
Mini Review
Austin Biomol Open Access. 2016; 1(2): 1009.
Cyanobacterial Toxin Cylindrospermopsin: It’s Possible Pathway from Poisoning to Cancer Curing
El-Deeb NM*
Biopharmacetical Product Research Department, Genetic Engineering and Biotechnology Research Institute, Egypt
*Corresponding author: Nehal M El-Deeb, Biopharmacetical Product Research Department, Genetic Engineering and Biotechnology Research Institute, City of Scientific Research and Technology Applications, Egypt
Received: July 25, 2016; Accepted: November 07, 2016; Published: November 10, 2016
Abstract
Cyanobacterial allelopathic interactions were being increasingly emerged for the pharmaceutical and environmental significance of the bioactive molecules. The biosynthetic pathways, regulatory mechanisms and genes involved in the cyanobacterial toxic compounds synthesis were well understood in relation to biotoxins, whereas the cytotoxins were being less investigated. Cyanotoxins comprised a rich natural source of cytotoxic compounds with potentiation to target specific uptake transporters in cancer cells. Moreover, their unique structure provided opportunities to resolve organ-specific toxicity issues and improve the therapeutic index.
In this review, we will discuss the possible role of cyanobacterial Cylindrospermopsin as a novel anticancer drug by summarizing the existing biomedical evidence of its toxicity, presenting structure-activity interaction and discussing developmental perspectives.
Keywords: Cyanobacterial toxins; Cylindrospermopsin; Toxicity; Anticancer
Introduction
Cyanobacteria (blue-green algae), among the Gram-negative photosynthetic prokaryotes, appeared approximately 3.5 billion years ago [1], cyanobacteria contain large group members 150 genera and about 2000 species of considerable diversity in physiology, metabolism and morphology [2]. They are prokaryotic that found in nature as unicellular species or in colonies (Figure 1) and rapidly grows in different habitats as terrestrial, fresh water, and marine ecosystems [2]. In addition, cyanobacteria also grow under various extremely conditions as they could found in Antarctic lakes and both saline and hot springs. Cyanobacteria consider as the primary first level organisms in food chains in water ecosystems due to their photosynthetic capacity. They also play an important role in the marine nitrogen cycle and have a role in balancing nitrogen (N) and CO dynamics in the biosphere [3]. Besides this role, certain species of cyanobacteria in water reservoirs (toxic cyanobacterial water-blooms) produce diverse toxic secondary metabolites as defense mechanisms against environmental stress factors present in a marine environment [4]. It is well known the potent cyanotoxins dangerous side effects to human as acute liver damage, neurotoxicity, gastrointestinal disturbances and liver cancer, all of which are demonstrated [5,6]. However, recently and by studying the physicochemical properties of cyanotoxins, the pharmacologists identify these molecules as potent anticancer agents. Cyanobacterial toxins such as Lipo Poly Saccharide (LPS), Anatoxin-a, Cylindrospermopsin and Microcystins could be pharmacologically active [7,8].
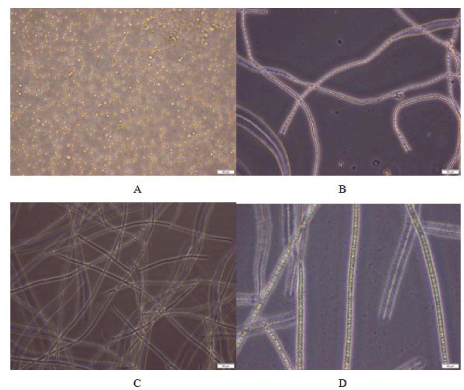
Figure 1: Different Cyanobacterial identified isolates from Egyptian habitat.
A) microcystisaeruginosa B) Nostoc commune C) Oscillatoria brevis D)
Arthrospiraplatensis.
More specifically a pharmacophore structure of cyanobacterial cyclopeptides cause cellular damages following cellular uptake through Organic Anion Transporting Polypeptides (OATPs). Interestingly, certain OATPs recorded to be prominently expressed in cancer cells in comparing with the normal cells, suggesting the possibility of using these toxins as targeted agents to cancer cells [9,10]. Many recorded cyanobacterial toxins have been studied for their anticancer prosperities in human cell lines, generating promising results for future research toward controlling of human cancers. To that end, the current review will focus on the possibility of Cylindrospermopsin usage as a novel anticancer compound in trail to identify its possible pathway from poisoning to healing.
