gastric mucosa to promote fusion of the opposed nonepithelized tissues. The endoclip jaw opening is limited so they can only be used in small GGFs (less than 10 mm). For larger fistulas, an endoscopic suturing device is needed. Complications of endoscopic procedures may include: perforation, bleeding and recurrence of the fistula in the long term. Moreover, the outcomes for endoscopic repairs are not encouraging [3,4]. Fernandez-Esparrach and colleagues [3] reviewed 95 cases of the closure of GGFs using an endoscopic approach. The long term re-opening rate was 81% and in fistulas of 20 mm or more, the long term re-opening rate was 100% [3]. The authors concluded that the endoscopic closure was feasible and safe but should be left for fistulas smaller than 10 mm diameter to achieve better long term outcomes [3].
Surgical management of GGFs is the standard of care when endoscopy fails. A minimally invasive robotic approach can offer some benefits in complex revisional bariatric surgeries [13]. Moreover, revisional surgeries have higher technical difficulties, rates of complication and longer length of hospitalizations [13]. Minimally invasive robotic surgeries versus open surgeries have helped to reduce these issues [13]. Different surgical techniques for the resolution of GGFs have been proposed. There are several important features in any surgical technique to repair GGFs, which include the identification of the anatomy (alimentary limb, fistula, gastric pouch and remnant), complete dissection of all the structures and the transection of the fistula. An intraoperative endoscopy is also recommended to confirm the findings. Dissection and transection of the fistula is a commonly used technique, with or without interposition of omentum or jejunum [4]. Other authors have reported the remnant gastrectomy to treat GGFs [9,10]. A remnant gastrectomy is technically more challenging but a lower recurrence rate of GGFs has been reported [4,9].
Few authors have reported the use of percutaneous procedures with intragastric laparoscopy and endoscopy to treat GGFs [2]. These procedures combine the use of radiology, ultrasound, laparoscopy and endoscopy in a minimally invasive approach [2]. These techniques require proficient specialists in laparoscopic and endoscopic interventions. This type of endo-organ surgery might show benefits especially in patients with multiple abdominal surgeries and acute inflammation [2]. However, further research on the use of these techniques is needed in order to provide additional information about the feasibility of these approaches to repair GGFs.
Conclusion
Gastrogastric fistulas are a rare complication in divided RYGB with complete transection of the stomach. Initially, conservative medical management may be sought, but persistently symptomatic patients require endoscopic or surgical interventions. Recommendations for the management of GGFs include the initial conservative medical treatment [proton pump inhibitors, avoid gastro toxic drugs (NSAIDS), antibiotics and sucralfate when helicobacter pylori and marginal ulcer are present respectively] for 6 to 8 weeks. When patients are persistently symptomatic, an endoscopic or surgical intervention is required. Endoscopy can be attempted in small fistulas (less than 10 mm) but even with a correct closure, the recurrence rate is high. There is no standardized surgical treatment for GGFs. Among the most important features for the surgical repair of GGFs are the correct identification of the anatomy and the complete dissection of the fistula before the transection are performed. According to the characteristics of the fistula, a remnant gastrectomy might be needed for a correct repair. The use of the robotic platform and intraoperative endoscopy are tools that allow precise dissection, better visualization and identification of the distorted anatomy, thereby reducing morbidity rates in complex cases.
References
- Filho AJ, KondoW, Nassif LS, Garcia MJ, Rafael de Almeida T, Dotti CM. Gastrogastric fistula: a possible complication of Roux-en-Y gastric bypass. JSLS. 2006; 10: 326-331.
- Torres-Villalobos G, Leslie D, Kellogg T, Andrade R, Maddaus M, Hunter D, et al. A new approach for treatment of gastro-gastric fistula after gastric bypass. Obes Surg. 2007; 17: 242-246.
- Fernandez-Esparrach G, Lautz DB, Thompson CC. Endoscopic repair of gastrogastric fistula after Roux-en-Y gastric bypass: a less-invasive approach. Surg Obes Relat Dis. 2010; 6: 282-288.
- Pauli EM, Beshir H, Mathew A. Gastrogastric fistulae following gastric bypass surgery-clinical recognition and treatment. Curr Gastroenterol Rep. 2014; 16: 405.
- Eisendrath P, Deviere J. Major complications of bariatric surgery: endoscopy as first-line treatment. Nat Rev Gastroenterol Hepatol. 2015.
- Ponce J, Nguyen NT, Hutter M, Sudan R, Morton J. American Society for Metabolic and Bariatric Surgery estimation of bariatric surgery procedures in the United States, 2011-2014. Surg Obes Relat Dis. 2015.
- Carrodeguas L, Szomstein S, Soto F, Whipple O, Simpfendorfer C, Gonzalvo JP, Villares A, et al. Management of gastrogastric fistulas after divided Rouxen- Y gastric bypass surgery for morbid obesity: analysis of 1,292 consecutive patients and review of literature. Surg Obes Relat Dis. 2005; 1: 467-474.
- Griffith PS, Daniel W Birch, Arya M. Sharma, Karmali S. Managing complications associated with laparoscopic Roux-en-Y gastric bypass for morbid obesity. Can J Surg. 2012; 55: 329-336.
- Tucker ON, Szomstein S, Rosenthal RJ, Surgical management of gastrogastric fistula after divided laparoscopic Roux-en-Y gastric bypass for morbid obesity. J Gastrointest Surg. 2007; 11: 1673-1679.
- Salimath J, Rosenthal RJ, Szomstein S. Laparoscopic remnant gastrectomy as a novel approach for treatment of gastrogastric fistula. Surg Endosc. 2009; 23: 2591-2595.
- Carucci LR, Conklin RC, Turner MA, Roux-en-Y gastric bypass surgery for morbid obesity: evaluation of leak into excluded stomach with upper gastrointestinal examination. Radiology. 2008; 248: 504-510.
- Gumbs AA, Duffy AJ, Bell RL. Management of gastrogastric fistula after laparoscopic Roux-en-Y gastric bypass. Surg Obes Relat Dis. 2006; 2: 117- 121.
- Hagen ME, Pugin F, Chassot G, Huber O, Buchs N, Iranmanesh P, Morel P. Reducing cost of surgery by avoiding complications: the model of robotic Roux-en-Y gastric bypass. Obes Surg. 2012; 22: 52-61.