
Review Article
Austin Biomol Open Access. 2016; 1(2): 1010.
Aluminum Phthalocyanine Derivatives: Potential in Antimicrobial PDT and Photodiagnosis
Zafar I¹*, Arfan M², Nasir RP³ and Shaikh AJ¹
¹Department of Chemistry, COMSATS Institute of Information Technology, Abbottabad, 22060, Pakistan
²Department of Chemistry, School of Natural Sciences, University of Sciences and Technology (NUST), Pakistan
³Department of Chemistry, University of Sargodha, Pakistan
*Corresponding author: Zafar I, Department of Chemistry, COMSATS Institute of Information Technology, Pakistan
Received: October 30, 2016; Accepted: December 07, 2016; Published: December 09, 2016
Abstract
Photodynamic Therapy (PDT) is minimally invasive therapeutic technique based on effective transfer of energy from the excited photosensitizer to the oxygen molecules present in tissues. Subsequent generation of reactive oxygen species is responsible for the cell damage, through apoptosis or necrosis. The use of phthalocyanines and their metal derivatives (MPcs) in this field is not new, and has been studied extensively around the world. Among other phthalocyanine derivatives, AlPcs are particularly interesting owing to the capability of Al for axial ligation. Alongside peripheral substitution, axial ligation provides the opportunity of linking biologically benign ligands to the AlPc core forming a variety of derivatives possessing variable cytotoxic efficacies. This review focuses on the photophysical and photochemical properties, cellular uptake, in vitro and in vivo photodynamic cytotoxicity of AlPc derivatives. In addition, photodiagnostic and antimicrobial PDT applications of AlPcs have been discussed.
Keywords: Photodynamic therapy; Aluminium phthalocyanine; Photodiagnosis; Antimicrobial PDT; cytotoxicity; Photophysical and photophysical properties
Introduction
Photodynamic Therapy (PDT) is now regarded as the new emerging minimally invasive therapeutic technique to cure various lesions especially solid tumors. It is also equally effective in many non-oncological treatments in medical field [1,2]. It requires Photosensitizer (PS) to generate the Reactive Oxygen Species (ROS) responsible for cell damage. Despite the fact that none of the PS meets the criteria of an ideal sensitizer [3,4], a moderate number of compounds are commercially available [4] in the market with Photofrin® being the pioneer one. Among other classes of macrocyclic complexes (see ref. for comparative details of macrocyclic classes) [5], phthalocyanines [6,7] (Pcs) are extensively explored to formulate third generation photosensitizers [8]. This is due to enormous possibility of modification in the Pc macrocycle which leads to a vast variety of complexes. In addition to peripheral substitution the metal phthalocyanines (MPcs) may be axially modified in the presence of metals allowing axial substitution, with variable photodiagnostic and photodynamic therapeutic properties.
Photophysical and photochemical properties [9] (fluorescence, singlet and triplet quantum yields and lifetimes, singlet oxygen quantum yield etc.) provide the primary data to establish the potentiality of a substance as an effective PS. In case of Pc complexes, these parameters can be tuned by either peripheral modification of macrocycle and/or insertion of metal inside the ring. The Pc macrocycle can accommodate almost all metals present in the periodic table; however for PDT point of view MPcs containing Zn, Al or Si as central metal have been studied extensively owing to their improved photophysical and photochemical properties [10-12]. MPcs capable of axial ligation show promising results in PDT and imaging due to their reduced tendency of aggregation at cellular level thus improving the ROS generation capability through maximal absorption of light [13]. Axial ligation also provides the opportunity of facile conjugation of biomolecules for receptor mediated targeting of PS.
Over the years a large number of AlPcs have been synthesized and studied for photodynamic activity and diagnostic imaging against a number of tumors [14,15]. Much attention has been devoted to the development of modified sulfonated AlPcs to improve their PDT efficacy. Although distilled water formulation of mixture of sulfonated AlPc (Photosens) has been approved in Russia to cure a range of oncological and non-oncological lesions. However, it requires further refinement to overcome post-treatment skin sensitivity of patient. After PDT treatment using this PS the patient has to remain in dark for 6 to 10 weeks [16]. To cope with this deficiency the AlPc derivatives have been modified in a number of ways e.g. attachment with biomolecules, encapsulation into biocompatible polymers and conjugation with nanoparticles or quantum dots etc. To the best of our knowledge till now there is no compilation in the literature highlighting the potential of AlPc derivatives in PDT, antimicrobial PDT and photodiagnosis of neoplastic tissues. Therefore, we attempted to discuss the photophysical/photochemical, photodynamic and imaging properties of the AlPcs against various cell lines performed during last two decades. To reach the logical conclusion only those publications have been included which indicate in vitro and/or in vivo experimental results.
Photophysical and Photochemical Properties
Photophysical and photochemical properties such as singlet oxygen quantum yield (ΦΔ), fluorescence quantum yields (Φf) as well as fluorescence and singlet/triplet lifetimes, are the initial parameters for the recognition of an effective diagnostic marker and PDT sensitizer (these properties of macrocyclic complexes have been reviewed by a number of times e.g.) [17,18]. These parameters are affected by the presence of central metal [19,20] and peripheral substituents [10,21,22] on the Pc macrocycle. For example, insertion of a diamagnetic metal is responsible for higher ΦΔ of Pcs as compared to the corresponding molecules containing paramagnetic metals [10]. This is due to the formation of comparatively stable triplet excited state of diamagnetic MPc which enables the energy transfer from excited PS to the ground state oxygen molecule.