Cylindrospermopsin
Cylindrospermopsin (CYN) is an important cyanobacterial toxin founded in water bodies worldwide. CYN was identified first in Cylindrospermopsis raciborskii culture while, other cyanobacteria species have been identified to produce CYN as Umezakianatans [11], Aphanizomenonovalisporum [12], Anabaena bergii [13], Raphidiopsiscurvata [14], Aphanizomenonflos-aquae [15], Anabaena lapponica [16] and Lyngbyawollei [17]. It was described first in Palm Island, northern Queensland, Australia, in 1979 after an outbreak of hepatoenteritis [18].
Chemical Characterization of CYN
CYN is alkaloid, a highly water soluble toxin it is a polycyclic uracil derivative containing guanidine and sulphate groups (Figure 2).
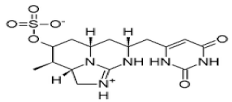
Figure 2: Structures of the most common isolatedCylindrospermopsin. IUPAC
(International Union of Pure and Applied Chemistry ) name; 2,4(1H,3H)-
Pyrimidinedione, 6-[(R)-hydroxy[(2aS,3R,4S,5aS,7R) -2,2a,3,4,5,5a,6,7-
octahydro-3-methyl-4-(sulfooxy) -1H-1,8,8b-triazaacenaphthylen-7-yl]
methyl]-.
Almost CYN complete structure was illustrated by Ohtani et al. [19] using Mass Spectroscopy (MS) and Nuclear Magnetic Resonance (NMR). However, later, it was found a false feature in the first identification as the orientation of the hydroxyl group, that was adjacent to uracil ring, it was corrected from epimerto 7-epicylindrospermopsin by Banker et al. [20]. This toxin was identified as a minor metabolite from A.ovalisporum that was isolated first from Lake Kinneret in Israel. In addition, Norris et al. in 1999 were able to isolate CYN (a toxic minor metabolite of A. ovalisporum) by Solid Phase Extraction (SPE) followed by semipreparative chromatography.
Toxicology
The only human toxicity associated cases with CYN were reported at the Palm Island hepato enteritis in 1979, where 148 people were hospitalized. The epidemiological investigation was summarized by Bourke et al. [18] revealed that people affected were mainly children they which had drunk CYA contaminated water that were supplied by reticulated system. The epidemic that consisted of three clear stages: a hepatitis phase (lasted 2 days), a lethargic phase (lasted 1-2 days) and a diarrhoeal phase (5 days of duration). While; the first report of the toxic effects of CYN in mouse model was noticed by Hawkins et al. [21] who used an extract of the original Palm Island strain in his experiment. Hawkins indicated that; the poisoned mice displayed anorexia, diarrhoea and gasping respiration. The autopsy data showed haemorrhages in the lungs, livers, kidneys, small intestines and adrenal glands. While, the Histopathological data showed a dose dependent necrosis in the hepatocytes, lipid accumulation and fibrinthrombi formation in blood vessels of the liver and lungs, along with varying epithelial cell necrosis in areas of the kidneys. Consequently and after identifying the chemical structure of CYN, Ohtani et al. [19] reported the LD50 of the pure CYN after i.p. administration in mice, he indicated that’ CYN had LC50 of 2.1mg/kg over 24 h and 0.2mg/kg lasting 5–6 days. Recently, Reid et al. [22] used Embryonic Stem Cell (mES) to evaluate the in vitro embryo-toxicity of CYN. They clarified that, after 24 hr, mES cells could tolerate toxins concentration up to 0.5 μg/mL without any morphological changes and the recorded toxin IC50 reached CYN 0.86 μg/mL. Another study was done by Humpage, et al. [23], the authors tested the effect of the purified cylindrospermopsin by daily gavage to male Swiss albino mice with doses 0, 30, 60, 120, or 240 μg/kg-day (10 mice per dose group) for 11 weeks. No deaths were reported in the study and they explained that, the kidney was the most sensitive organ to the toxin that increased in weigh in a dose-dependent manner starting at 60 μg/kg-day. Starting with the previous reports that support the finding; CYN was not toxic at concentrations up to 10 μg /L [24], we could mention that Low concentrations of cylindrospermopsin could be a promising therapeutic anticancer agent.
