
Research Article
Austin Biom and Biostat. 2015;2(1): 1011.
Analysis of Buruli Ulcer Prevalence in Amansie West District: A Geostatistical Approach
Frank B Osei1* and Alfred A Duker2
1Department of Mathematics and Statistics, University of Energy and Natural Resources, Ghana
2Department of Geomatic Engineering, Kwame Nkrumah University of Science and Technology, Ghana
*Corresponding author: Osei FB, Department of Mathematics and Statistics, University of Energy and Natural Resources, Sunyani, Ghana.
Received: October 27, 2014; Accepted: January 05, 2015; Published: January 06, 2015
Abstract
Buruli Ulcer (BU) is a disease caused by Mycobacterium Ulcerans (MU). The exact mode of transmission is yet unknown. However, the occurrences of BU cases in some specific discrete foci suggest a space-confined distribution pattern; this motivates this study. We incorporate a geostatistical technique to investigate the spatial patterns of BU prevalence in part of the Amansie West district of Ghana. A semivariogram model was computed to summarize the spatial variation and to determine the strength and spatial scale of the pattern. Ordinary kriging was used to produce a spatially continuous risk map of BU. The semivariogram model indicated a wider range of spatial dependence. Such nature of spatial dependence could be attributed to poor nature of public health interventions and the nature of BU risk factors. Spatial distribution of BU was observed to be high at the southern parts of the study area. Empirical classification of the study area into low, moderate and high risk zones showed that20% of the communities are within the high risk zone; 7% within the moderate risk zone; and 73% within the low risk zone.
Keywords: Mycobacterium ulcerans; Buruli ulcer; WHO; Topographic maps
Introduction
Buruli Ulcer (BU) is a disease caused by Mycobacterium Ulcerans (MU) that manifests as disfiguring skin ulceration which is difficult to treat. In its advanced stage the disease does not respond to drugs and requires surgery, sometimes even limb amputation. It often starts as a painless, mobile swelling under the skin called a nodule. The disease can present as a large area of indurations- plaque- or a diffuse swelling of the legs and arms - edema. Because of the local immunosuppressive properties of mycolactone, or perhaps as a result of other unknown mechanisms, the disease progresses with no pain and fever, which may partly explain why those affected often, do not seek prompt treatment [1]. If untreated BU may lead to extensive soft tissue destruction, with inflammation extending to deep fascia. The parts of the body most affected are the extremities. Subsequent complications may include contracture and deformities. The main form of treatment was excision surgery and skin grafting while, occasionally amputation of limb is unavoidable. Today, however, according to WHO the overwhelming evidence is that, 8 weeks of streptomycin-rifampicin or 4 weeks of rifampicin-streptomycin followed by 4 weeks of rifampicin-clarithromycin or 8 weeks of other oral regimens all achieve recurrence-free healing with an acceptable level of side-effects. This is true for ulcers of all sizes, even without additional surgery to remove necrosis or skin grafting to accelerate healing [2].
In recent years, there has been increased incidence of BU in West Africa (including Benin, Burkina Faso, Cote d'Ivoire, Ghana, Guinea, Liberia and Togo), Mexico, French Guyana, Papua New Guinea and Australia. The disease seems to affect mostly impoverished inhabitants in remote and rural areas; children are the most vulnerable, accounting for approximately 70% of the cases [3]. The World Health Organization (WHO) has recognized BU as the third most prevalent mycobacterium disease after tuberculosis and leprosy and has called for urgent action to control [3,4].
The disease was first described by Cook in Uganda in1897 [5] and the etiologic agent was characterized by [6] and others in Australia. Since [7] reported the first case of Buruli ulcer identified in Ghana in 1971 awareness of the public health importance of the disease has been increasing [8]. Due to the ugly appearance of the deformities it leaves, Buruli ulcer is greatly feared and stigmatized in the endemic areas, and is often attributed to witchcraft and curses [9].