Unsubstituted AlPcs are in fact lipophilic in nature, therefore show aggregation in biological tissues and aqueous solutions. Consequently the AlPcs show lower values of photophysical and photochemical parameters. Aggregation may be minimized either by encapsulation or conjugation of the PS with nanomaterials, quantum dots and liposomes which in turn improves these properties. As observed, ClAlPc can be dispersed in water using Polymeric Nanoparticles (PNPs) thereby hampering the aggregation in aqueous medium [23]. Nanocapsulation of ClAlPc shows red shift in Q-band, increased the triplet lifetime (80 μs to 141±0.06 μs) [24], and ΦΔ (0.30-0.80) [25]. Similarly surfactants also improve the ΦΔ values of oligomeric carboxy AlPc up to two fold [26].
Quantum Dots (QDs) are capable of transferring energy to the Pc macrocycle through Fluorescence Resonance Energy Transfer (FRET), thus altering the photophysical parameters and photodynamic efficacy [27] of the Pcs. In general decrease in Q-band maximum and Φf is noticed [28] whereas increased ΦΔ values are observed for AlPcs conjugated with CdTe-QDs [28,29]. Similar results are reported for sulfonated AlPcSn attached with Human Serum Albumin (HSA) [30].
Cellular Internalization and Localization
Preferential uptake of the drugs by tumor tissues is crucial factor to be under taken while designing the third generation PS. Transportation of PS from blood stream to the cell is affected by the interaction of sensitizer with cell membrane which is lipoprotein in nature [31,32]. It has been observed that active transport of PS through cell membrane is facilitated by the metal-phosphate coordination of the lipid bilayer of cell membrane [33] while fluoride or hydroxyl ions limit this process [32,34,35]. This might be due to inhibitory effect of these ions on the metal-phosphate binding. Lipid binding is another essential factor which helps traversing the PS through cell membranes. It has been confirmed that cationic AlPcs efficiently bind to the phospholipid membranes as compared to the anionic and neutral Pcs such as AlPcS4 and ZnPc. This interaction is related with the presence of slightly negatively charged nature of the membranes and coordinating capability of the central metal in MPcs [34].
Sulfonated AlPcs demonstrate higher cellular uptake by the tumor tissues as compared to the porphyrins of similar lipophilicity whereas increasing lipophilic character in sulfonated AlPcs increases the cellular internalization in vitro [36].
Encapsulation of sensitizers in nanoparticles (NPs) not only reduces the aggregation but also takes advantage of Enhanced Permeability and Retention (EPR) effect [37], which demonstrates the higher cellular internalization of larger particles by the tumor cells due to their leaky vasculature. Therefore researchers are exploiting the nanoparticles for targeted drug delivery to the tumor tissues. The ClAlPc encapsulated in polymeric nanoparticles are taken up in higher concentration in tumor tissues than non cancerous cells [23].
After cell internalization, the sensitizers are localized in different cellular compartments depending on the lipophilic character of PS. The successful uptake and intracellular localization are important factors for effective PDT [38]. The AlPcs are localized in different cell organelles depending on the cell line used in vitro, e.g. the hydrophobic ClAlPc [39] is distributed throughout the cytoplasm of human meningioma and RR1022 cell lines [39] whereas the hydrophilic sulfonated Pcs are localized in the lysosomes [40]. More hydrophilic AlPcS4 aggregated in the cells in vitro, show subcellular relocation during in vivo experiments observed through enhanced fluorescence [39]. In general the nuclei are not among the primary seats of location by the PS [41].
In Vitro and In Vivo Cytotoxicity
In vitro photocytotoxicity is essayed followed by incubation of the tumor cell line with sensitizer at variable concentrations. The cell viability is determined in dark or under laser light irradiation of wavelength equivalent to the Q-band of the sensitizer at different power [42]. The cellular concentration, localization, absorptivity, intensity and wavelength of incident light, oxygen concentration, aggregation and physical properties of the PS are among the few parameters which determine their efficiency during PDT. Lipophilic ClAlPc has been found potent sensitizer against various cancer cell lines [40,43] however it also alters the morphology of cytoplasm or nuclei of the normal HeLa cells in vitro [43]. Evaluation through MTT essay proved ClAlPc as better PS in comparison with hydrophilic AlPcS1, AlPcS4 and Hematoporphyrin Derivative (HpD) against human meningioma cells [40]. Cytotoxicity of the sulfonated AlPcs depends on the lipophilic character of the complex which is controlled by degree of sulfonation and decreases with increasing the sulfonic groups on the macrocycle. As determined by using a number of cell lines, the in vitro photocytotoxicity of AlPcSn decreases in the order AlPcS1 >AlPcS2 >AlPcS3 >AlPcS4, however this sequence may not be followed in in vivo studies using the same cell line [44]. Contrary to the above mentioned observations AlPcS2 was found to be more potent than monosulfonated analog and Photofrin [45] in vivo, due to its amphiphilic nature. In addition to its photodynamic applications, AlPcS2 was also tested as a potential sonosensitizer for sonodynamic therapy against G361 melanoma cells sensitized by ultrasonic treatment of 1MHz frequency at 2W/cm intensity [46]. The oxidative cell damage affected by sulfonated AlPcs is generally through apoptotic mechanism as confirmed by flow cytometry [43].
Introduction of the hydrophobic functional groups on the Pc macrocycle generally improves the PDT efficacy on effect of decreased aggregation of PS and increased cellular internalization. Reduction in the hydrophilic character in AlPcS4 was achieved by substitution with 4, 8, 12 or 16 carbon long aliphatic chain. Complete regression of EMT-6 mouse mammary tumor cell line implanted in Balb/c mice was observed with the Pc containing longest chain [47]. In another experiment in vivo photo-inactivation of the EMT-6 tumor cells increased by a factor of ten by decreasing the number of sulfonic groups from four to two [48]. Photosensitizers also damage the blood vessels and it has been deduced that cancer tissue vasculature is more susceptible to PD damage and increases with decreasing the degree of sulfonation [49].
