Mini Review
Austin J Bioorg & Org Chem. 2014;1(1): 4.
Glucosinolates: Novel Sources and Biological Potential
Ivica Blaževic*
Department of Organic Chemistry, University of Split, Croatia
*Corresponding author: Ivica Blaževic, Department of Organic Chemistry, University of Split, Teslina 10, 21000 Split, Croatia, Europe.
Received: August 08, 2014; Accepted: September 10, 2014; Published: September 11, 2014
Abstract
Glucosinolates investigation is an ongoing research activity and new structures are documented. Extraction and purification of fair amounts of glucosinolates from suitable plant species that contain high concentrations of a single or a small number of glucosinolates represent one of the most used sources of these compounds. In some cases, for obtaining enough amounts several synthesis paths are available. Occurrence in nature of some glucosinolates, such as long-chain olefinic ones, during the years of investigation are reported only by their breakdown products and not confirmed with spectroscopic data, and as such have to be re-evaluated. Despite the generally adopted scheme of glucosinolate degradation, there is further evidence that some isothiocyanates degrade further into various compound classes. As precursors of biologically active compounds, glucosinolates represent compounds with wide possible implementation and thus the novel sources by the isolation and synthesis are reviewed.
Keywords: Brassicaceae; Glucosinolates; Isolation; Synthesis; Isothiocyanates; Biological activity
Abbreviations
GL: Glucosinolate; ITC: Isothiocyanate; GC-MS: Gas Chromatography-mass Spectrometry; HPLC: High-performance Liquid Chromatography; NMR: Nuclear Magnetic Resonance; AChE: Acetylcholinesterase
Introduction
Glycosides are very large and diverse group of secondary metabolites, which usually occur in higher plants, but are also found in some lower species. They consist of a sugar part-carbohydrate moiety and non-sugar part-aglycone components, together connected by glycosidic bonds. Given the type of atoms through which realizes glycosidic bond, glycosides are divided into four groups: O-, S-, N-and C-glycosides.
Glucosinolates (GLs) are natural S-glucosides, and are abundant in sixteen families Capparales order in which the family Brassicaceae, which encompasses many of our daily vegetables (cabbages, radishes, mustards, cauliflower, broccoli, horseradish, mustard, turnip, oilseed rape, etc.), is by far the most important. All known GLs display a remarkable structural homogeneity based on a hydrophilic β-D-glucopyrano unit, a NO-sulfated anomeric (Z)-thiohydroximate function connected to aglucone part with different R-groups. The variation of R-groups, whose constitution include mostly aliphatic, arylaliphatic and indole side chain, is responsible for the variation in biological activities of these plant compounds. GL investigation is an ongoing research activity and new structures, after Fahey's review [1], have been documented by Agerbirk and Olsen [2], now including more than 130 compounds.
Glucosinolate sources
The lack of commercially available desulfo-GL standards represents one of the major troubles in investigations of GLs. This major drawback is overcomed through the purification of fair amounts of GLs and their desulfo-counterparts starting from suitable species that contain high concentrations of a single or a small number of GLs. The content of GLs in plants varies between cultivars, plant individuals and part of the plants, due to factors such as genetics, environment and plant nutrients. GLs can be found in the roots, seeds, leaf and stem of the plant, while youngest tissues contain the highest amount [3]. The sound seed-screening reported by Bennett et al. has brought to light clear subdivisions based on GL content: (i) only short- to medium chain-length aliphatic (C-3, or C-3 and C-4 with traces of C-5); (ii) only long-chain-length aliphatic; (iii) only simple arylaliphatic (such as benzyl, 4-hydroxybenzyl, 2-phenylethyl GL); and (iv) highly substituted arylaliphatic (such as 3,4-dihydroxybenzyl, 3,4dimethoxybenzyl, 3,4,5-trimethoxybenzyl GLs) [4,5]. C3-C5 aliphatic glucosinolates are a common characteristic of most Brassica species [4]. Recent reports of the plants included in tribe Alysseae (Aurinia species, Degenia velebitica and Fibigia triquetra) showed high GL contents ranging from 9.9 to 135.4 μmol/g of dried material in different plant parts - especially in the seeds (over 4.0% w/w with the highest, 6.1% w/w in F. triquetra). Those Alysseae are found to represent appropriate sources for GLs bearing a C-4 and/or C-5 olefinic aglycon chain (gluconapin, glucoberteroin) and/or a thiofunctionalized chain (glucoerucin, glucoberteroin, glucoraphanin, glucoalyssin) [5-7]. Long-chain-length aliphatic glucosinolates (C7-C10) are generally restricted to a few species within the Brassicaceae such as Arabis, Nasturtium and certain wild Lepidium species [4]. Seeds of Arabis, Barbarea, Lepidium, Moringa, and Sinapis species were good sources of aromatic glucosinolates [4].
