
Review Article
Austin J Biomed Eng. 2024; 7(1): 1046.
Biogenic Synthesis of MgNOPs: A Revolutionary Frontier in Biomedical Advancements
Sidra Fatima1; Ayesha Abid2; Syed Muhammad Asad Ali1; Qandeel Bajwa3; Mustaqeem Khan4; Sami Ullah5; Areeha Amjad6; Muhammad Hammad Bilal6*
1Centre for Applied Molecular Biology, University of the Punjab, Lahore, Pakistan
2Govt college University, Lahore, Pakistan
3Faculty of Life Sciences, University of Central Punjab, Lahore, Pakistan
4University of Malakand, KPK, Pakistan
5Shaheed Benazir Bhutto University Sheringal dir upper, Pakistan
6Center of Agriculture Biochemistry and Biotechnology, University of Agriculture Faislabad, Pakistan
*Corresponding author: Muhammad Hammad Bilal Center of Agriculture Biochemistry and Biotechnology, University of Agriculture Faislabad, Pakistan. Email: mhamadbilal740@gmail.com
Received: January 17, 2024 Accepted: February 29, 2024 Published: March 07, 2024
Abstract
At present, nanotechnology is developing at exponential rate because of its widespread applications in biomedical field. Traditionally, nanoparticles are fabricated through variety of physical and chemical approaches but because of their limited efficiency in life sciences, biogenic synthesis of nanoparticles has gained much attention. Biogenically fabricated MgONPs possess unique features that enabled their use in therapeutics and environmental biotechnology. Biogenically MgONPs can be fabricated by employing plant extracts (Manihot esculenta, Rhizophora lamarckii, Ocimum sanctum, Rosemary, Aloe vera, Orange, Artemisia abrotanum, Neem, Bauhinia purpurea), bacteria (S. coelicolor, A. johnsonii RTN), fungi (Aspergillus flavus TFR-12, Aspergillus brasiliensis TFR 2, white-button mushroom’s) and algae (S. whigti). These biogenically synthesized MgONPs are being reported as anti-fungal, antioxidant, antibacterial, anti-pyretic, anti-inflammatory and anti-cancer agents. Moreover, their photocatalytic activity is being discovered against certain organic dyes. This review focuses on biogenic synthesis of MgONPs, their applications in therapeutics and as antimicrobial agents as well as future prospects associated with their application in biomedical field.
Introduction
In 21st century, nanotechnology has emerged as one of the most promising and innovative research areas. This technology is progressing day by day as it possesses extensive applications in biomedical sciences, energy sciences, biotechnology, tissue engineering, material sciences and environmental technology [1-3]. Nanoparticles of metals and their oxides display unique physical and chemical properties e.g., precise shape, nanoscale size, biomimetic nature, distribution, immense area to volume ratio, massive surface energy, biocompatibility which makes them suitable for wide range of applications in different fields of life sciences [4-6]. Various physical and chemical procedures are employed to manufacture nanoparticles that requires specific conditions e.g., vacuum, high temperature, sophisticated apparatus, toxic chemicals [3].
Some physical methods which are widely used to manufacture nanoparticles involve radiolysis, UV irradiation, laser ablation, evaporation followed by condensation and ultrasonication [2,7]. Large scale use of these methods is limited because of high operational cost associated with high power consumption, instrumentation and radiative heating [5,8]. Similarly, progresses in chemical synthesis of nanoparticles also pose serious threats to environment as they involve use of toxic chemicals that not only contaminate nanoparticles but are also non-biodegradable [3,9]. These factors limit the use of chemically synthesized nanoparticles in biomedical field [10]. Thus, biogenic synthesis of nano-particles has gained attention all over the world as these methods are relatively safe, simple, environment friendly, avoid use of toxic chemicals and involve biodegradable products which makes them best choice for widespread applications in life sciences [11]. Studies reported that in contrast with physio-chemical methods biogenically synthesized nanoparticles exhibit precise morphology and well-defined size [12].
Nano particles of metals (Gold, silver, copper, platinum etc.) and metal oxides (zinc oxide, magnesium oxide, Nickle oxide, cadmium oxide etc.) are renowned for their pharmacological applications [13]. Among all types of metal oxide nano particles, magnesium oxide nano particles (MgONPs) are well known for their biomedical usage because of their higher stability and biocompatible nature. These nanoparticles are widely used as antibacterial, antitumor, anticancer agents and also exhibit photocatalytic effect. MgONPs can be used in the treatment of heart burn and bone regeneration. As well as these particles can also be used as super conducting products, essential element in bioremediation, additives and catalysts [3].
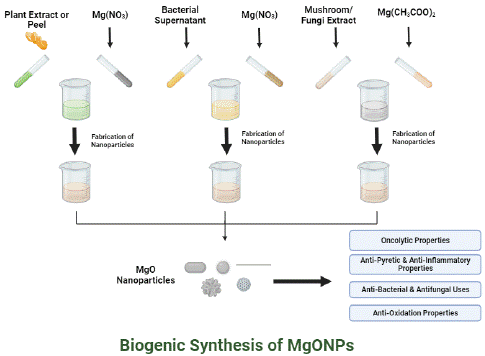
Graphical Abstract
Biogenically MgONPs may be synthesized by employing plants leaves, roots, flower and bark extracts as well as from bacteria (Acinetobacter johnsonii strain RTN1, Streptomyces coelicolor strain E72) [14,15], fungi (Aspergillus flavus) [16] and algae (Sargassum wighitii) [17]. However, biogenic synthesis of magnesium-oxide nanoparticles from plant is a preferred method because of cost effectiveness, high yield and ease of cultivation [18]. Plants possess wide variety of bioactive metabolites that contribute in biogenic synthesis of nanoparticles [19].