The Possibility of usage the Cyanobacterial Cylindrospermopsin toxins as Anticancer Agent
In cancer patients, the tumor cells that could survive during the chemotherapy often emerge with increased resistant to the treated drug in phenomenon could Multidrug Resistant (MDR). Different genera of Cyanobacteria as Anabaena, Oscillatoria and Microcystis considered as rich source of cytotoxic compounds that have a potential effect to target specific active uptake transporters in MDR cancer cells [24]. Theses toxins could be found in form of acyclic peptides, linear decadepsipeptides, lipophylic cyclic peptides and macrolactones that are very toxic to various cancer cell lines [24], these toxins could be potent 100-1000 times than the available anticancer drugs [25].
Marine cyanobacterial toxic metabolites as cylindrospermopsin exhibit important biological features by interfering in signal transduction either through sodium channels activation or by targeting signaling apoptosis proteins (Figure 3). The pharmacological importance of these toxins were not explained well up till now while; few reports explained that its important resides in their abilities to alter the cellular proliferation and growth rates of cancer cell lines [26]. The possible role of the tumor suppressor protein p53 in cylindrospermopsin-induced gene expression in human hepatocellular carcinoma cell line HepG2 was examined by Bain et al. [27]. The author clarified that After 24 hr, in HepG2 treated cells with cylindrospermopsin, there are an early concentration-dependent increases in mRNA levels of the p53 target genes CDKN1A, GADD45alpha, BAX and MDM2.
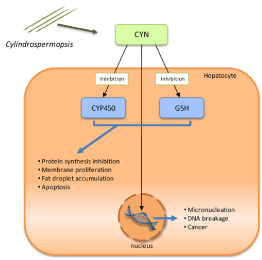
Figure 3: The molecular mechanisms of Cylindrospermopsin (CYN) toxicity
through the inhibition of Glutathione (GSH) and protein synthesis, as well as
Cytochrome P450 (CYP450), and CYN interaction with DNA. Valério et
al. [30].
In addition, the transcriptomic profile of differentiated Caco- 2 cells exposed to CYN, a sub-toxic concentration of CYN (1.6 μM) after 24hr of exposure was analysed by Huguet et al. [28] using pangenomic microarrays, Go Miner application and IPA software. The obtained data indicated that, after 24hr. of exposure, the differentiated Caco-2 cells over-expressed transcription and DNA damage repair genes, including modifications of nucleosomal histones. In parallel, in human hepatoma cells, HepG2, CYN showed abilities to induce DNA Double Strand Breaks (DSBs) formation after prolonged exposure (72 hrs). In addition, CYN (0.1-0.5 μg/mL, 24-96 h) induced morphological changes and reduced cell viability in a dose and time dependent manner without significant changes in Lactate dehydrogenase (LDH) leakage and with arresting cells in G0/G1 after 24 h and S-phase arrest after longer exposure. This results indicated that the reduction in cell numbers were due to the reduction in cell proliferation rates and not due to cytotoxicity [29].
However, if the cytotoxicity of cylindrospermopsin that mediated by the inhibition of protein synthesis, cell cycle arrest or create an imbalance in cellular redox potential could be used to target the tumor cells with doses that do not affect the healthy organs or can we hypothesize other mechanisms? Can we in the future explain other receptors that are specifically expressed by tumor cells that can be targeted by Cylindrospermopsin or a cylindrospermopsin-carrier conjugate? Can we find other cylindrospermopsin intracellular mechanisms that can induce tumor cells’ apoptosis or autophagy? Theses hypotheses need further future studies.