Most of the research has aimed at identifying GLs by GC-MS of their breakdown products (mostly isothiocyanates) and HPLC analysis of the enzymatically desulfated GLs. Characterization and quantification of GLs based on the HPLC analysis of desulfo-GLs is described in the ISO 9167-1 official method [8]. However, some GL breakdown products are unstable in the conditions applied for determination and in the case of desulfated GLs there are difficulties in interpreting results of the individual GLs, due to concerns over the effect of pH value, time, and enzyme sulfatase (EC 3.1.6.1) concentration on desulfation products. Some GLs in nature, were reported only through their breakdown products, such as the presence of long chain C8-C10 unsaturated isothiocyanates in autolyzates of Nasturtium montanum [9]. Their existence were not compared with authentic standards or literature spectra of such, the interpretation of mass spectra was not discussed, NMR data were not provided, and the origin from GLs was not tested [2]. Therefore, the direct analysis of intact GLs by LC-MS and nuclear magnetic resonance (NMR) spectrometry is needed for more specific and accurate determination and for better interpretation of analytical results. In other respects, the NMR spectral data are indispensable for structure elucidation of novel GLs [2].
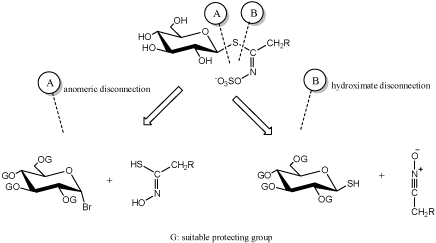
Dedicated extractive methods allow one to isolate a number of GLs from adequate plant material, but in many cases, organic synthesis is an alternative neccesary to obtain necessary quantities of natural GLs [10]. In other respects, synthesis is the only way to elaborate a diversified range of artificial GL analogues. From a chemical synthetic point of view, two major approaches (Figure 1) for the elaboration of GLs structures have been developed by a limited number of groups over the past 50years and are summerized in the recent review [10]. These are based on a retrosynthetic scheme where a single specific bond formation affords the GL skeleton: two types of disconnection have been considered, either on the anomeric center (A) or on to the hydroximoyl moiety (B) (Figure 1).
Figure 1 : Main chemical approaches for glucosinolate synthesis [10].
Recent reports include a new method for accessing the thiohydroximate function allows an alternative synthetic pathway to glucosinolates [11], total synthesis of (R,S)S-glucoraphanin, a novel, simple and convenient synthesis od natural and unnatural (methylsulfinyl) alkyl GLs [12], synthesis of aromatic GLs [13], and the nitronate and nitrovinyl methods to synthesize indole glucosinolates [14].