Receptor mediated targeting of tumor cells has led the modification of photosensitizers with biomolecules to be recognized by the specific tumor tissues containing overexpressed biomolecule receptors [50]. Several biomolecules such as carbohydrates, peptides, albumins, oligonucleotides and lipoproteins have been attached with Pcs aimed at targeted cellular internalization [51]. AlPcS4 has been conjugated with a number of receptor oriented peptides or proteins (Figure 1) such as bombesin [52], Monoclonal Antibodies (MABs) [53] and RGD [54]. The conjugates were taken preferentially by the tumor tissues due to higher binding affinity with neoplastic cells as compared to the normal cells. AlPcS4-bombesin conjugate significantly reduced the cell viability of human prostate cancer cell PC-7 in vitro, when compared with AlPcS4 at concentration range between 1-20 μM. Higher toxicity of the conjugate can be correlated with its enhanced uptake mediated by Gestrin Releasing Peptide Receptors (GRPR) [52] present in the prostate cancers, even at very early stage [55]. Overexpression of integrin receptors [56] was targeted by AlPcS4-RGD conjugates in vitro as well as in vivo. Arginine-glycine-aspartic acid (RGD) is a peptidic sequence present in adenovirus penton base proteins which binds with great affinity and high specificity to integrin receptors [56]. The AlPcS4-RGD conjugate was equally effective against human cell lines expressing integrin receptors (A549 and HEp2), and one lacking RGD receptors (EMT-6). In this case the regression of EMT-6 is due to more photo susceptibility of these cells [54].
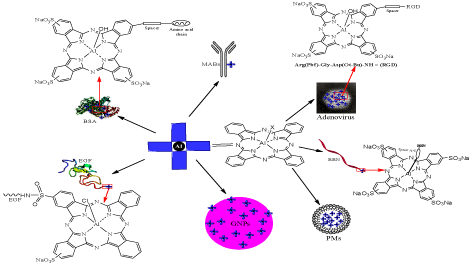
Figure 1: Schematic of AlPc derivatives conjugated with biomolecules, NPs and PNPs.
Receptor mediated internalization of albumin by specific cell lines such as monocytes, macrophages, hepatocytes etc., containing albumin binding proteins is exploited for drug delivery [57]. Bovine Serum Albumin (BSA) is an alternative to Human Serum Albumin (HSA), to be employed as drug delivery vehicle for human subjects. Therefore BSA is coupled to photosensitizers to target the tumors containing phagocytic cells e.g. J774. AlPcS4-BSA was tested for singlet oxygen production, receptor-mediated cell uptake and phototoxicity toward J774 and non-phagocytic EMT-6 cells. Competition studies of the conjugate showed higher cellular concentration and photocytotoxicity towards phagocytic cells [33]. Similarly Epidermal Growth Factor (EGF) conjugates of ClAlPcS2 were synthesized to determine the cytotoxic activity against human breast carcinoma MCF-7 in vitro [58]. Liposomal formulation of ClAlPc has been utilized for targeted delivery of PS in breast [59] and oral squamous cell [60,61] carcinoma. Dose dependent reduction in cell viability of Oral Squamous Cell Carcinoma (OSCC-3) was detected in vitro [60] and in in vivo experiments [61] using liposomal formulations. Polymeric Micelles (PMs) are also used as pH responsive drug delivery vehicles. PMs consisting of N-isopropylacrylamide (NIPAM) show low cytotoxicity than surfactant Cremophore EL (CRM) when tested on intradermal EMT-6 tumor implanted in Balb/c mice. Unlike the above observation, in vivo uptake of PM formulation of ClAlPc showed similar uptake in tumor tissues but improved photodynamic activity than its formulation in CRM [62].
Nanoparticles (NPs) are employed as carriers of photosensitizers due to their stability and biocompatibility in tissue fluids as well as their potential to deliver the PS to the target. Phthalocyanines have been conjugated with various NPs including gold, polymeric and silica based nanomaterials as well as Quantum Dots (QD) to improve the drug availability and internalization in the tumors [63] for PDT and diagnostic applications. Quantum dot conjugated aluminium phthalocyanines easily penetrate into human nasopharyngeal carcinoma and cell damage proceeds through FRET mediated PDT when the conjugates are excited at 532 nm laser irradiation [27].
It has been observed that photodynamic activity of ClAlPc is compromised when it is encapsulated into polymeric nanoparticles such as poly(methyl vinyl ether-co-maleic anhydride, PMA/MA), poly(D,L-lactide-co-glycolide), poly(D,L-lactide) and polyethylene glycol-block-poly(D,L-lactide). For example, ClAlPc-NP(PMA/MA) proved to be cytotoxic against murine cancerous and noncancerous (4T1 and NIH/3T3) cells and human breast cancer cells (MCF-7) even at 0.25 μM [23] concentration. This indicates nonspecific uptake of the composite by these cell lines in vitro. Therefore, polymeric encapsulation of AlPcs may not be suitable for targeted oncological applications.