Conclusion
Cyanotoxins are responsible for severe poisoning in aquatic organisms and humans. Although these toxins could cause severe damages, they are also considered a rich source of natural cytotoxic compounds with potential anticancer action. Several compounds of various cyanobacterial species were found to inhibit cancer cell growth while the exact pathways by which cancer cells are inhibited are still poorly elucidated. Marine cyanobacteria toxins especially Cylindrospermopsin seems to be clearly a promising source of anticancer drugs. However, more explanations are still need to clarify the specific targets and pathways that are behind cancer cell cytotoxicity but an immense field of research is still waiting for further developments. Liver toxicity still might be a significant challenge in using cylindrospermopsin as anticancer drug making it necessary to chemically synthesized another derivatives of cylindrospermopsin that could be metabolically detoxified in healthy liver cells and/or effluxed into the bile, and thus take advantage of hepatic detoxification and clearance mechanisms that absent in tumor cells.
References
- Awramik SM. The oldest records of photosynthesis. Photosynth Res. 1992; 33; 75-89.
- Berg KA, Lyra C, Sivonen K, Paulin L, Suomalainen S, Tuomi P, et al. High diversity of cultivable heterotrophic bacteria in association with cyanobacterial water blooms. ISME J. 2009; 3: 314-325.
- Zehr JP, Waterbury JB, Turner PJ, Montoya JP, Omoregie E, Steward GF, et al. Unicellular cyanobacteria fix N2 in the subtropical North Pacific Ocean. Nature. 2001; 412: 635-638.
- Tan LT. Bioactive natural products from marine cyanobacteria for drug discovery. Phytochemistry. 2007; 68: 954-979.
- Gaudin J, Le Hegarat L, Nesslany F, Marzin D, Fessard V. In vivo genotoxic potential of microcystin-LR: a cyanobacterial toxin, investigated both by the unscheduled DNA synthesis (UDS) and the comet assays after intravenous administration. Environ Toxicol. 2009; 24: 200-209.
- van Apeldoorn ME, van Egmond HP, Speijers GJ, Bakker GJ. Toxins of cyanobacteria. Mol Nutr Food Res. 2007; 51: 7-60.
- Chorus I, Falconer IR, Salas HJ, Bartram J. Health risks caused by freshwater cyanobacteria in recreational waters. J Toxicol Environ Health B Crit Rev. 2000; 3: 323-347.
- Kounnis V, Chondrogiannis G, Mantzaris MD, Tzakos AG, Fokas D, Papanikolaou NA, et al. Microcystin LR Shows Cytotoxic Activity Against Pancreatic Cancer Cells Expressing the Membrane OATP1B1 and OATP1B3 Transporters. Anticancer Res. 2015; 35: 5857-5865.
- Monks NR, Liu S, Xu Y, Yu H, Bendelow AS, Moscow JA. Potent cytotoxicity of the phosphatase inhibitor microcystin LR and microcystin analogues in OATP1B1- and OATP1B3expressing HeLa cells. Mol Cancer Ther. 2007; 6: 587-598.
- Herfindal L, Kasprzykowski F, Schwede F, Lankiewicz L, Fladmark KE, Lukomska J. Acyloxymethyl esterification of nodularin-R and microcystin-LA produces inactive protoxins that become reactivated and produce apoptosis inside intact cells. J Med Chem. 2009; 52: 5758-5762.
- Harada KI, Ohtani I, Iwamoto K, Suzuki M, Watanabe MF, Watanabe M et al. Isolation of cylindrospermopsin from a cyanobacteriumUmezekianatans and its screening method. Toxicon. 1994; 32: 73-84.
- Banker R, Carmeli S, Hadas O, Teltsch B, Porat R. Sukenik A. Identi?cation of cylindrospermopsin in Aphanizomenon ovalisporum isolated from Lake Kinneret, Israel. J Phycol. 1997; 33: 613-616.
- Schembri MA, Neilan BA, Saint CP. Identi?cation of genes implicated in toxin production in the cyanobacterium Cylindrospermopsis raciborskii. Environ Toxicol. 2001; 16: 413-421.
- Li R, Carmichael WW, Brittain S, Eaglesham GK, Shaw GR, Liu Y, et al. First report of the cyanotoxinscylindrospermopsin and deoxycylindrospermopsin from Raphidiopsiscurvata. J Phycol. 2001; 37: 1121-1126.