Biological Synthesis oF MgONPs
Conventionally, physical and chemical synthetic approaches have been employed to manufacture MgONPs [15,20]. However, MgONPs fabricated through these complex and expensive techniques were found to be contaminated with toxic residues which in turn limit their efficacy in therapeutics [3,21]. Hence, biogenic synthesis has proven to be an efficient alternative way for synthesis of non-hazardous MgONPs that can be effectively used for biological applications. Biogenically, MgONPs can be synthesized by bacteria, yeast, algae, plant extracts and fungi [22]. Bioactive metabolites (alkaloids, enzymes, flavonoids, proteins, phenols, polysaccharides and other biochemicals) obtained from these biological synthesizers serve as reducing, capping or stabilizing agent for green synthesis of stable MgONPs [23]. Several factors affect biological fabrication of nanoparticles that include safety of nanoparticle’s synthesizers, temperature, pH, high bio-reluctant concentration and reaction time [15].
Plant Name
Part Used
Reaction Conditions
Average Size
(nm)Shape
References
Bauhinia purpurea
Leaves
90°C, 3 hours
10-11 nm
Very thin, flake like structures
[29]
Neem (Azadirachta indica)
Leaves
80°C, 4 hours
43 nm
Cubic
[30]
Nephelium lappaceum
Peel
80°C, 2 hours
60-70 nm
Spherical
[63]
Manihot esculenta (Crantz)
Leaves
Room temperature, 12 hours
36.7 nm
Hexagonal
[33]
Orange
Peel
25°C, 4 hours, pH: 12
5-100 nm
Spherical
[28]
Aloe vera
Leaves
130°C, 2 days
140 nm
Spherical
[18]
Artemisia abrotanum
Herb powder
90°C, 6 hours
around 10 nm
Spherical
[34]
Ocimum sanctum
Leaves
50-100 nm
[36]
Rosmarinus officinalis L. (Rosemary)
Flowers
70°C, 4 hours
>20 nm
Flower like morphology
[38]
Rhizophora lamarckii
Leaves
Room temperature, 24 hours
20 and 50 nm
Nano-hexagonal and spherical
[39]
Table 1: Plant mediated synthesis of MgONPs.
Synthesis by Plant Extracts
Numerous studies have reported that plant-based fabrication of nanoparticles has been more effective than microbial synthesis as by employing plant extract, synthetic process can be scaled-up easily and harsh reaction conditions as high pressure,temperature, concentrated hazardous chemicals are not required [24]. Furthermore, plant mediated manufacturing eliminate the requirements of microbial isolation, media preparation for culturing, maintenance of culture [25]. Plant mediated fabrication of nanoparticles involve simple and rapid synthetic procedures [26]. These processes involve mixing of metal salts with plant’s extract at room temperature [27]. Then reducing agents in plant-derived extract reduce metal ions in metal salts. Resulting atoms clustered together and eventually leads to the formation of nanoscale particles [28].
Various studies have demonstrated the plant mediated fabrication of MgONPs. Biogenic synthesis of MgONPs was reported by using leaves extract of Bauhinia purpurea through alkaline precipitation method and color changing indicated the presence of MgONPs (as colorless solution turned to cloudy brown) [29]. UV-visible spectroscopy showed adsorption spectrum in the range of 200-800 nm that further confirmed the formation of MgO nanoflakes. Study found that leaves extract of B. purpurea contains significant proportion of flavonoids, antioxidants and phenolics that possibly contribute to the manufacturing of MgONPs (10-11 nm). 250μg/ml dose of these MgO nanoflakes was documented to be the minimum inhibitory concentration that efficiently inhibit the growth of S. aureus MTCC-3384 [29]. S. Krishna Moorthy et al. reported the manufacturing of MgONPs through Neem leaves extract by employing magnesium nitrate as precursor. Color transformation from yellow to yellowish-brown confirmed the presence of MgONPs. 5 ml neem leaves extract, 500°C calcination temperature and 80°C stirring temperature was found to be optimum for the synthesis of MgONPs [30]. These MgONPs was found to improve chlorophyll and carotenoids content in Cicer arietinum but not in Solanumly copersicum in which seed germination did not display variations [30].
Furthermore, MgONPs has been synthesized efficiently by employing Nephelium lappaceum peels that serve as natural ligation agent [31]. These biogenically synthesized MgONPs possessed spherical shape with particle size of 60-70 nm determined by XDR and SEM analysis [32]. MgONPs has also been manufactured successfully by employing aqueous leaf extract of Manihot esculenta (crantz) [33]. Essien et al. reported that 50 ml of Mg(NO3)2·6H2O (0.1M) used as precursor in which 250 ml leaf extract of crantz was added dropwise under constant stirring conditions in period of about 12 hours. Obtained product was then centrifuged (at 7000 rpm, 10 minutes) to separate reaction mixture into supernatant and pellets, that were dried in oven (at 70°C, 2 hours) followed by calcination (500°C, 2 hours) to gain MgONPs [33].
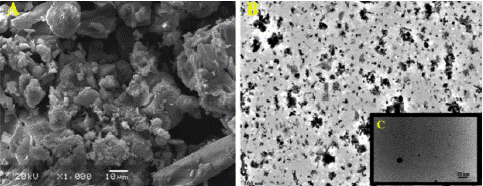
Figure 1: (A) SEM analysis of MgONPs fabricated by using endophytic S. coelicolor strain E72 extract with scale 10 μm. (B) and (C). TEM images with scale 200nm and 50 nm respectively.