Photodiagnosis
Clinical diagnosis and location of tumors is the crucial event in successful photodynamic treatment. A number of imaging techniques are applied for the distinction between neoplastic and normal tissues [42,64]. The photodiagnostic imaging involves the utilization of fluorescent markers, enabling the location of malignant tissues through photoluminescence. Recently Raman spectroscopic techniques such as confocal Raman imaging and Raman microspectroscopy, were used to distinguish tumors in human breast cells. Determined by Raman spectroscopy, the distribution of AlPcS4, was significantly higher in the cancer cells than that in normal tissues [65,66]. AlPc derivatives have been studied as fluorescent markers for the flow cytometric imaging of the malignancies in in vitro and in vivo experiments. The in vivo imaging is advantageous being rapid and more reliable, therefore has been employed for the pharmacokinetic studies of the PS [67].
Preferential accumulation of the sensitizers in the tumor tissues and its fluorescence quantum yield play pivotal role in photodiagnostic process. The AlPcs are preferentially localized in tumor cells as compared to the ZnPc, however increasing hydrophilicity through sulfonation lowers the tumor over normal tissue fluorescence ratio, indicating lower tumor uptake of tetrasulphonated Pcs [68]. On the other hand, introduction of hydrophobic moieties such as long aliphatic chains on the periphery of AlPcSn makes the PS amphiphilic in nature thus improving its photodiagnostic and photodynamic activity [69]. That’s why AlPcS2 has been most widely used for the imaging of the premalignant and malignant disorders reaching to maximum fluorescence in 2-10 hrs following injection [70].
Gold nanoparticles (AuNPs) have been extensively studied in biomedical applications due to their nontoxic behavior towards living cells. AuNPs conjugated with AlPcSn enhance the fluorescence up to 150 times than unconjugated Pc. In vitro experiments revealed excellent fluorescence of AlPcS-AuNP conjugates when excited at 405 nm (single photon excitation) or 800 nm (two photon excitation) laser irradiation [71]. Covalently conjugated carboxylated AlPcs with silica shell NPs were targeted towards the folate receptor positive cancer cells. The conjugates were taken up preferentially by these tumor cells due to the presence of folic acid used for the surface modification of the silica nanoshells [72]. The high accumulation of the conjugates in the tumor tissues and subsequent high fluorescence makes them multifunctional theranostic agents to be employed both in PDT and diagnosis [72]. It is noted that liposomal formulation of AlPcS4 and ZnPc against RR 1022 cell line showed significant necrosis by ZnPc. Therefore, AlPcS4 being nontoxic in liposomal formulation proved more feasible for diagnosis [39].
Antimicrobial Activities
PDT is now emerging as the treatment modality against a number of microorganisms including pathogenic bacteria and viruses. Aluminum phthalocyanines have been extensively employed in antimicrobial PDT (a-PDT) as effective photosensitizers. In vitro and clinical studies performed against cariogenic bacteria (Streptococcus mutans and Lactobacillus acidophilus), using cationic liposomal formulation of ClAlPc showed preferential uptake of the sensitizer in the bacterial cells as compared to the eukaryotic dental pulp cell line. Clinical investigations proved the overall 82% regression of the bacterial cavities after photodynamic action [73].
Cutaneous leishmaniasis is an infectious disease caused by a protozoan belonging to the genus Leishmania. Phthalocyanines such as ZnPc and AlPc derivatives have been studied as promising cytotoxic photosensitizers against Leishmania species in vitro as well as in vivo. Cellular regression in both species of Leishmania namely L. major and L. braziliensis was observed when the cell cultures were treated with AlPcS4 at 1.0μM or 10.0μM concentration followed by one hour incubation and irradiation with 659 nm laser at 5 or 10 J/ cm². L. braziliensis showed the highest mortality rate (~99%) after treatment with 10.0 μM concentration of the sensitizer at 10 J/ cm² of laser light dosage [74]. ClAlPc also effectively inhibited the growth of L. chagasi and L. panamensis promastigotes on exposure of visible light (670 nm). The Pc was especially potent against L. chagasi promastigotes with inhibitory dose 50 (ED50) concentration values of 0.0033, 0.0083 and 0.0093 μM upon 10.0, 5.0, and 2.5 J/cm² light intensities respectively [75].
Nanoemulsion of chloroaluminum phthalocyanine (ClAlPc/ NE) has been successfully examined to photoinactivate the fungus Cryptococcus neofromans [76] and Staphylococcus aureus [77] bacteria. Both melanized and nonmelanized cells of C. neoformans were photokilled with PS in dose dependent manner, however the melanized species showed more tolerance than others due to the less porous cell walls and reduced penetration of light through melanin. Two strains of S. aureus, antibiotic susceptible (MSSA) and resistant (MRSA), were incubated with different delivery systems containing ClAlPc. Photosensitizer formulated with cationic nanoemulsion (ClAlPc/NE) and free ClAlPc were particularly effective in photokilling the both strains of S. aureus, at light dosage of 25 J/cm² for MSSA and 50 J/cm² for MRSA. The anionic formulation of PS was not effective against MRSA strain. In general amphiphilic and cationic AlPcs possess higher antiviral activity as compared to their anionic counterparts against Herpes Simplex Virus type 1 (HSV- 1) [78] and Vesicular Stomatitis Virus (VSV) [79, 80]. Moreover, cationic AlPcs selectively eradicate the viruses such as HIV [81] in blood concentrates without photohemolysis [82] in the presence of red light whereas anionic AlPcS4 proved ineffective against Trypanosoma cruzi in plasma and red blood cell concentrates [83].