Glucosinolate degradation
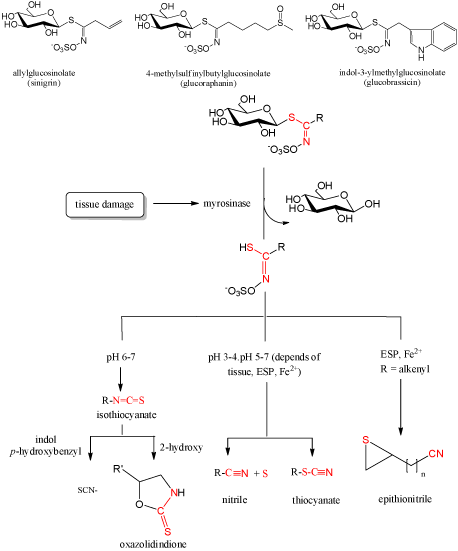
GLs may break down enzymatically, chemically, or to some extent thermally [15]. During the years of GLs investigation, many reports included their identification only by their breakdown products produced enzymatically. Enzymatical degradation includes enzyme myrosinase (EC 3.2.1.147) in plant or within the gastrointestinal tract by the actions of commensal microflora and the products obtained were mostly isothiocyanates (ITCs). Under the influence of different conditions (enzymatic, thermal and chemical (acidic and basic)) GLs, beside ITCs (at pH > 7), can produce other organic classes such as nitriles, thiocyanates, oxazolidinethiones (Figure 2). At pH < 4, and in the presence of Fe2+ ions at all pH values, there is an increase of nitriles. Allyl-, benzyl- and 4-methylthiobuthyl glucosinolates generate thiocyanates. The presence of OH-group in β-position of side chains causes spontaneous cyclization to produce the corresponding oxazolidine-2-thiones, and the terminal double bond in the side chain results in generation of epithionitriles in the presence of Fe2+ and epithiospecifier protein [3,5-7,18,19,22-25].
Figure 2: General scheme of glucosinolate degradation.
Several studies dealt with GL degradation by hydrodistillation in a Clevenger-type apparatus (or Likens-Nickerson-type apparatus) at 100OC under which GL can thermally degrade [3,6,16-21]. In order to improve qualitative and quantitative analysis, a novel method of hydrodistillation-adsorption on activated carbon was developed and used in the isolation of GL breakdown products from Eruca sativa [25] and Degenia velebitica [24]. The volatiles were simultaneously isolated during hydrodestillation and separated into three fractions: the main fraction A, containing the H2O-soluble compounds adsorbed on charcoal, fraction B, consisting of the H2O-insoluble compounds, and fraction C, composed of the highly volatile compounds adsorbed on charcoal in the upper column from the gaseous phase compounds. Recent report dealt with the influence of different conditions (enzymatic, thermal and chemical (acidic and basic)) on GLs degradation of Lunaria annua seeds. In comparison to enzymatic degradation, thermal degradation and very acidic conditions were not a good approach when the indirect method for glucosinolate identification is used. On the other hand, basic and acidic conditions were good for glucosinolate degradation and can be used instead of expensive enzyme myrosinase when necessary [23].
Although the GLs degradation scheme is generally accepted, as shown in Figure 2., some isothiocyanates formed can further degrade under certain conditions. Jin et al. reported that broccoli constituent sulforaphane, one of the most promising ITCs, degraded in an aqueous solution at 50 and 100°C into dimethyldisulphide, S-methyl methylthiosulfinate, S-methyl methylthiosulfonate, methyl(methylthio) methyl disulphide, 1,2,4-trithiolane, 4-isothiocyanato-1-(methylthio)-1-butene, and 3-butenyl ITC [26]. Accordingly to the sulforaphane degradation, it was suggested that its analogue alyssin can form 4-pentenyl ITC [18]. More recent report included testing of diversely substituted benzylic-type isothiocyanates under standard hydrodistillation-mimicking conditions. 2-Methoxybenzyl, 4-methoxybenzyl, 3,4-dimethoxybenzyl, and 3,4,5-trimethoxybenzyl isothiocyanates underwent conversion into 2-methoxybenzyl, 4-methoxybenzyl, 3,4-dimethoxybenzyl, and 3,4,5-trimethoxybenzyl alcohols, respectively, whereas benzyl, 3-methoxybenzyl, and 4-chlorobenzyl isothiocyanates were converted into the corresponding benzylamines [27].