- PreuBel K, Stuken A, Wiedner C, Chorus I, Fastner J. First report on cylindrospermopsin producing Aphanizomenon ?os-aquae (Cyanobacteria) isolated from two German lakes. Toxicon. 2006; 47: 156-162.
- Spoof L, Berg KA, Rapala J, Lahti K, Lepisto L, Metcalf JS, et al. First observation of cylindrospermopsin in Anabaena lapponica isolated from the Boreal Environment (Finland). Environ Toxicol. 2006; 21: 552-560.
- Seifert M, McGregor G, Eaglesham G, Wickramasinghe W, Shaw G. First evidence for the production of cylindrospermopsin and deoxy-cylindrospermopsin by the freshwater benthic cyanobacterium, Lyngbyawollei. Harmful Algae. 2007; 6: 73-80.
- Bourke ATC, Hawes RB, Neilson A, Stallman ND. An outbreak of hepato-enteritis (the Palm Island mystery disease) possibly caused by algal intoxication. Toxicon. 1983; 21: 45-48.
- Ohtani I, Moore RE, Runnegar MTC. Cylindrospermopsin: a potent hepatotoxin from the blue-green alga Cylindrospermopsis raciborskii. J Am Chem Soc. 1992; 114: 7942-7944.
- Banker R, Teltsch B, Sukenik A, Carmeli S. Epicylindro spermopsin, a toxic minor metabolite of the cyanobacterium Aphanizomenonovalisporum from Lake Kinneret, Israel. J Nat Prod. 2000; 63: 387-389.
- Hawkins PR, Runnegar MT, Jackson AR, Falconer IR (November 1985). Severe hepatotoxicity caused by the tropical cyanobacterium (blue-green alga) Cylindrospermopsis raciborskii (Woloszynska) Seenaya and Subba Raju isolated from a domestic water supply reservoir. Applied and Environmental Microbiology. 1985, 50: 1292-1295.
- Reid KJ, Lang K Froscio S, Humpage AJ, Young FM. Undifferentiated murine embryonic stem cells used to model the effects of the blue-green algal toxin cylindrospermopsin on preimplantation embryonic cell proliferation. Toxicon. 2015; 106: 79-88.
- Humpage AR, Falconer IR. Oral toxicity of the cyanobacterial toxin cylindrospermopsin in male Swiss albino mice: determination of no observed adverse effect level for deriving a drinking water guideline value. Environ Toxicol. 2003; 18: 94-103.
- Samuel Liebel, Ciro Alberto de Oliveira Ribeiro, Valéria Freitas de Magalhães, Rodrigo de Cássio da Silva. Low concentrations of cylindrospermopsin induce increases of reactive oxygen species levels, metabolism and proliferation in human hepatoma cells (HepG2). Toxicology in vitro. 2015; 29: 479-488
- Nagarajan M, Maruthanayagam V, Sundararaman M. A review of pharmacological and toxicological potentials of marine cyanobacterial metabolites. J Appl Toxicol. 2012; 32: 153-185.
- Trimurtulu G, Ohtani I, Patterson GML, Moore RE, Corbett TH, Valeriote FA, et al. Total structures of cryptophycins, potent antitumor desipeptides from blue green alga Nostoc sp. strain GSV 224. J Am Chem Soc. 1994; 116: 4729-4737.
- Bain P, Shaw G, Patel B. Induction of p53-regulated gene expression in human cell lines exposed to the cyanobacterial toxin cylindrospermopsin. J Toxicol Environ Health A. 2007; 70: 1687-1693.
- Huguet A, Hatton A, Villot R, Quenault H, Blanchard Y, Fessard V. Modulation of Chromatin Remodelling Induced by the Freshwater Cyanotoxin Cylindrospermopsin in Human Intestinal Caco-2 Cells. Plos 1. 2014; 9: e99121.
- Štern A, Filipic M, Novak M, Zegura B. Double Strand Breaks and Cell-Cycle Arrest Induced by the Cyanobacterial Toxin Cylindrospermopsin in HepG2 Cells. Marine Drugs. 2013; 11: 3077-90.
- Valério E , Chaves S and Tenreiro R. Diversity and Impact of Prokaryotic Toxins on Aquatic Environments: A Review. Toxins. 2010; 2: 2359-2410.