
Research Article
Austin J Bioorg & Org Chem. 2023; 2(1): 1005.
Quinoline Derivatives: Design and Synthesis as a Potential COVID-19 Protease Inhibitor
Mani R* and Ranjith WAC
Centre for Molecular and Nanomedical Sciences, International Research Centre, Sathyabama Institute of Science and Technology (Deemed to be University), India
*Corresponding author: Rajeshekar Mani Centre for Molecular and Nanomedical Sciences, International Research Centre, Sathyabama Institute of Science and Technology (Deemed to be University), Chennai - 600 119, India
Received: November 21, 2022; Accepted: January 30, 2023; Published: February 06, 2023
Abstract
A multicomponent one-pot reaction involving Phenylacetylene, 4-Aminofluorescein and aromatic aldehydes using Cu (I) as a catalyst is described, which provides an efficient and practical route to synthesize quinoline in good yield. The compound was analyzed for its potential to act on 3C-like proteinase using in silico docking studies, 4b and 4c showed a docking score of -8.2 kcal/mol (2 hydrogen bonds) and -8.1 kcal/mol (2 hydrogen bonds) respectively for monomer of 3C-like protease, and -8.88 kcal/mol (3 hydrogen bonds) and -8.9 kcal/mol (4 hydrogen bonds) respectively for dimer of 3C-like protease. These provide an insight of using Quinolines as a potential drug to target COVID 19 protein target 3C-like protease.
Keywords: Aminofluorescein; Quinoline; One-Pot Reaction; Phenylacetylene; COVID 19
Introduction
An outbreak of a series of acute respiratory illness caused by a novel coronavirus, SARS-CoV-2, caused a global threat in 2020. The World Health Organization (WHO) named the disease “COVID-19” and declared it as a world health emergency pandemic. Quinolines play an important role in organic chemistry. They are important structural motifs that exist in numerous natural products are shown in (Figure 1) [1-3]. Quinolines are heterocyclic molecules composed off used benzene and pyridine rings. The quinolines can possess various biological activities, including antiproliferative [4], antiviral [5], antibacterial [6], antifungal [7], anti-infl ammatory [8], and antiparasitic [9]. Members of the quinoline family, such as chloroquine and hydroxychloroquine, have shown antiviral activity against several viruses, such as coronaviruses [5], human immunodeficiency virus [10], and respiratory syncytial virus [11]. Concerning Flavivirus, quinoline derivatives have proved active against the Hepatitis C virus [12], West Nile virus [13], Japanese Encephalitis virus [14], Zika virus [15], and dengue virus. It also found wide utility as efficient organo-catalysts and used as a ligand for the preparation of phosphorescent complexes. They are useful tools for the highly enantio selective syntheses of chiral molecules [16,17].
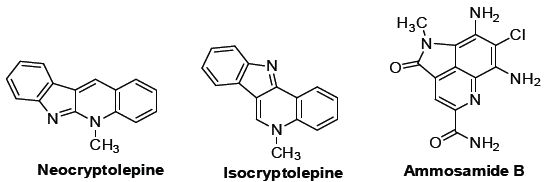
Figure 1: Structures of quinoline ring containing natural products.
Among these Multicomponent Reactions (MCRs) provides easy access to the preparation of quinoline derivatives, because Multicomponent Reactions (MCRs) have emerged as bond-forming efficient tools in medicinal chemistry [18,19]. All these procedures are through strong acid/metal-catalyzed sequential intermolecular addition of alkynes onto imines and subsequent intramolecular ring closure by arylation. The quinoline core in both biological and chemical fi elds, new direct approaches remain highly valuable to the contemporary collection of synthetic methods [20,21]. The study shows that most of the one-pot reaction involves amine, aldehyde, and acetylene moieties to synthesize quinoline derivatives [22]. In the context of our studies in the area of MCRs, we would like to report an efficient approach for the one-pot synthesis of quinolone and target the protein of COVID 19.