Conclusion
Despite the synthesis and efficacy of a vast variety of photosensitizers, effective for various cell lines, the quest for the third generation photosensitizers aimed at targeted PDT remains there. In case of phthalocyanines, the role of central metal in determining the photophysical/photochemical properties and therefore photocytotoxcity is inevitable. In this review, we described the photophysical and photochemical parameters of the AlPc derivatives, as well as their in vitro and in vivo cytotoxic activities, diagnostic aspects and antimicrobial efficacy. From the data, it can be concluded that certain parameters, such as liphophilicity/hydrophilicity, charge on the molecule and aggregation in the tissue fluid, determine the cellular uptake, localization and photodynamic activity of AlPcs. In general lipophilic derivatives proved to be more cytotoxic as compared to the hydrophilic ones. Receptor mediated targeting is helpful in recognition and enhanced cellular internalization of AlPc derivatives although cytotoxicity is not much improved. Any factor capable of reducing the aggregation, e.g. the use of polymeric micelles, surfactants and substitution of bulky groups on the axial or peripheral position of Pc ring also improves the cellular internalization and photocytotoxcity. Taking into account the antimicrobial PDT, cationic derivatives show promising results against virus, bacteria and antibiotic resistant bacteria as well. In addition to the PDT, AlPcs possess high fluorescence especially hydrophilic AlPcS4 derivative which is least cytotoxic and may be applied in photodiagnosis.
References
- Nowak-Stepniowska A, Pergol P, A. Padzik-Graczyk A. Photodynamic method of cancer diagnosis and therapy--mechanisms and applications. Postepy Biochem. 2013; 59: 53-63.
- Juarranz A, Jean P, Sanz-Rodriguez F, Cuevas J, Salvador G. Photodynamic therapy of cancer: Basic principles and applications. Clin Transl Oncol. 2008; 10: 148-154.
- Plaetzer K, Krammer B, Berlanda J, Berr F, Kiesslich T. Photophysics and photochemistry of photodynamic therapy: fundamental aspects. Lasers Med Sci. 2009; 24: 259-268.
- Allison RR, Sibata CH. Oncologic photodynamic therapy photosensitizers: a clinical review. Photodiagnosis Photodyn Ther. 2010; 7: 61-75.
- Brown SB, Brown EA, Walker I. The present and future role of photodynamic therapy in cancer treatment. The lancet oncology. 2004; 5: 497-508.
- Tedesco AC, Primo FL, Beltrame M. Phthalocyanines: Synthesis, Characterization and Biological Applications of Photodynamic Therapy (PDT), Nanobiotechnology, Magnetohyperthermia and Photodiagnosis (Theranostics). In book. Reference Module in Materials Science and Materials Engineering. 2016.
- Claessens CG, Hahn U, Torres T, Phthalocyanines: from outstanding electronic properties to emerging applications. The Chemical Record. 2008; 8: 75-97.
- Josefsen LB, Boyle RW. Unique diagnostic and therapeutic roles of porphyrins and phthalocyanines in photodynamic therapy, imaging and theranostics. Theranostics. 2012; 2: 916-66.
- Ion RM. The use of phthalocyanines and related complexes in photodynamic therapy, in Photosensitizers in Medicine, Environment and Security. 2012; 315-349.
- Nyokong T. Effects of substituents on the photochemical and photophysical properties of main group metal phthalocyanines. Coordination Chemistry Reviews. 2007; 251: 1707-1722.
- Ogunsipe A, Nyokong T. Effects of central metal on the photophysical and photochemical properties of non-transition metal sulfophthalocyanine. Journal of Porphyrins and Phthalocyanines. 2005; 9: 121-129.
- Iqbal Z, Chen J, Chen Z, Huang M. Phthalocyanine-Biomolecule Conjugated Photosensitizers for Targeted Photodynamic Therapy and Imaging. Current drug metabolism. 2015; 16: 816-832.
- Viola A, Jeunet A, Decreau R, Chanon M, Julliard M. ESR studies of a series of phthalocyanines. Mechanism of phototoxicity. Comparative quantitation of O2-. using ESR spin-trapping and cytochrome c reduction techniques. Free Radic Res. 1998; 28: 517-532.
- Zhang Y, Lovell JF. Recent applications of phthalocyanines and naphthalocyanines for imaging and therapy. Wiley Interdisciplinary Reviews: Nanomedicine and Nanobiotechnology. 2016.
- Asem H, El-Fattah AA, Nafee N, Zhao Y, Khalil L,Muhammad M, et al. Development and biodistribution of a theranostic aluminum phthalocyanine nanophotosensitizer. Photodiagnosis and photodynamic therapy. 2016; 13: 48-57.
- Jiang Z, Shao J, Yang T, Wang J, Jia L. Pharmaceutical development, composition and quantitative analysis of phthalocyanine as the photosensitizer for cancer photodynamic therapy. J Pharm Biomed Anal. 2014; 87: 98-104.
- DeRosa MC, and Crutchley RJ. Photosensitized singlet oxygen and its applications. Coordination Chemistry Reviews. 2002; 233: 351-371.
- Singh S, Aggarwal A, Bhupathiraju NV, Arianna G, Tiwari K, Drain CM. Glycosylated Porphyrins, Phthalocyanines and Other Porphyrinoids for Diagnostics and Therapeutics. Chemical reviews. 2015; 115: 10261-10306.
- Kobayashi N, Oquata H, Nonaka N, Luk’yanets EA. Effect of peripheral substitution on the electronic absorption and fluorescence spectra of metal-free and zinc phthalocyanines. Chemistry. 2003; 9: 5123-5134.
- Furuyama T, Satosh K, Kushiya T, Kobayashi N. Design, synthesis, and properties of phthalocyanine complexes with main-group elements showing main absorption and fluorescence beyond 1000 nm. J Am Chem Soc. 2014; 136: 765-776.