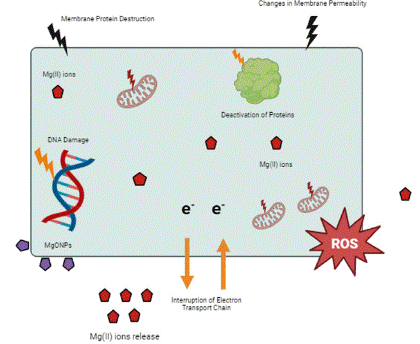
Figure 2: Cytotoxic Activity of MgONPs.
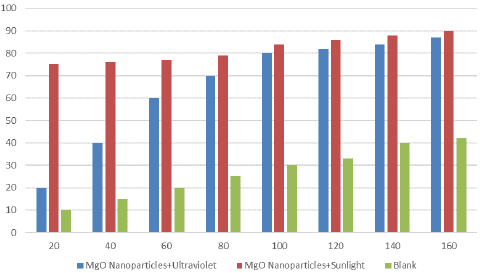
Figure 3: Catalytic Effect of MgONPs for Methylene Blue dye degradation in presence of Sunlight and Ultraviolet Radiations [4].
Green synthesis of MgONPs has also been reported by employing Artemisia abrotanum (herb) extract [34]. A. abrotanum herb extract was added to Mg(NO3)2 solution in ratio of 90 (%w): 10 (%w) and kept at 90°C under constant stirring for 6 hours [34]. XDR analysis showed crystalline nature of fabricated MgONPs and crystal size identified by Scherrer’s formula was found to be about 10 nm. These biogenically manufactured MgONPs exhibited excellent photocatalytic activity as they degraded methyl-orange dye and showed antioxidant activity [34].
Orange peel has also been employed to fabricate MgONPs from Mg (NO3)2 solution that was made alkaline through the addition of Na2CO3 under constant stirring for 4 hours [35]. White colored nanoparticles were obtained with spherical morphology and particle size in range of 5-100 nm. These biogenically fabricated MgONPs displayed antimicrobial action [35]. Soma Prabha A. and Prabakaran V. reported the biogenic manufacturing of MgONPs by employing Aloe Vera leaf extract from magnesium-acetate tetrahydrate. SEM displayed spherical shape and particle-size of these MgONPs was found to be 140 nm shown by XDR analysis. These biogenically fabricated MgONPs exhibited excellent anti-inflammatory activity upon oral administration that was tested through formalin induced oedema in mouse model. 100mg/Kg dose of these MgONPs displayed maximum efficacy of 60% oedema inhibition after 4 hours [18]. Furthermore, MgONPs synthesized by aloe vera leaf extract have also exhibited antibacterial action against Staphylococcus aureus and displayed antifungal activity against Aspergillus flavus and Aspergillus nigerand [18].
Green synthesis of MgONPs have also been reported by using Ocimum sanctum’s leaves extract [36]. In a study where 5.21 g of Mg (NO3) 2.6H2O was added to 200 ml distilled water, then blended with 5.21g of Ocimum sanctum leaf extract and kept under stirring condition for 30 minutes and NaOH was added dropwise that eventually cause fabrication of MgONPs at bottom of flask. In UV-Visible analysis peak was observed at 244nm that ensure manufacturing of MgONPs [37]. These biogenically synthesized MgONPs have shown little antibacterial action against E. coli and Staphylococcus aureus but exhibited strong antioxidant activity identified by DPPH method [22].
Plant mediated fabrication of MgO nano-flowers has also been stated by employing Rosmarinus officinalis L. (Rosemary) flower’s extract. 100 ml of 1 mM magnesium oxide solution was added to 100 ml Rosemary flower’s extract and solution was kept at 70°C under constant stirring for 4 hours [38]. 2 peaks were found in UV-Visible analysis in range of 200-800 nm. Highest peak was at 250 nm that confirmed the fabrication of MgONPs [38]. These MgO nano-flowers exhibited excellent antibacterial activity (at concentration of 4μg/ml, 8μg/ml and 16μg/ ml) against Xoo strain GZ 0005 that is causative agent of bacterial blight disease in rice plant. Most probably antibacterial potential of these MgONFs was because of inhibition of growth, motility, biofilm formation along with devastation of cell wall [38]. Prasanth et al. successfully fabricated MgONPs by using mangrove plant “R. lamarckii leaf extract. 5g Mg (NO3)2 was added to R. lamarckii leaf’s extract and kept under constant stirring for 24 hours. Eventually, synthesis of MgONPs was confirmed by color transformation from yellow to yellowish-brown [39]. FTIR analysis deduce that alkane and amine functional groups in R. lamarckii extract probably contribute in reduction and stabilization of newly fabricated MgONPs. These biogenically synthesized MgONPs displayed excellent antibacterial action against gram-negative as well as gram positive bacteria [39].
Microbial Synthesis of MgONPs
Since the last decade, microbes including bacteria, yeast, actinomycetes and fungi has been recognized as effective system for biogenic fabrication of NPs [40]. Miro-organisms possess intrinsic potential to precipitate nanoparticles either extracellularly or intracellularly through their metabolic action [41]. Bacteria possesses special reducing enzymes e.g., nitrate dependent reductase or NADH-dependent reductase that are probably involved in fabrication of NPs [42]. While membrane-bound quinones and reductase possibly serve as reducing agents in yeast to manufacture biogenic NPs [43]. Although, microbial approach to synthesize NPs seems to be attractive as it is ecofriendly, energy-efficient and necessitate moderate pressure and temperature requirements [44] but it also have certain limitations including specific culture maintaining requirements, sterile conditions and high mutation rate that limit commercialization of microbial based NPs synthetic procedures [45].