Biological potential
Consumption of cruciferous vegetables, via GL hydrolysis products-isothiocyanates, is more strongly associated with cancer protection than vegetable consumption in general [28]. Isothiocyanates show interesting chemopreventive activities against several chronic-degenerative diseases, including cancer, cardiovascular diseases, neurodegeneration, and diabetes. The electrophilic carbon residue in the isothiocyanate moiety reacts with biological nucleophiles and modification of proteins is recognized as a key mechanism underlying the biological activity of isothiocyanates [29]. In vitro and in vivo studies have reported that isothiocyanates affect many steps of cancer development including modulation of phase I and II detoxification enzymes, functioning as a direct or as an indirect antioxidant by phase II enzyme induction, modulating cell signalling, induction of apoptosis, control of the cell cycle and reduction of heliobacter infections [1,30-32]. In particular, such evidences are reported for the well known sulforaphane, one of the most studied ITC, which is released during the hydrolysis of the precursor glucoraphanin (Figure 2). In addition, some direct antioxidant behavior has been observed for a limited number of ITCs. Paradoxically relevant pro-oxidant properties have also been documented, possibly related to the simultaneous induction of phase-1 enzymes [33].
Insecticidal, nematicidal, fungicidal and phytotoxic effects are also often associated with plants cabbage-related plants. Reports on the antimicrobial activities of various volatile extracts obtained from the Brassicaceae plants such as Aurinia sinuata [18], Cardaria draba [34], Degenia velebitica [24], and Sisymbrium officinale [19] have suggested that GL degradation products are responsible for their broad and strong antimicrobial activity. These volatile extracts were found to inhibit a wide range of Gram-positive bacteria, Gram-negative bacteria, and fungi. In some cases, these volatile extracts showed higher sensitivity against tested microorganisms in comparison to tested antibiotics, indicating their promising antimicrobial potential. High antimicrobial activity of many ITCs revealed in previous studies merits search for more active representatives of this class and thorough investigation of their action mechanism in order to use the compounds to combat infections. The studies of ITCs as potential antimicrobial agents are yet at the starting stage [35].
Having a small molecular weight and lipid solubility, volatile components of essential oils, probably can pass the blood-brain barrier, and thus represents a potential for treating neurodegenerative diseases. The WHO estimates there are at present 35.6 million people living in dementia worldwide [36]. Alzheimer disease is the most frequent cause of dementia in Western societies. It is assumed that the dysfunction of colinergic neurotransmission in the brain contribute to the relevant cognitive decline in Alzheimer disease. The loss of colinergic cells is accompanied by the loss of the neutrotransmitter acetylcholine, thus, one of the most accepted strategies in Alzheimer disease treatment is the use of cholinesterase inhibitors [37]. To our knowledge, acetylcholinesterase (AChE) inhibitory activities of Brassicaceae plant extracts, as well as their components, are rare. Report of Alyssoides utriculata volatile extract AChE inhibition activity was attributed to the presence of the main GL degradation products, such as 3-butenyl ITC, erucin, and sulforaphane, previously known for various biological activities. This research confirmed the importance of a further study of the correlation between the chemical composition of volatile isolates characterized by GLs degradation products and their AChE inhibitory activity.
Conclusion
Glucosinolates and their breakdown products show versatile biological potential. Due to the fact that they are found in plants of Brassicales, widely used as food and medicinal plants, they are subject of various disciplines. Knowledge about glucosinolates sources, provided by the isolation from plants, or by the synthesis is important for biological testing. Moreover, investigating novel GL-rich plants with a biological potential may stimulate the development of new horticultural crops.
Acknowledgement
Part of the investigation reviewed was done in the frame of the COGITO project "Glucosinolates - Novel Sources and Biological Potential" which was supported by the Ministry of Science, Education and Sports, Republic of Croatia.