The mode of MU infection is still unknown; yet several hypotheses have been described. The first hypothesis is trauma to the skin by a contaminated environment (e.g., soil, water, vegetation, insect vector) [10]. The second hypothesis is that, as MU has been shown to be aerosolized from suspensions of tap water [11] it could be inhaled or ingested [11-13] and then reactivated in low temperature areas of the body at the sites of trauma. The second hypothesis is demonstrated in an extensive outbreak among residents of Philip Island [14,15]. One could argue, however, that there are either three hypotheses, or basically only one hypothesis: i.e., whatever mechanism or vector transmission, the basic idea is that there is some unknown environmental reservoir (in water-or mud plant bio films; snails; amoebae; fish; faecal pellets of certain rodents; etc.). These suggest some space-confined environmental reservoir, which justifies the definition of the risk of infection to space or place-defined risk; and therefore the analysis based on a geostatistical approach. Since environmental processes that play a role in mediating BU disease are spatially continuous in nature [16] BU rates are expected to display some spatial pattern, i.e. BU rates measured in communities that are geographically closer should be more similar than the rates recorded over larger separation distances.
Past studies of spatial patterns have relied mostly on methods based on the examination of the mean and variance or on the frequency distribution of observed disease incidence [17]. Deterministic approaches to interpolation (trend surface, inverse distance weighting, triangulation, and splinting) are based upon a priori mathematical models of spatial variation. It is assumed that the sampled data has no errors, which is often an incorrect assumption. In practice, error cannot be eliminated but only minimized. Therefore, in most cases one cannot produce the best representative map of estimated values in un-sampled locations with these techniques. However, these methods do not incorporate information on the location of the samples and in particular they fail to consider the degree of dependency between neighbouring observations (i.e., spatial dependence). Recently, methods that recognize such dependency have been proposed [18]. Geostatistics, as introduced by geologists, quantifies the spatial dependence and has been applied successfully in agro forestry, agronomy, and entomology [19]. In this study, we incorporate a geostatistical technique to investigate the spatial patterns of BU prevalence.
Materials and Methods
Study area
In Ghana, the first case of BU was reported in 1971 and, between 1991 and 1997, more than 2000 cases have been reported [4]. Approximately 6000 cases were recorded in a national survey in 1999. BU is the third most widespread mycobacterium infection in the country with an overall prevalence of 22.7 per 100,000. Cases have been identified in all ten regions of the country and in 90 out of 110 districts [20]. The Ashanti Region (which accounts for 10.2% of the land area and 19.1% of the population of Ghana) is the worst affected, accounting for 60% of all reported cases (Figure 2), with the Amansie West District (of the Ashanti Region) having the highest prevalence of 150.8 per 100,000 [20]. Figure 1 shows the prevalence of suspected active cases of Buruli Ulcer by region. In the Amansie West District high incidence of BU occurs in communities in close proximity to the off in River. Reports also show that 44% of BU patients are farmers [21]. This district is therefore placed in a suitable setting for studying the relation between BU and environmental factors that may potentially contribute to infection. The Amansie West District lies between latitudes 6°00'N and 6°45'N and longitudes 1°30'W and 2°15'W and covers an area of approximately 1,136 km2. The District is underlain by Lower Proterozoic volcanic greenstones with intervening sedimentary rocks and granitoid intrusions [22]. The District is drained by the off in and Oda rivers and the landscape varies from gentle to broken. Vegetation thrives on ferric fluvisols (the major soil types), which have been developed through yearly rainfall ranging from 125 to 200 cm and temperatures of 22 to 30° C. Vegetation is secondary forests, thicket, swamp and for b re growth (i.e., soft-stemmed leafy herbs, mostly the weeds, which appear on farms and have to be cut regularly). Of the 310 settlements in the Amansie West District, 19 have a population of 1000 or more, and their total population in 2000 was 108,726. There are approximately equal percentages of males and females. In terms of occupation, 70% are farmers and 22% are engaged in legal and 'Galamsey' (or illegal) gold mining. Since the national BU case search in 1999, the Amansie West District Health Administration and the mission hospital (St. Martin's Hospital) at Agroyesum have consistently collected data on BU.