MgONPs has been fabricated successfully from endophytic actinomycete, Streptomyces coelicolor strain E72 [15]. Study reported that intracellular metabolic fraction containing 6.3 g/l proteins, 7.2μg/μl carbohydrates and 5.2nmol/hr/ml nitrate reductase has been employed to fabricate MgONPs [15]. These MgONPs possess highly pure-crystalline structure determined by XDR analysis with spherical and ellipsoidal morphology (25nm) shown by TEM and SEM images [15], These biogenically synthesized MgONPs have displayed excellent antibacterial action against variety of tested multidrug tolerant human pathogens [15]. T. Ahmad et al. reported the manufacturing g of MgONPs by employing Acinetobacter johnsonii strain RTN1. Study reported that 50 mL of 7mM Mg (NO3) 2.H2O solution was added to equal volume of RTN1 strain’s supernatant and kept under shaking conditions of 140rpm at 28 ± 2°C for about 24 hours. Visible clumps at base of flask guaranteed the synthesis MgONPs [1]. Further characterization analysis demonstrated that these MgONPs possessed spherical morphology with average size in range of 18 to 45nm. Strong antibacterial action of these biogenically fabricated MgONPs against Acidovorax oryzae (rice pathogen) suggested that these MgONPs have potential to be used as nano-pesticides [14].
Synthesis by Fungi
In nanotechnology research, fungi have gained attention as attractive source for biogenic fabrication of NPs because of high tolerance and metal bioaccumulation capacity [46]. Biomimetic mineralization and intra cellular or extracellular reduction of metal ions by enzymatic action is the most probable mechanism through which fungus can synthesize NPs [47]. Raliya et al. reported the biogenic synthesis of MgONPs from fungus Aspergillus flavus strain TFR-12. Average hydrodynamic diameter of these MgONPs was found to be 5.8nm [48]. Study reported that foliar application of these fungus derived MgONPs improved not only chlorophyll content but also shoot-root ratio in Cyamopsis tetragonoloba [48]. MgONPs has also been fabricated successfully by employing Aspergillus brasiliensis TFR 2 at concentration of 20ppm, with an average diameter of <5.9 nm [49]. It was reported that foliar application of these biogenically synthesized MgONPs in wheat crop improved chlorophyll content, light absorption, biomass yield and grain yield [49].
Biogenic manufacturing of MgONPs by employing white-button mushroom’s extract has also been reported [50]. Study reported that process involves mixing of mushroom’s extract with 0.1M, Mg (CH3COO)2.4H2O solution followed by constant stirring of solution at 40°C for 2 hours [50]. XDR spectrum revealed that synthesized MgONPs have different average size (20, 18.5, 18, 16.5 and 15nm) at specific concentrations [50]. Study found that smaller size (15nm) MgONPs enhance seedling development and overall growth in peanut plant as compared to control and other MgONPs [50].
Synthesis by Seaweed/ Marine Algae
Currently, seaweeds have gained attention as prevalent source for biological fabrication of NPs because of high efficiency and ease access. Generally, algal cell-wall contain wide variety of polysaccharides, carotenoids, proteins, polyphenols, minerals, amino-acids and vitamins that serve as reducing and stabilizing agents in biogenic manufacturing of NPs [3]. Pugazhendhi et al. reported the synthesis of MgONPs by mixing S.whigti extract and magnesium-nitrate solution in 9:1 followed by constant stirring at 90°C for 6 hours. XDR analysis displayed that these biogenically fabricated MgONPs (68.08 nm) were crystalline with face-centered cubic structure. Sulphated polysaccharide’s binding function groups were identified in FTIR analysis that possibly serve as reducing and stabilizing agents [3].
Applications
Nanoparticles are widely employed in different areas of life and it is not possible to prevent human exposure from it. Nanoparticle’s use with different composition and implementations are expanding day by day but their cytotoxicity investigation on biological systems is less. MgONPs find their extensive medicinal applications particularly in therapeutic and diagnostic devices to better cope with illness. These nanoparticles also find their use as anticancer, antibacterial, antifungal, anti-inflammatory, anti-pyretic and photocatalytic agents [51].
Anti-Bacterial
MgONPs are renowned for their antibacterial activity reliant on their composition, size, shape and pathogen. Ahmed et al. observed antibacterial activity of these particles against Acidovorax oryzae. Treatment of A. oryzae cells at concentration of 20μg mL-1 ruptured morphological structures that leads to leaching out of nuclear material and death of bacteria [14]. MgONPs synthesized from Saussurea costus showed dose dependent antibacterial activity against P.aeruginosa, B. subtilis, S. aureus and E.coli. Highest activity of these MgONPs was noticed against P.aeruginosa and E.coli, at a concentration of 35μg mL-1 [4]. These biogenically fabricated MgONPs stick to bacterial surface and cross peptidoglycan membrane that cause release of cell components and ultimately leads to cell death. This damage might be due to the oxidative stress caused by release of RNS and ROS free radicals or might be attributed to electrochemical interaction between Mg2+ and LPS [52].
Antibacterial potential of MgO nano flakes synthesized from Bauhinia purpurea is also investigated against gram positive bacteria S. aureus. Altering the concentration of these nano flakes have varying toxicity against bacteria. Complete growth inhibition of S. aureus was reported at a concentration of 500 – 1000 μg mL-1 with 8 hours incubation. FESEM analysis depicted that MgO nanoflakes cause disruption of bacterial cell leading to leakage of cellular components and ultimately death occurs [53].