References
- Fahey JW, Zalcmann AT, Talalay P. The chemical diversity and distribution of glucosinolates and isothiocyanates among plants. Phytochemistry. 2001; 56: 5-51.
- Agerbirk N, Olsen CE. Glucosinolate structures in evolution. Phytochemistry. 2012; 77: 16-45.
- Blaževic I, Mastelic J. Glucosinolate degradation products and other bound and free volatiles in the leaves and roots of radish (Raphanus sativus L.). Food Chem. 2009; 113: 96-102.
- Blaževic I, Mastelic J. Glucosinolate degradation products and other bound and free volatiles in the leaves and roots of radish (Raphanus sativus L.). Food Chem. 2009; 113: 96-102.
- Blaževic I, De Nicola GR, Montaut S, Rollin P. Glucosinolates in two endemic plants of the Aurinia genus and their chemotaxonomic significance. Nat Prod Commun. 2013; 8: 1463-1466.
- Blaževic I, Radonic A, Skocibušic M, De Nicola GR, Montaut S, Iori R, et al. Glucosinolate profiling and antimicrobial screening of Aurinia leucadea (Brassicaceae). Chem Biodivers. 2011; 8: 2310-2321.
- De Nicola GR, Blaževic I, Montaut S, Rollin P, Mastelic J, Iori R. Glucosinolate distribution in aerial parts of Degenia velebitica. Chem Biodivers. 2011; 8: 2090-2096.
- Wathelet J-P, Iori R., Leoni O, Rollin P, Quinsac A, Palmieri S. Guidelines for glucosinolate analysis in green tissues used for biofumigation. Agroindustria. 2004; 3: 257-266.
- Songsak T, Lockwood GB. Glucosinolates of seven medicinal plants from Thailand. Fitoterapia. 2002; 73: 209-216.
- Rollin P, Tatibouët A. Glucosinolates: The synthetic approach. C. R. Chim. 2011; 14: 194-210.
- Cerniauskaite D, Rousseau J, Sackus A, Rollin P, Tatibouët A. Glucosinolate synthesis: A hydroxamic acid approach. European J Org Chem. 2011; 12: 2293-2300.
- Vo QV, Trenerry C, Rochfort S, Hughes AB. A total synthesis of (R,S)S-glucoraphanin. Tetrahedron. 2013; 69: 8731-8737.
- Vo QV, Trenerry C, Rochfort S, Wadeson J, Leyton C, Hughes AB. Synthesis and anti-inflammatory activity of aromatic glucosinolates. Bioorg Med Chem. 2013; 21: 5945-5954.
- Vo QV, Trenerry C, Rochfort S, Wadeson J, Leyton C, Hughes AB. Synthesis and anti-inflammatory activity of indole glucosinolates. Bioorg Med Chem. 2014; 22: 856-864.
- Bones AM, Rossiter JT. The enzymic and chemically induced decomposition of glucosinolates. Phytochemistry. 2006; 67: 1053-1067.
- Blaževic I, Mastelic J. Free and bound volatiles of rocket (Eruca sativa Mill.). Flavour Fragr J. 2008; 23: 278-285.
- Blaževic I, Mastelic J. Free and bound volatiles of garlic mustard (Alliaria petiolata). Croat Chem Acta. 2008; 81: 607-613.
- Blaževic I, Radonic A, Mastelic J, Zekic M, Skocibušic M, Maravic A. Glucosinolates, glycosidically bound volatiles and antimicrobial activity of Aurinia sinuata (Brassicaceae). Food Chem. 2010; 121:1020-1028.
- Blaževic I, Radonic A, Mastelic J, Zekic M, Skocibušic M, Maravic A. Hedge mustard (Sisymbrium officinale): chemical diversity of volatiles and their antimicrobial activity. Chem Biodivers. 2010; 7: 2023-2034.