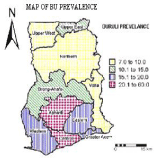
Figure 1: A map of Ghana showing prevalence of suspected active cases of Buruli Ulcer, by region, 1999.
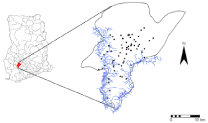
Figure 2: Location of the Amansie West District, Ghana.
Materials
- The following relevant materials were used for this study:Incidence of BU per settlement from 1999 - 2005, Amansie West District.
- Settlement population estimates for 2005, Amansie West District.
- Topographic maps (Sheet 0602C1, C3, 0602D2 and D4 at a scale of 1:50,000) showing location of settlements of Amansie West District were obtained from the Survey Department, Accra, Ghana.
- Amansie West District map (at a scale of 1:250,000).
- Aster image (2002)
- ArcGIS and ILWIS software's.
Methods
Feature classes such as settlement locations, rivers, roads and district boundary were digitized from the topographic sheet and district map. Settlement locations were digitized as points, rivers and roads digitized as lines and the district boundary as polygon. This operation was performed using ArcGIS and the digitizing board. These features were imported into ILWIS for further analysis. Each feature digitized has a unique identified number linked to an attribute table. To bring the incidences of the disease to a common standard, we calculated the disease prevalence by dividing the incidence by the population for each community in the study area. The spatial location of each community is given by the coordinate of its centroid. The input data was examined to check the distribution (random, clustered, regular, or paired, etc) of sample points.
The experimental semi-variogram values were computed using
γ(h)=1/2(E[Z(x)-Z(x+h)]2).............. (1)
Where Z(x) and Z(x+h) are the values of Z at any two places, x and x+h separated by h, and E denotes the expectation. The function γ(h) that relates the variance to the lag is the semi-variogram. When the distance becomes great, the sample values may become independent of one another and then γ(h) tends towards a maximum value.
Spatial correlation operation was used to investigate whether the values are spatially correlated and until which distance from any point this correlation occurs. The omnidirectional method which simply determines all distances between all point pairs, regardless of any direction, was used. Thus, all point pairs that have a certain distance towards each other were counted in a certain distance class. When using the omnidirectional method, wij=1 when point pairs belong to a certain distance class, otherwise wij=0.
From the results of the spatial correlation operations, a semi-variogram was plotted. In the semi-variogram, the discrete experimental semi-variogram values that are the outcome of spatial correlation analyses were modelled by a continuous function so that a semivariogram value γ(h) will be available for any distance h. Several models (Circular, Exponential, Gaussian, and Spherical) were fitted but Gaussian model gave the best R2.
Spherical model was fitted for each graph plotted by specifying values for the sill, range and nugget. Formulae used for calculating the Gaussian model is given below:
Where θs>0 is the partial sill parameter, θr>0 is the range parameter, and h is the lag distance.
The model that best fitted the variogram was obtained by calculating the Goodness of fit.
Where γ is the experimental semi-variogram values calculated from the spatial correlation analyses γ is the estimated semivariogram values from the model, and N is the total number of lags. The maximum value of R2 is 1, meaning an exact match of calculated semivariogram values and experimental semi-variogram values.
In this study, ordinary kriging was chosen over the other interpolation methods. Unlike interpolation methods like simple kriging, ordinary kriging assumes that the randomized spatial function is non-stationary and the mean varies over the area of interest. It amounts to re-estimating the mean at each new location. Ordinary kriging also provides a measure of the probable error associated with the estimates. ILWIS software was used to undertake all geostatistical analyses.