MgONPs can also be synthesized from brown algae, and these NPs have effective bactericidal activity against gram negative bacteria such as Aeromonas baumannii, Pseudomonas aeruginosa, E. coli. and gram-positive bacteria such as MRSA 56, MRSA 11 and Streptococcus pneumonia. Among these strains, synthesized NPs exhibit highest inhibitory effect against P. aeruginosa and MRSA56. Antibacterial efficiency of these MgONPs might be attributed to their small size [3]. MgONPs from brown algae cause cell damage mediated by oxidative stress, electrochemical interaction between LPS phosphate group and magnesium ions thus disturbing membrane integrity leading to membrane leakage.
MgONPs fabricated from Streptomyces coelicolor have antibacterial avtivity against multiple drug resistant human pathogens like Salmonella typhimurium, Shigella flexneri, Klebsiella pneumonia, Streptococcus pneumonia, Proteus vulgaris and Bacillus cereus. All these bacterial strains are susceptible to MgONPs, at different concentrations. These NPs attack pathogenic cells through vital components like peptidoglycan cell wall, cell membrane and other molecules to alter permeability of membrane, leading to cell death. Inside cell these NPs interact with sulphur or phosphorus containing components thereby inhibiting their function. It is also reported that aggregation of NPs inside target cells does not provide desirable results than well spread nano particles [53]. Efficiency of MgONPs may also depend upon chemical nature of cell being targeted [54].
Anti-Pyretic Activity
Anti-pyretic activity of aloe vera derived MgONPs was tested in albino mice that was subcutaneously injected with brewer’s yeast. This suspension was then massaged to spread it in surrounding tissues. Rectal temperature was recorded before and after yeast administration. It was reported that rectal temperature was elevated after brewer’s yeast inoculation. But a considerable decrease in temperature was recorded after treatment with MgONPs. Further increasing the concentration of NPs positive results were obtained. This experiment revealed anti-pyretic activity of biogenically synthesized MgONPs [4].
Anti-Fungal
The antifungal activity of Saussurea costus derived MgONPs was detected at different concentrations against two fungal strains i.e. Candida tropicalis and Candida glabrata. These NPs penetrate into the fungal cells and induce oxidative stress due to release of ROS free radicals thus causing cell denaturation [55]. In another study antifungal activity of Sargassum wightii derived MgONPs was conducted against three fungal strains Fusarium solani, Aspergillus fumigates and Aspergillus niger. Among these, antifungal activity was more prominent against Aspergillus niger and Fusarium solani. During its activity Mg2+ in MgONPs interact electrostatically with phosphate group present in cell membrane, upon entry into cell Mg2+ ions bind with thiol group thus causing cell disruption. Moreover, oxidative stress due to ROS may cause fungal cell death [3]. Antifungal activity of aloevera derived MgONPs was also tested against two fungal strains Aspergillus niger and Aspergillus flavus. The results clearly depicted sensitivity of both strains to these NPs [56].
Anti-Inflammatory Activity
In a mice model, anti-inflammatory activity of aloe vera derived MgONPs was tested by means of paw edema, induced by formalin. Paw size was determined at different time intervals and it continued to increase. But when MgONPs were administered paw size was reduced. This experiment confirms the anti-inflammatory activity of green synthesized MgONPs [57].
Anticancer
Cancer is a leading cause of mortality among people. According to an estimate about 17.6% of people die because of lungs cancer. Multiple environmental and life-style features including smoking, lack of physical exercise, alcohol and passive exposure, leads to lung cancer. Depending on cancer stage different treatments like drugs, chemotherapy, surgery and radiation therapy are recommended but these procedures also possess some side effects on body. Thus, there was an urgent need of cost effective, target specific, eco-friendly and non-toxic treatment [3].
Nanoparticles having size less than 100nm with distinctive chemical and physical properties, interact with lipids, protein and nucleic acid inside cell that offer new ways for cancer treatment and diagnosis. MgONPs derived from seaweeds are examined against lung cancer cell line and positive results are obtained. These NPs induce several morphological changes in target cells including cell shrinkage, chromatin condensation, blabbing and ultimately formation of apoptotic bodies. These NPs denature cancerous cells through ROS production and activation of apoptosis. ROS cause rearrangement and breakage in DNA thus altering DNA sequence [58]. Gene expression regulation through ROS have potential approach in therapeutics. Many antioxidant compounds are known to inhibit DNA damage, ROS generation and cell mortality e.g., curcumin [59]. In general, production of ROS and gene expression alteration are responsible for cancer cell pathogenesis [60].
A crucial challenge to face during cancer treatment is to increase the target specificity of anticancer agent and reduce its effect on normal cells. MgONPs are much more target specific due to their colloidal stability. It Is also suggested that cell membrane of cancer cells is much more permeable to nanoparticles than normal cells, So, larger NPs can easily infiltrate cancer cells [61]. Anticancer potential of MgONPs was reported against Breast adenocarcinoma cells. Different concentrations of MgONPs synthesized from S.costus have different cytotoxicity against MCF-7 cells at different time intervals. These nanoparticles inhibit cell viability and proliferation. Endocytosis of MgO nanoparticles cause morphological changes due to activation of apoptotic pathway that leads to cell death [52]. Apoptosis causes pathological changes in cell such as lipid peroxidation, DNA damage, inflammation and protein oxidation [62]. Furthermore, MgONPs cause loss of mitochondrial membrane potential. Lactate dehydrogenase is a cytoplasmic enzyme that is released during cell damage and its assay can be used to confirm cytotoxicity of MgO nanoparticles against cancerous cells [51].