Experimental Section
General Methods
4-Aminofluorescein, Aldehydes, Phenylacetylene, Copper chloride and other solvents were obtained from SRL, Chennai, Tamil Nadu, India. Column chromatography was performed on Silica Gel (100-200 mesh). Melting points were measured on a Sigma micro melting point apparatus and are uncorrected. NMR spectra were recorded on Bruker DRX 300 MHz in CDCl3 or DMSO-d6 at the University of Madras (Chennai, Tamil Nadu, India). TMS was used as the internal standard (d = 0.00 ppm) and all the J values are given in hertz. Elemental analyses were performed using Perkin-Elmer 2400 elemental analyzer.
General Procedure for the Synthesis of Quinoline (4a-e)
To a solution of the aldehydes (1a-e, 1.0 mmol), 4-Aminofluorescein (2, 1.0 mmol), Phenylacetylene (3, 1.0 mmol) and dry THF (10 mL) were added copper chloride (0.3 mmol). After stirring at 80°C (oil bath temperature) for a given period of time, the reaction mixture was evaporated under reduced pressure and extracted by DCM-water. The DCM layer was dried over anhyd. Na2SO4 and concentrated to dryness. The product was further purifi ed by flash column chromatography.
Physicochemical and spectral data for 2-(4-chlorophenyl)-3',6'-dihydroxy-4-phenyl-8H-spiro[furo[3,4-g]quinoline-6,9'-xanthen]-8-one (4a): A mixture of 4-Chlorobenzaldehyde (1a, 0.14 g, 1.0 mmol), 4-Aminofluorescein (2, 0.34 g, 1.0 mmol), Phenylacetylene (3, 0.10 g, 1.0 mmol) and CuCl (0.07 g, 0.3 mmol) in 10 mL of THF afforded compound 4a as an pale yellow solid (0.41 g, 73%); mp 152-154oC; [a]D31 + 58.8 (c 0.1, CHCl3); ¹H NMR: (CDCl3, 300 MHz): d 6.74 (d, 2H, J = 8.4 Hz, Ar-H), 6.77 (d, 1H, J = 8.4 Hz, Ar-H), 6.98 (t, 2H, J = 7.8 Hz, Ar-H), 7.08 (t, 2H, J = 9.9 Hz, Ar-H), 7.32 (t, 1H, J = 8.0 Hz, Ar-H), 7.58 (q, 4H, J = 7.5 Hz, Ar-H), 7.70 (d, 3H, J = 6.8 Hz, Ar-H), 8.09 (q, 1H, J = 8.5 Hz, Ar-H), 8.23 (s, 2H, Ar-OH), 8.25 (d, 2H, J = 7.8 Hz, Ar-H). 13C NMR: (CDCl3, 75 MHz): d 82.8, 110.2, 116.4, 117.9, 118.4, 119.4, 124.2, 125.3, 125.6, 126.1, 128.4, 128.8, 128.9, 129.0, 130.2, 130.3, 130.4, 130.6, 133.9, 134.3, 135.4, 149.8, 150.2, 150.6, 150.8, 152.8, 164.5, 169.1, Anal. Calcd for C35H20ClNO5: C, 73.75; H, 3.54; N, 2.46. Found: C, 73.76; H, 3.52; N, 2.44.