The slicing function available in ILWIS software was used to define the kriged surface low, moderate, and high risk zones. The threshold for classification was based on the distribution of the interpolated risks. Values below the mean were considered to be low risk; values between the man and the mean plus half of the standard deviation were considered to be moderate; and those higher than that were considered to be high risk.
Results
Experimental semivariograms were computed from the 62 communities using the Equation 1. The semivariogram was estimated using 10 lags of 6km. Figure 3 shows the results of the semivariogram model. Since the spatial variability of BU prevalence was assumed to be isotropic, directional semivariograms were not considered in this study, hence only the omnidirectional semivariogram is displayed. The semivariogram is a Gaussian model with a practical range of almost 36km. The semivariogram also shows a relatively larger range of spatial autocorrelation compared with the maximum distance between pairs of communities i.e. almost 60km.
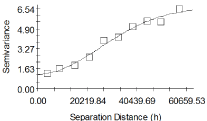
Figure 3: Omni directional semivariogram model describing the spatial
structure of BU prevalence estimated using 10 lags of 6km.
Figure 4 shows the results of the kriging interpolation. A visual inspection reveals clustering of high rates at the southern locations. After slicing the BU risk map, almost20% of the communities fell within the high risk zone; 7% within the moderate risk zone; and 73% within the low risk zone.
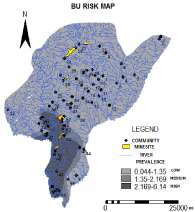
Figure 4: Results of the kriged map sliced into three classes, i.e. low,
moderate and high risk zones.
Discussion
This study utilised geostatistical methods to describe and map the spatial patterns of BU prevalence in the Amansie West District. The findings from the semivariogram model reveal that there is a relatively larger range of spatial autocorrelation, suggesting a better spatial structure of BU transmission. The larger range of spatial autocorrelation may be explained by two main reasons. (1) Public health interventions: Public health has carried out little interventions to reduce the risk of MU transmission in the study area. Therefore, local similarities in socioeconomic condition and health behaviour among neighbouring communities due fewer interventions programs may explain the larger range of spatial autocorrelation displayed by the semivariogram model. (2) Nature of BU risk factors: The larger range of spatial autocorrelation also shows that there are common elevated risk factors of BU among high prevalent communities. This is also an indication of large scale environmental/ecological variables contributing to BU transmission within the study area. Although the main mode of BU transmission is quite unclear, several studies have related BU transmission to environmental factors. It has been observed that the common MU host environments are related to rivers, swampy areas [23], or through environmental disturbances such as flooding [11,24] and mining [25]. Such environmental variables are often large scale which can influence several communities within a particular spatial region. Example, through spatial analyses, Duker et al [26] asserted that an important environmental disturbance that influences BU transmission within the study area is artisanal mining. After the enactment of Ghana's Small Scale Mining Law of 1989, several artisanal mining companies, popularly known as 'Galamsey' have sprung up within the study area. Whiles artisanal mining has become an important economic sector within this study area, lack of training, resources and environmental awareness amongst them leads exposure to environmental toxics such as heavy metals. Runoff during heavy rainfall may results in the downward transport of substantial fluxes of some heavy metals from artisanal mining workings [27], which consequently may pollute surface water bodies used for various household activities. Studies suggest that exposure to some heavy metals such as arsenic interferes with several enzymes in the body [28,29] and could predispose to defects in the immune system [30], lowering resistance to mycobacterial infections [31].
The high rates of BU within the southern locations may be explained by the numerous floodplains and agricultural activities within such areas. Outbreaks of BU have in many cases been attributed to environmental disturbances such as flooding, riverine and swampy areas [12,32]. If an environmental disturbance such as mining is a contributory factor to BU transmission, then the high rates of BU within the southern locations may be explained by the downstream transport of substantial fluxes of heavy metals such as arsenic from artisanal mine workings during heavy rains [27]. Also, in times of flooding, which are frequent in the study area, these arsenic-bearing sediments are deposited on river floodplains, which constitute the farmland soils of the local inhabitants. Food crops grown on arsenicenriched soils tend to take up arsenic [33,34].