- Chernonosov AA, Ermilov EA, Roder B, Solovyova LI, Fedorova OS. Effect of Some Substituents Increasing the Solubility of Zn(II) and Al(III) Phthalocyanines on Their Photophysical Properties. Bioinorg Chem Appl. 2014.
- Lo PC, Crystal MH, Liu JY, Fong WP, Dennis KP. Highly photocytotoxic glucosylated silicon (IV) phthalocyanines. Effects of peripheral chloro substitution on the photophysical and photodynamic properties. J Med Chem. 2007; 50: 2100-2107.
- Muehlmann LA, Muehlmann LA, Chiyin MB, Paulo Figueiró LJ, Almeida Santos FM, Azevedo RB. Aluminum-phthalocyanine chloride associated to poly(methyl vinyl ether-co-maleic anhydride) nanoparticles as a new third-generation photosensitizer for anticancer photodynamic therapy. Int J Nanomedicine. 2014; 9: 1199-1213.
- de Paula CS, Tedesco AC, Primo FL, Vilela JM, Andrade MS, Mosqueira VC. Chloroaluminium phthalocyanine polymeric nanoparticles as photosensitisers: photophysical and physicochemical characterisation, release and phototoxicity in vitro. Eur J Pharm Sci. 2013; 49: 371-381.
- Siqueira-Moura MP, Primo FL, Espreafico EM, Tedesco AC. Development, characterization, and photocytotoxicity assessment on human melanoma of chloroaluminum phthalocyanine nanocapsules. Mater Sci Eng C Mater Biol Appl. 2013; 33: 1744-1752.
- Zhao P, Song Y, Dong S, Niu L, Zhang F. Synthesis, photophysical and photochemical properties of amphiphilic carboxyl phthalocyanine oligomers. Dalton Trans, 2009; 32: 6327-6334.
- Li L, Zhao JF, Won N, Jin H, Kim S, Chen JYl. Quantum dot-aluminum phthalocyanine conjugates perform photodynamic reactions to kill cancer cells via fluorescence resonance energy transfer. Nanoscale Res Lett. 2012; 7: 386.
- Tekdas DA, Durmus M, Yanik H, Ahsen V. Photodynamic therapy potential of thiol-stabilized CdTe quantum dot-group 3A phthalocyanine conjugates (QD-Pc). Spectrochim Acta A Mol Biomol Spectrosc, 2012; 93: 313-320.
- Ma J, Chen JY, Idowu M, Nyokong T. Generation of singlet oxygen via the composites of water-soluble thiol-capped CdTe quantum dots-sulfonated aluminum phthalocyanines. J Phys Chem B. 2008; 112: 4465-4469.
- Foley MS, Beeby A, Parker AW, Bishop SM, Phillips D. Excited triplet state photophysics of the sulphonated aluminium phthalocyanines bound to human serum albumin. J Photochem Photobiol B. 1997; 38: 10-7.
- Rokitskaya TI, Block M, Antonenko YN, Kotova EA, Pohl P. Photosensitizer binding to lipid bilayers as a precondition for the photoinactivation of membrane channels. Biophys J. 2000; 78: 2572-2580.
- Pashkovskaya AA, Sokolenkob EA, Sokolovb VS, Kotovaa EA, Antonenkoa YN. Photodynamic activity and binding of sulfonated metallophthalocyanines to phospholipid membranes: contribution of metal-phosphate coordination. Biochim Biophys Acta. 2007; 1768: 2459-2465.
- Brasseur N, Langlois R, La Madeleine C, Ouellet R, van Lier JE. Receptor-mediated targeting of phthalocyanines to macrophages via covalent coupling to native or maleylated bovine serum albumin. Photochem Photobiol. 1999; 69: 345-352.
- Pashkovskaya AA, Perevoshchikova IV, Maizlish VE, Shaposhnikov GP, Kotova EA, Antonenko YN. Interaction of tetrasubstituted cationic aluminum phthalocyanine with artificial and natural membranes. Biochemistry (Mosc). 2009; 74: 1021-1026.
- Shapovalov VL, Rokitskaya TI, Kotova EA, Krokhin OV, Antonenko YN. Effect of fluoride anions on gramicidin photoinactivation sensitized by sulfonated aluminum phthalocyanines. Photochem Photobiol. 2001; 74: 1-7.
- Berg K, Bommer JC, Moan J, Evaluation of sulfonated aluminum phthalocyanines for use in photochemotherapy. Cellular uptake studies. Cancer Lett. 1989; 44: 7-15.
- Kobayashi H, Turkbey B, Watanabe R, Choyke PL. Cancer Drug Delivery: Considerations in the Rational Design of Nanosized Bioconjugates. Bioconjug Chem. 2014; 25: 2093-2100.
- Rosenkranz AA, Jans DA, Sobolev AS. Targeted intracellular delivery of photosensitizers to enhance photodynamic efficiency. Immunology and Cell Biology. 2000; 78: 452-464.
- Ruck A, Beck G, Bachor R, Akgun N, Gschwend MH, Steiner R. Dynamic fluorescence changes during photodynamic therapy in vivo and in vitro of hydrophilic A1(III) phthalocyanine tetrasulphonate and lipophilic Zn(II) phthalocyanine administered in liposomes. J Photochem Photobiol B. 1996; 36: 127-133.
- Malham GM, Thomsen RJ, Finlay GJ, Baguley BC. Subcellular distribution and photocytotoxicity of aluminium phthalocyanines and haematoporphyrin derivative in cultured human meningioma cells. Br J Neurosurg, 1996; 10: 51-57.
- Peng Q, Moan J, Nesland JM. Correlation of subcellular and intratumoral photosensitizer localization with ultrastructural features after photodynamic therapy. Ultrastruct Pathol. 1996; 20: 109-129.