- Valette L, Fernandez X, Poulain S, Loiseau AM, Lizzani-Cuvelier L, Levieil R, et al. Volatile constituents from Romanesco cauliflower. Food Chem. 2003; 80: 353-358.
- Mastelic, J, Blaževic I., Jerkovic I. Free and bound sulphur containing and other volatile compounds from evergreen candytuft (Iberis sempervirens L.). Croat Chem Acta. 2006; 79: 591-597.
- Blazevic I, Burcul F, Ruscic M, Mastelic J. Glucosinolates, volatile constituents, and acetylcholinesterase inhibitory activity of Alyssoides utriculata. Chem Nat Comp. 2013; 49: 374-378.
- Blazevic I, Males T, Ruscic M. Glucosinolates of Lunaria annua: Thermal, enzymatic, and chemical degradation. Chem Nat Comp. 2014; 49: 1154-1157.
- Mastelic J, Blaževic I, Kosalec I. Chemical composition and antimicrobial activity of volatiles from Degenia velebitica, a European stenoendemic plant of the Brassicaceae family. Chem Biodivers. 2010; 7: 2755-2765.
- Mastelic J, Jerkovic I, Blaževic I, Radonic A, Krstulovic L. Hydrodistillationadsorption method for the isolation of water-soluble, non-soluble and high volatile compounds from plant materials. Talanta. 2008; 76: 885-891.
- Jin Y, Wang M, Rosen RT, Ho CT. Thermal degradation of sulforaphane in aqueous solution. J Agric Food Chem. 1999; 47: 3121-3123.
- De Nicola GR, Montaut S, Rollin P, Nyegue M, Menut C, Iori R, et al. Stability of benzylic-type isothiocyanates in hydrodistillation-mimicking conditions. J Agric Food Chem. 2013; 61: 137-142.
- Verhoeven DT, Verhagen H, Goldbohm RA, van den Brandt PA, van Poppel G. A review of mechanisms underlying anticarcinogenicity by Brassica vegetables. Chem Biol Interact. 1997; 103: 79-129.
- Fimognari C, Turrini E, Ferruzzi L, Lenzi M, Hrelia P. Natural isothiocyanates: genotoxic potential versus chemoprevention. Mutat Res. 2012; 750: 107-131.
- Barillari J, Cervellati R, Paolini M, Tatibouët A, Rollin P, Iori R, et al. Isolation of 4-methylthio-3-butenyl glucosinolate from Raphanus sativus sprouts (Kaiware Daikon) and its redox properties. J Agric Food Chem. 2005; 53: 9890-9896.
- Hanlon PR, Robbins MG, Hammon LD, Barnes DM. Aqueous extract from the vegetative portion of Spanish black radish (Raphanus sativus L. var. niger) induces detoxification enzyme expression in HepG2 cells. J Funct Foods. 2009; 1: 356-365.
- Takaya Y, Kondo Y, Furukawa T, Niwa M. Antioxidant constituents of radish sprout (Kaiware-daikon), Raphanus sativus L. J Agric Food Chem. 2003; 51: 8061-8066.
- Valgimigli L, Iori R. Antioxidant and pro-oxidant capacities of ITCs. Environ Mol Mutagen. 2009; 50: 222-237.
- Radonic A, Blaževic I, Mastelic J, Zekic M, Skocibušic M, Maravic A. Phytochemical analysis and antimicrobial activity of Cardaria draba (L.) Desv. volatiles. Chem Biodivers. 2011; 8: 1170-1181.
- Kurepina N, Kreiswirth BN, Mustaev A. Growth-inhibitory activity of natural and synthetic isothiocyanates against representative human microbial pathogens. J Appl Microbiol. 2013; 115: 943-954.
- World health organization (WHO), Dementia: a public health priority. Geneva. 2012.
- de Paula AA, Martins JB, dos Santos ML, Nascente Lde C, Romeiro LA, Areas TF. New potential AChE inhibitor candidates. Eur J Med Chem. 2009; 44: 3754-3759.