It is also true that cases of the disease have reduced due to education concerning how individuals could protect themselves against it. This reduction or fluctuations could also be due to climatic changes, environmental changes, immunological factors including blunting of the epidemic due to lack of susceptible individuals, coinfection driving the immune response towards enhanced or reduced protection, e.g., changing the Th1/Th2 balance etc., indicating that other factors apart from geology may be driving the epidemic and explaining the risk for BU infection.
Limitations of the Study
This study also has several limitations. (1): In this study, only the omnidirectional semivariogram model was used to describe the spatial structure of the data, neglecting possible directional influences on the semivariogram. (2): Also, we ignored the fact that the BU rates computed consist of a numerator (cases) and a denominator (population). Therefore, there is the likelihood of variance instability in BU rates as a result of heterogeneity in the distribution of BU cases and population data.
References
- WHO: Surveillance and control of Mycobacterium ulcerans disease (Buruli ulcer). In: Fifty-seventh World Health Assembly. Resolutions and decisions. Geneva. 2004.
- WHO. World Health Organization. Treatment of mycobateriumulcerans disease (Buruli ulcer): guidance for health workers: World Health Organization. 2014.
- Asiedu K, Scherpbier R, Raviglione M. Buruli ulcer: Mycobacterium ulcerans infection. Geneva: World Health Organization. 2000.
- Grosset J, Kanga JM, Portaels F, Guedenon A, Tignokpa N, Scherpbier R, et al. Country assessment reports (Annex 5). In: Asiedu K, Scherpbier R, Raviglione M, editors. Buruli Ulcer: Mycobacterium ulcerans infection, World Health Organisation, Global Buruli Initiative. 2000; 87-92.
- Strickland GT. Hunter's Tropical Medicine and Emerging Infectious Diseases. 8th edition. Philadelphia: W. B. Saunders Company. 2000; 524-525.
- MacCallum P, Tolhurst JC. A new mycobacterial infection in man. J Pathol Bacteriol. 1948; 60: 93-122.
- Bayley AC. Buruli ulcer in Ghana. Br Med J. 1971; 2: 401-402.
- van der Werf TS, van der Graaf WT, Groothuis DG, Knell AJ. Mycobacterium ulcerans infection in Ashanti region, Ghana. Trans R Soc Trop Med Hyg. 1989; 83: 410-413.
- Stienstra Y, van der Graaf WT, Asamoa K, van der Werf TS. Beliefs and attitudes toward Buruli ulcer in Ghana. Am J Trop Med Hyg. 2002; 67: 207-213.
- Portaels F, Johnson P, Meyers WM, editors. Buruli ulcer: Diagnosis of Mycobacterium ulceransdisease. Geneva: World Health Organization. 2001.
- Hayman J. Postulated epidemiology of Mycobacterium ulcerans infection. Int J Epidemiol. 1991; 20: 1093-1098.
- Johnson PD, Stinear TP, Hayman JA. Mycobacterium ulcerans--a mini-review. J Med Microbiol. 1999; 48: 511-513.
- Connor DH, Lunn HF. Mycobacterium ulcerans infection (with comments on pathogenesis). Int J Lepr. 1965; 33: 698-709.
- Ross BC, Johnson PD, Oppedisano F, Marino L, Sievers A, Stinear T, et al. Detection of Mycobacterium ulcerans in environmental samples during an outbreak of ulcerative disease. Appl Environ Microbiol. 1997; 63: 4135-4138.
- Stinear T, Davies JK, Jenkin GA, Hayman JA, Oppedisano F, Johnson PD. Identification of Mycobacterium ulcerans in the environment from regions in Southeast Australia in which it is endemic with sequence capture-PCR. Appl Environ Microbiol. 2000; 66: 3206-3213.