- JF Calvete, Mário C, Simoes VC, Henriques AC, Pinto MA, Pereira M, et al. Tetrapyrrolic macrocycles: potentialities in medical imaging technologies. Current Organic Synthesis. 2014; 11: 127-140.
- Pazos dCM, Pacheco-Soares C, Soares da Silva N, DaMatta RA, Pacheco MT. Ultrastructural effects of two phthalocyanines in CHO-K1 and HeLa cells after laser irradiation. Biocell. 2003; 27: 301-309.
- Chan WS,West CML, Moore JV, Hart IR. Photocytotoxic efficacy of sulphonated species of aluminium phthalocyanine against cell monolayers, multicellular spheroids and in vivo tumours. Br J Cancer. 1991; 64: 827-832.
- Peng Q, Moan J. Correlation of distribution of sulphonated aluminium phthalocyanines with their photodynamic effect in tumour and skin of mice bearing CaD2 mammary carcinoma. Br J Cancer. 1995; 72: 565-574.
- Kolarova H, Tomankova K, Bajgar R, Kolar P, Kubinek R. Photodynamic and sonodynamic treatment by phthalocyanine on cancer cell lines. Ultrasound Med Biol. 2009; 35: 1397-1404.
- Allen CM, Langlois R, Sharman WM, La Madeleine C, Van Lier JE. Photodynamic properties of amphiphilic derivatives of aluminum tetrasulfophthalocyanine. Photochem Photobiol. 2002; 76: 208-216.
- Boyle RW, Paquette B, van Lier JE. Biological activities of phthalocyanines. XIV. Effect of hydrophobic phthalimidomethyl groups on the in vivo phototoxicity and mechanism of photodynamic action of sulphonated aluminium phthalocyanines. Br J Cancer. 1992; 65: 813-817.
- van Leengoed HL, van der Veen N, Versteeg AA, Ouellet R, van Lier JE, Star WM. In vivo photodynamic effects of phthalocyanines in a skin-fold observation chamber model: role of central metal ion and degree of sulfonation. Photochem Photobiol,1993; 58: 575-580.
- Weyergang A., Selbo PK, Berstad ME, Bostad M, Berg K. Photochemical internalization of tumor-targeted protein toxins. Lasers in surgery and medicine. 2011; 43: 721-733.
- Taquet JP, Frochot C, Manneville V, Barberi-Heyob M. Phthalocyanines covalently bound to biomolecules for a targeted photodynamic therapy. Current medicinal chemistry. 2007; 14: 1673-1687.
- Dubuc C, Langlois R, Benard F, Van Lier EJ. Targeting gastrin-releasing peptide receptors of prostate cancer cells for photodynamic therapy with a phthalocyanine-bombesin conjugate. Bioorg Med Chem Lett. 2008; 18: 2424-2427.
- Morgan J, Lottman H, Abbou CC, Chopin DK. A comparison of direct and liposomal antibody conjugates of sulfonated aluminum phthalocyanines for selective photoimmunotherapy of human bladder carcinoma. Photochem Photobiol. 1994; 60: 486-496.
- Allen CM, Sharman WM, La Madeleine C, Weber JM, Langlois R, Ouellet R, et al. Photodynamic therapy: tumor targeting with adenoviral proteins. Photochem Photobiol, 1999; 70: 512-523.
- Markwalder R, Reubi JC. Gastrin-releasing peptide receptors in the human prostate: relation to neoplastic transformation. Cancer Res. 1999; 59: 1152-1159.
- Desgrosellier JS, Cheresh DA. Integrins in cancer: biological implications and therapeutic opportunities. Nature Reviews Cancer. 2010; 10: 9-22.
- Heukers R, Altintas I, Raghoenath S, De Zan E, Pepermans R, Roovers RC, et al. Targeting hepatocyte growth factor receptor (Met) positive tumor cells using internalizing nanobody-decorated albumin nanoparticles. Biomaterials. 2014; 35: 601-610.
- Lutsenko SV, Feldman NB, Finakova GV, Posypanova GA, Severin SE, Skryabin KG, et al. Targeting phthalocyanines to tumor cells using epidermal growth factor conjugates. Tumour Biol. 1999; 20: 218-224.
- Rocha MS, Lucci CM, Longo JP, Galera PD, Simioni AR, Lacava ZG, et al. Aluminum-chloride-phthalocyanine encapsulated in liposomes: activity against naturally occurring dog breast cancer cells. J Biomed Nanotechnol. 2012; 8: 251-257.
- Velloso NV, Muehlmann A, Figueiró Longo PJ, da Silva JR, Zancanela DC, Tedesco CA, et al. Aluminum-Phthalocyanine Chloride-Based Photodynamic Therapy Inhibits PI3K/Akt/Mtor pathway in Oral Squamous Cell Carcinoma Cells In Vitro. Chemotherapy. 2012; 1: 1-4.
- Figueiro Longo J, Muehlmann LA, Vieira Velloso N, Simioni RA, Paulino Lozzi S, Oliveira CE, et al. Effects of photodynamic therapy mediated by liposomal aluminum-phthalocyanine chloride on chemically induced tongue tumors. Chemotherapy. 2012; 1: 2.
- Le Garrec D, Taillefer J, Van Lier JE, Lenaerts V, Leroux JC. Optimizing pH-responsive polymeric micelles for drug delivery in a cancer photodynamic therapy model. J Drug Target. 2002; 10: 429-437.
- Jia X, L. Jia. Nanoparticles Improve Biological Functions of Phthalocyanine Photosensitizers Used for Photodynamic Therapy. Current Drug Metabolism. 2012; 13: 1119-1122.