Photocatalytic Activity
Organic dyes are commonly used in industries. Excessive use of these organic dyes causes environmental pollution and it is necessary to control these industrial effluents. Thus, there was an immediate need of efficient method for degradation of these dyes. Metal oxide NPs provide one such solution as they possess catalytic activity against organic dyes. A study reported the use of Artemisia abrotanum derived MgONPs for degradation of methyl orange (an organic dye used in paper, textile and chemical industries). Increasing the concentration of MgONPs increase the degradation of methyl orange [34]. A similar study conducted by Amina et al. reported photocatalytic effect of MgONPs by determining optimum absorbance of methylene blue at different time intervals and 660nm wavelength. They reported light absorbance and charge separation of MgONPs that can reduce or oxidize the organic compounds.
Photocatalytic activity of MgONPs is because of their activation under visible or UV light that causes the excited electrons to move from valance band to conduction band leading to redox interaction of organic dyes adsorbed on nanoparticles surface [4].
Conclusion and Future Perspective
Biocompatible, less toxic and ecofriendly single step synthetic procedures for biogenic fabrication of nanoparticles attract attention of researchers and offer great potential for future progresses in areas of biosensors, cancer therapy, drug delivery, integrated insect or pest management programs for sustainable agricultural development and healthcare. As studies demonstrated that plant mediated manufacturing of MgONPs has proved to be cost effective and easy to scale-up for commercial production. Hence, there exist possibility that vast plant diversity that has not been investigated so far might hold great potential as biological source for manufacturing of MgONPs that may serve important role in advancement of pharmaceutical industry as well as in developing new therapies for cancer treatment. Furthermore, photocatalytic activity of MgONPs offers possibility that these biologically fabricated MgONPs might serve important role in environmental biotechnology by developing new tools for pollutant degradation and waste management.
References
- Hasan A, Morshed M, Memic A, Hassan S, Webster TJ, Marei HE-S. Nanoparticles in tissue engineering: Applications, challenges and prospects. International journal of nanomedicine. 2018; 13: 5637-5655.
- Khandel P, Yadaw RK, Soni DK, Kanwar L, Shahi SK. Biogenesis of metal nanoparticles and their pharmacological applications: Present status and application prospects. Journal of Nanostructure in Chemistry. 2018; 8: 217-254.
- Pugazhendhi A, Prabhu R, Muruganantham K, Shanmuganathan R, Natarajan S. Anticancer, antimicrobial and photocatalytic activities of green synthesized magnesium oxide nanoparticles (mgonps) using aqueous extract of sargassum wightii. Journal of Photochemistry and Photobiology B: Biology. 2019; 190: 86-97.
- Amina M, Al Musayeib NM, Alarfaj NA, El-Tohamy MF, Oraby HF, Al Hamoud GA, et al. Biogenic green synthesis of mgo nanoparticles using saussurea costus biomasses for a comprehensive detection of their antimicrobial, cytotoxicity against mcf-7 breast cancer cells and photocatalysis potentials. PLoS One. 2020; 15: e0237567.
- Khan H, Sakharkar M, Nayak A, Kishore U, Khan A. Nanoparticles for biomedical applications: An overview. Nanobiomaterials. 2018; 357-384.
- Yoon Y, Truong PL, Lee D, Ko SH. Metal-oxide nanomaterials synthesis and applications in flexible and wearable sensors. ACS Nanoscience Au. 2021; 2: 64-92.
- Ijaz I, Gilani E, Nazir A, Bukhari A. Detail review on chemical, physical and green synthesis, classification, characterizations and applications of nanoparticles. Green Chemistry Letters and Reviews. 2020; 13: 223-245.
- Ertl B, Hartmann FG, Heine JH. Analyzing large-scale studies: Benefits and challenges. Frontiers in Psychology. 2020; 11: 577410.
- Ying S, Guan Z, Ofoegbu PC, Clubb P, Rico C, He F, et al. Green synthesis of nanoparticles: Current developments and limitations. Environmental Technology & Innovation. 2022; 26: 102336.
- Egbuna C, Parmar VK, Jeevanandam J, Ezzat SM, Patrick-Iwuanyanwu KC, Adetunji CO, et al. Toxicity of nanoparticles in biomedical application: Nanotoxicology. Journal of Toxicology. 2021: 1-21.
- Haneefa MM. Green synthesis characterization and antimicrobial activity evaluation of manganese oxide nanoparticles and comparative studies with salicylalchitosan functionalized nanoform. Asian Journal of Pharmaceutics (AJP). 2017; 11.
- Patil S, Chandrasekaran R. Biogenic nanoparticles: A comprehensive perspective in synthesis, characterization, application and its challenges. Journal of Genetic Engineering and Biotechnology. 2020; 18: 1-23.
- Vandhana T, Lourduraj AC. Biogenic synthesis of mn-ag co-doped feo (fe1-2xmnxagx) nanoparticles: As an effective disinfectant and anticancer agent. Inorganic Chemistry Communications. 2020; 112: 107712.
- Ahmed T, Noman M, Shahid M, Shahid MS, Li B. Antibacterial potential of green magnesium oxide nanoparticles against rice pathogen acidovorax oryzae. Materials Letters. 2021; 282: 128839.
- El-Moslamy SH. Bioprocessing strategies for cost-effective large-scale biogenic synthesis of nano-mgo from endophytic streptomyces coelicolor strain e72 as an anti-multidrug-resistant pathogens agent. Scientific reports. 2018; 8: 3820.
- Kanjana D. Foliar application of magnesium oxide nanoparticles on nutrient element concentrations, growth, physiological, and yield parameters of cotton. Journal of Plant Nutrition. 2020; 43: 3035-3049.