- Webster R, Oliver MA, Munir KR, Man JR. Kriging the local risk of rare disease from a register of diagnoses. Geographical Analysis. 1994; 26: 168-185.
- Nicot PC, Rouse DI, Yandell BS. Comparison of statistical method for studying spatial patterns of soil borne plant pathogens in the field. Phytopathology. 1984; 74: 1399-1402.
- Reynolds KM, Madden LV. Analysis of epidemics using spatio-temporal autocorrelation. Phytopathology 1988; 78: 240-246.
- Burgess TM, Webster R, McBratney AM. Optimal interpolation and isarithmic mapping of soil properties. J SoilSci. 1980; 31: 505-524.
- Amofah G, Bonsu F, Tetteh C, Okrah J, Asamoa K, Asiedu K, et al. Buruli ulcer in Ghana: results of a national case search. Emerg Infect Dis. 2002; 8: 167-170.
- Amofah GK, Sagoe-Moses C, Adjei-Acquah C, Frimpong EH. Epidemiology of Buruli ulcer in Amansie West district, Ghana. Trans R Soc Trop Med Hyg. 1993; 87: 644-645.
- Robb LJ, Yao Y, Armstrong RA, Murphy PJ. Gold in the Birimian granites of Ghana: a metamorphic origin. In: Stanley, editor. Mineral Deposits: Processes to Processing, Balkema AA, Rotterdam. 1999; 1033-1036.
- Portaels F. Epidemiology of mycobacterial diseases. Clin Dermatol. 1995; 13: 207-222.
- Barker DJ. Epidemiology of Mycobacterium ulcerans infection. Trans R Soc Trop Med Hyg. 1973; 67: 43-50.
- Aguiar J, Domingo MC, Guedenon A, Meyers W, Stenou C, Portaels F. Buruli ulcer, a significant increase in mycobacterial disease in Benin. Bull SeancAcad R. Overseas. 1997; 43: 325-356.
- Duker AA, Carranza EJ, Hale M. Spatial dependency of Buruli ulcer prevalence on arsenic-enriched domains in Amansie West District, Ghana: implications for arsenic mediation in Mycobacterium ulcerans infection. Int J Health Geogr. 2004; 3: 19.
- Craw D, Wilson N, Ashley PM. Geochemical controls on the environmental mobility of Sb and As at mesothermal anatomy and gold deposits. Appl Earth Sci (Trans. Inst. Min. Metall. B). 2004.
- Abernathy CO, Liu YP, Longfellow D, Aposhian HV, Beck B, Fowler B, et al. Arsenic: health effects, mechanisms of actions, and research issues. Environ Health Perspect. 1999; 107: 593-597.
- NRC (National Research Council): Arsenic in drinking water. National Research Council. Washington, D. C: National Academy Press. 1999.
- Harrison MT, McCoy KL. Immunosuppression by arsenic: a comparison of cathepsin L inhibition and apoptosis. Int Immunopharmacol. 2001; 1: 647-656.
- Stienstra Y, van der Graaf WT, te Meerman GJ, The TH, de Leij LF, van der Werf TS. Susceptibility to development of Mycobacterium ulcerans disease: review of possible risk factors. Trop Med Int Health. 2001; 6: 554-562.
- Portaels F. [Epidemiology of ulcers due to Mycobacterium ulcerans]. Ann Soc Belg Med Trop. 1989; 69: 91-103.
- Sarkodie PH, Nyamah D, Amonoo-Niezer EH. Speciation of arsenic in some biological samples from Obuasi and its surrounding villages. National Symposium Proceedings - The Mining Industry and the Environment. 1997; 146-154.
- Alam MG, Snow ET, Tanaka A. Arsenic and heavy metal contamination of vegetables grown in Samta village, Bangladesh. Sci Total Environ. 2003; 308: 83-96.