- Subramanian V, Ragunath K. Advanced endoscopic imaging: a review of commercially available technologies. Clinical Gastroenterology and Hepatology. 2014; 12: 368-376.
- Brozek-Pluska B, Jarota A, Jablonska Gajewicz J, Abramczyk H. Distribution of phthalocyanines and Raman reporters in human cancerous and noncancerous breast tissue as studied by Raman imaging. Technology in Cancer Research and Treatment. 2012; 11: 317-331.
- Abramczyk H, Brozek-Pluska B, Surmacki J, Musial J, Kordek R. Oncologic photodynamic diagnosis and therapy: confocal Raman/fluorescence imaging of metal phthalocyanines in human breast cancer tissue in vitro. Analyst. 2014; 139: 5547-59.
- Chen JY, Chen W, Cai HX, Dong RC. Studies on pharmacokinetics of sulfonated aluminum phthalocyanine in a transplantable mouse tumor by in vivo fluorescence. J Photochem Photobiol B. 1993; 18: 233-237.
- van Leengoed HL, van der Veen N, Versteeg AA, Ouellet R, van Lier JE, Star WM. In vivo fluorescence kinetics of phthalocyanines in a skin-fold observation chamber model: role of central metal ion and degree of sulfonation. Photochem Photobiol. 1993; 58: 233-237.
- Rousseau J, Boyle RW, Maclennan AH, Truscott TG, Van Lier JE. Biodistribution and tumor uptake of [67Ga] chlorogallium-tetraoctadecyloxy phthalocyanine and its sulfonation products in tumor bearing C3H mice. Int J Rad Appl Instrum B. 1991; 18: 777-782.
- Witjes MJ, Speelman OC, Nikkels PG, Nooren CA, Nauta JM, van der Holt B, et al. In vivo fluorescence kinetics and localisation of aluminum phthalocyanine disulphonate in an autologous tumour model. Br J Cancer. 1996; 73: 573-580.
- Xu YK, Hwang S, Kim S, Chen JY. Two orders of magnitude fluorescence enhancement of aluminum phthalocyanines by gold nanocubes: a remarkable improvement for cancer cell imaging and detection. ACS Appl Mater Interfaces. 2014; 6: 5619-5628.
- Wang F, et al. Synthesis of magnetic, fluorescent and mesoporous core-shell-structured nanoparticles for imaging, targeting and photodynamic therapy. Journal of Materials Chemistry. 2011; 21: 11244-11252.
- Longo JP, Leal SC, Simioni AR, Almeida-Santos FM, Tedesco AC, Azevedo RB. Photodynamic therapy disinfection of carious tissue mediated by aluminum-chloride-phthalocyanine entrapped in cationic liposomes: an in vitro and clinical study. Lasers Med Sci. 2012; 27: 575-584.
- JG Pinto, Pacheko Soares C, Mittman J. Assessment of Leishmania major and Leishmania braziliensis promastigote viability after photodynamic treatment with aluminum phthalocyanine tetrasulfonate (AlPcS4). Journal of Venomous Animals and Toxins including Tropical Diseases. 2011; 17: 300-307.
- Escobar P, Hernandez IP, Rueda CM, Martínez F, Páez E. Photodynamic activity of aluminium (III) and zinc (II) phthalocyanines in Leishmania promastigotes. Biomedica. 2006; 26: 49-56.
- Rodrigues GB, Primo FL, Tedesco AC, Braga GU. In vitro photodynamic inactivation of Cryptococcus neoformans melanized cells with chloroaluminum phthalocyanine nanoemulsion. Photochem Photobiol. 2012; 88: 440-447.
- Ribeiro AP, Andrade MC, Bagnato VS, Vergani CE, Primo FL, Tedesco AC, et al. Antimicrobial photodynamic therapy against pathogenic bacterial suspensions and biofilms using chloro-aluminum phthalocyanine encapsulated in nanoemulsions. Lasers Med Sci. 2013.
- Smetana Z, Ben-Hur E, Mendelson E, Salzberg S, Wagner P, Malik Z. Herpes simplex virus proteins are damaged following photodynamic inactivation with phthalocyanines. J Photochem Photobiol B. 1998; 44: 77-83.
- Rywkin S, Ben-Hur E, Malik Z, Prince AM, Li YS, Kenney ME, et al. New phthalocyanines for photodynamic virus inactivation in red blood cell concentrates. Photochem Photobiol. 1994; 60: 165-170.
- Moor AC, Wagenaars-van Gompel AE, Brand A, Dubbelman MA, VanSteveninck J. Primary targets for photoinactivation of vesicular stomatitis virus by AIPcS4 or Pc4 and red light. Photochem Photobiol. 1997; 65: 465-470.
- Margolis-Nunno H, Ben-Hur E, Gottlieb P, Robinson R, Oetjen J, Horowitz B. Inactivation by phthalocyanine photosensitization of multiple forms of human immunodeficiency virus in red cell concentrates. Transfusion. 1996; 36: 743-750.
- Ben-Hur E, Geacintov NE, Studamire B, Kenney ME, Horowitz B. The effect of irradiance on virus sterilization and photodynamic damage in red blood cells sensitized by phthalocyanines. Photochem Photobiol. 1995; 61: 190-195.
- Gottlieb P, Shen LG, Chimezie E, Bahng S, Kenney ME, Horowitz B, et al. Inactivation of Trypanosoma cruzi trypomastigote forms in blood components by photodynamic treatment with phthalocyanines. Photochem Photobiol. 1995; 62: 869-874.