- El-Naggar NE-A, Hussein MH, El-Sawah AA. Phycobiliprotein-mediated synthesis of biogenic silver nanoparticles, characterization, in vitro and in vivo assessment of anticancer activities. Scientific reports. 2018; 8: 8925.
- Jeevanandam J, Chan YS, Wong YJ, Hii YS. Biogenic synthesis of magnesium oxide nanoparticles using aloe barbadensis leaf latex extract. IOP Conference Series: Materials Science and Engineering. IOP Publishing. 2020; 943: 012030.
- Khan MF, Khan MA. Plant-derived metal nanoparticles (pdmnps): Synthesis, characterization, and oxidative stress-mediated therapeutic actions. Future Pharmacology. 2023; 3: 252-295.
- Nejati M, Rostami M, Mirzaei H, Rahimi-Nasrabadi M, Vosoughifar M, Nasab AS, et al. Green methods for the preparation of mgo nanomaterials and their drug delivery, anti-cancer and anti-bacterial potentials: A review. Inorganic Chemistry Communications. 2022; 136: 109107.
- Cai L, Chen J, Liu Z, Wang H, Yang H, Ding W. Magnesium oxide nanoparticles: Effective agricultural antibacterial agent against ralstonia solanacearum. Frontiers in microbiology. 2018; 9: 790.
- Tabrez S, Khan AU, Hoque M, Suhail M, Khan MI, Zughaibi TA. Investigating the anticancer efficacy of biogenic synthesized mgonps: An in vitro analysis. Frontiers in Chemistry. 2022; 10: 970193.
- Abdel-Aziz MM, Emam TM, Elsherbiny EA. Bioactivity of magnesium oxide nanoparticles synthesized from cell filtrate of endobacterium burkholderia rinojensis against fusarium oxysporum. Materials Science and Engineering: C. 2020; 109: 110617.
- Mustapha T, Misni N, Ithnin NR, Daskum AM, Unyah NZ. A review on plants and microorganisms mediated synthesis of silver nanoparticles, role of plants metabolites and applications. International Journal of Environmental Research and Public Health. 2022; 19: 674.
- Sarhan MS, Hamza MA, Youssef HH, Patz S, Becker M, El Sawey H, et al. Culturomics of the plant prokaryotic microbiome and the dawn of plant-based culture media–a review. Journal of Advanced Research. 2019; 19: 15-27.
- Rani N, Singh P, Kumar S, Kumar P, Bhankar V, Kumar K. Plant-mediated synthesis of nanoparticles and their applications: A review. Materials Research Bulletin. 2023; 112233.
- Khan F, Shariq M, Asif M, Siddiqui MA, Malan P, Ahmad F. Green nanotechnology: Plant-mediated nanoparticle synthesis and application. Nanomaterials. 2022; 12: 673.
- Singh J, Dutta T, Kim K-H, Rawat M, Samddar P, Kumar P. ‘Green’synthesis of metals and their oxide nanoparticles: Applications for environmental remediation. Journal of nanobiotechnology. 2018; 16: 1-24.
- Das B, Moumita S, Ghosh S, Khan MI, Indira D, Jayabalan R, et al. Biosynthesis of magnesium oxide (mgo) nanoflakes by using leaf extract of bauhinia purpurea and evaluation of its antibacterial property against staphylococcus aureus. Materials Science and Engineering: C. 2018; 91: 436-444.
- Moorthy SK, Ashok C, Rao KV, Viswanathan C. Synthesis and characterization of mgo nanoparticles by neem leaves through green method. Materials Today: Proceedings. 2015; 2: 4360-4368.
- Sharma G, Soni R, Jasuja ND. Phytoassisted synthesis of magnesium oxide nanoparticles with swertia chirayaita. Journal of Taibah University for Science. 2017; 11: 471-477.
- Shahid S, Ejaz A, Javed M, Mansoor S, Iqbal S, Elkaeed EB, et al. The anti-inflammatory and free radical scavenging activities of bio-inspired nano magnesium oxide. Frontiers in Materials. 2022; 9: 875163.
- Essien ER, Atasie VN, Okeafor AO, Nwude DO. Biogenic synthesis of magnesium oxide nanoparticles using manihot esculenta (crantz) leaf extract. International Nano Letters. 2020; 10: 43-48.
- Dobrucka R. Synthesis of mgo nanoparticles using artemisia abrotanum herba extract and their antioxidant and photocatalytic properties. Iranian Journal of Science and Technology, Transactions A: Science. 2018; 42: 547-555.
- Munjal S, Singh A, Kumar V. Synthesis and characterization of mgo nanoparticles by orange fruit waste through green method. International Journal of Advanced Research in Computer Science. 2017; 4: 36-42.
- Miu BA, Dinischiotu A. New green approaches in nanoparticles synthesis: An overview. Molecules. 2022; 27: 6472.
- Ammulu MA, Vinay Viswanath K, Giduturi AK, Vemuri PK, Mangamuri U, Poda S. Phytoassisted synthesis of magnesium oxide nanoparticles from pterocarpus marsupium rox. B heartwood extract and its biomedical applications. Journal of Genetic Engineering and Biotechnology. 2021; 19: 1-18.
- Abdallah Y, Ogunyemi SO, Abdelazez A, Zhang M, Hong X, Ibrahim E, et al. The green synthesis of mgo nano-flowers using rosmarinus officinalis l.(rosemary) and the antibacterial activities against xanthomonas oryzae pv. Oryzae. BioMed Research International. 2019.
- Prasanth R, Kumar SD, Jayalakshmi A, Singaravelu G, Govindaraju K, Kumar VG. Green synthesis of magnesium oxide nanoparticles and their antibacterial activity. Indian Journal of Geo Marine Sciences. 2019; 48: 1210-1215.
- Koul B, Poonia AK, Yadav D, Jin J-O. Microbe-mediated biosynthesis of nanoparticles: Applications and future prospects. Biomolecules. 2021; 11: 886.
- Busi S, Rajkumari J. Microbially synthesized nanoparticles as next generation antimicrobials: Scope and applications, nanoparticles pharmacother. Elsevier. 2019; 485–524.
- Sánchez-Cañizares C, Prell J, Pini F, Rutten P, Kraxner K, Wynands B, et al. Global control of bacterial nitrogen and carbon metabolism by a ptsntr-regulated switch. Proceedings of the National Academy of Sciences. 2020; 117: 10234-10245.
- Boroumand Moghaddam A, Namvar F, Moniri M, Md. Tahir P, Azizi S, Mohamad R. Nanoparticles biosynthesized by fungi and yeast: A review of their preparation, properties, and medical applications. Molecules. 2015; 20: 16540-16565.
- Ghosh S, Ahmad R, Zeyaullah M, Khare SK. Microbial nano-factories: Synthesis and biomedical applications. Frontiers in Chemistry. 2021; 9: 626834.
- Wang L, Hu C, Shao L. The antimicrobial activity of nanoparticles: Present situation and prospects for the future. International journal of nanomedicine. 2017; 12: 1227-1249.
- Loshchinina EA, Vetchinkina EP, Kupryashina MA. Diversity of biogenic nanoparticles obtained by the fungi-mediated synthesis: A review. Biomimetics. 2022; 8: 1.
- Guilger-Casagrande M, Lima Rd. Synthesis of silver nanoparticles mediated by fungi: A review. Frontiers in bioengineering and biotechnology. 2019; 7: 287.
- Raliya R, Tarafdar J. Biosynthesis and characterization of zinc, magnesium and titanium nanoparticles: An eco-friendly approach. International Nano Letters. 2014; 4: 1-10.
- Rathore I, Tarafdar J. Perspectives of biosynthesized magnesium nanoparticles in foliar application of wheat plant. Journal of Bionanoscience. 2015; 9: 209-214.
- Jhansi K, Jayarambabu N, Reddy KP, Reddy NM, Suvarna RP, Rao KV, et al. Biosynthesis of mgo nanoparticles using mushroom extract: Effect on peanut (arachis hypogaea l.) seed germination. 3 Biotech. 2017; 7: 263.
- Behzadi E, Sarsharzadeh R, Nouri M, Attar F, Akhtari K, Shahpasand K, et al. Albumin binding and anticancer effect of magnesium oxide nanoparticles. International journal of nanomedicine. 2019; 14: 257-270.
- Verma SK, Jha E, Panda PK, Thirumurugan A, Suar M. Biological effects of green-synthesized metal nanoparticles: A mechanistic view of antibacterial activity and cytotoxicity. Advanced nanostructured materials for environmental remediation. 2019; 145-171.
- Das B, Dash SK, Mandal D, Ghosh T, Chattopadhyay S, Tripathy S, et al. Green synthesized silver nanoparticles destroy multidrug resistant bacteria via reactive oxygen species mediated membrane damage. Arabian Journal of Chemistry. 2017; 10: 862-876.
- Balakumaran M, Ramachandran R, Balashanmugam P, Mukeshkumar D, Kalaichelvan P. Mycosynthesis of silver and gold nanoparticles: Optimization, characterization and antimicrobial activity against human pathogens. Microbiological research. 2016; 182: 8-20.
- Sierra-Fernandez A, De la Rosa-García S, Gomez-Villalba LS, Gómez-Cornelio S, Rabanal ME, Fort R, et al. Synthesis, photocatalytic, and antifungal properties of mgo, zno and zn/mg oxide nanoparticles for the protection of calcareous stone heritage. ACS applied materials & interfaces. 2017; 9: 24873-24886.
- Saniasiaya J, Salim R, Mohamad I, Harun A. Antifungal effect of malaysian aloe vera leaf extract on selected fungal species of pathogenic otomycosis species in in vitro culture medium. Oman Medical Journal. 2017; 32: 41-46.
- Vergheese M, Vishal SK. Green synthesis of magnesium oxide nanoparticles using trigonella foenum-graecum leaf extract and its antibacterial activity. Journal of pharmacognosy and phytochemistry. 2018; 7: 1193-1200.
- Szewczyk OK, Roszczenko P, Czarnomysy R, Bielawska A, Bielawski K. An overview of the importance of transition-metal nanoparticles in cancer research. International Journal of Molecular Sciences. 2022; 23: 6688.
- Dai C, Li D, Gong L, Xiao X, Tang S. Curcumin ameliorates furazolidone-induced DNA damage and apoptosis in human hepatocyte l02 cells by inhibiting ros production and mitochondrial pathway. Molecules. 2016; 21: 1061.
- Pandurangan M, Veerappan M, Kim DH. Cytotoxicity of zinc oxide nanoparticles on antioxidant enzyme activities and mrna expression in the cocultured c2c12 and 3t3-l1 cells. Applied biochemistry and biotechnology. 2015; 175: 1270-1280.
- Bertrand N, Wu J, Xu X, Kamaly N, Farokhzad OC. Cancer nanotechnology: The impact of passive and active targeting in the era of modern cancer biology. Advanced drug delivery reviews. 2014; 66: 2-25.
- Liou G-Y, Storz P. Reactive oxygen species in cancer. Free radical research. 2010; 44: 479-496.
- Suresh J, Yuvakkumar R, Sundrarajan M, Hong SI. Green synthesis of magnesium oxide nanoparticles. Advanced Materials Research. 2014; 952: 141-144.