Physicochemical and spectral data for 2-(4-bromophenyl)-3',6'-dihydroxy-4-phenyl-8H-spiro[furo[3,4-g]quinoline-6,9'-xanthen]-8-one (4b): A mixture of 4-Bromobenzaldehyde (1b, 0.18 g, 1.0 mmol), 4-Aminofluorescein (2, 0.34 g, 1.0 mmol), Phenylacetylene (3, 0.10 g, 1.0 mmol) and CuCl (0.07 g, 0.3 mmol) in 10 mL of THF afforded compound 4b as an pale yellow solid (0.43 g, 70%); [a]D31 + 68.2 (c 0.1, CHCl3); 1H NMR: (CDCl3, 300 MHz): d 6.72 (d, 2H, J = 7.2 Hz, Ar-H), 6.75 (d, 1H, J = 8.4 Hz, Ar-H), 6.95 (t, 2H, J = 7.2 Hz, Ar-H), 7.09 (t, 2H, J = 7.2 Hz, Ar-H), 7.35 (d, 1H, J = 7.4 Hz, Ar-H), 7.55 (q, 2H, J = 7.5 Hz, Ar-H), 7.62 (d, 2H, J = 7.5 Hz, Ar-H), 7.72 (d, 3H, J = 6.8 Hz, Ar-H), 8.12 (q, 1H, J = 7.5 Hz, Ar-H), 8.22 (s, 2H, Ar-OH), 8.28 (d, 2H, J = 7.5 Hz, Ar-H). 13C NMR: (CDCl3, 75 MHz): d 82.6, 110.4, 116.6, 117.5, 118.2, 119.5, 124.4, 125.5, 125.8, 126.7, 128.2, 128.5, 128.8, 129.6, 130.1, 130.3, 130.5, 130.7, 133.5, 134.5, 135.4, 149.6, 150.4, 150.6, 150.8, 152.6, 164.6, 169.5, Anal. Calcd for C35H20BrNO5: C, 68.42; H, 3.28; N, 2.28. Found: C, 68.44; H, 3.26; N, 2.26.
Physicochemical and spectral data for 2-(4-fluorophenyl)-3',6'-dihydroxy-4-phenyl-8H-spiro[furo[3,4-g]quinoline-6,9'-xanthen]-8-one (4c): A mixture of 4-Fluorobenzaldehyde (1c, 0.12 g, 1.0 mmol), 4-Aminofluorescein (2, 0.34 g, 1.0 mmol), Phenylacetylene (3, 0.10 g, 1.0 mmol) and CuCl (0.07 g, 0.3 mmol) in 10 mL of THF afforded compound 4c as an pale yellow solid (0.37 g, 67%); [a]D31 + 65.7 (c 0.1, CHCl3); ¹H NMR: (CDCl3, 300 MHz): d 6.73 (d, 2H, J = 8.4 Hz, Ar-H), 6.78 (d, 2H, J = 8.4 Hz, Ar-H), 6.96 (d, 2H, J = 7.5 Hz, Ar-H), 7.09 (t, 2H, J = 7.5 Hz, Ar-H), 7.32 (d, 1H, J = 8.0 Hz, Ar-H), 7.58 (d, 4H, J = 7.2 Hz, Ar-H), 7.70 (d, 2H, J = 7.6 Hz, Ar-H), 8.08 (d, 1H, J = 8.2 Hz, Ar-H), 8.23 (s, 2H, Ar-OH), 8.25 (d, 2H, J = 7.2 Hz, Ar-H). 13C NMR: (CDCl3, 75 MHz): d 82.5, 110.3, 116.6, 117.6, 118.4, 119.5, 124.5, 125.3, 125.8, 126.5, 128.3, 128.8, 128.9, 129.6, 130.1, 130.3, 130.4, 130.8, 133.8, 134.3, 135.6, 149.6, 150.2, 150.6, 150.8, 152.8, 164.6, 169.2. Anal. Calcd for C35H20FNO5: C, 75.94; H, 3.64; N, 2.53. Found: C, 75.92; H, 3.66; N, 2.54.
3.2.4 Physicochemical and spectral data for 3',6'-dihydroxy-2-(4-iodophenyl)-4-phenyl-8H-spiro[furo[3,4-g]quinoline-6,9'-xanthen]-8-one (4d): A mixture of 4-Iodobenzaldehyde (1d, 0.23 g, 1.0 mmol), 4-Aminofluorescein (2, 0.34 g, 1.0 mmol), Phenylacetylene (3, 0.10 g, 1.0 mmol) and CuCl (0.07 g, 0.3 mmol) in 10 mL of THF afforded compound 4d as an pale yellow solid (0.45 g, 68%); [a]D31 + 56.8 (c 0.1, CHCl3); ¹H NMR: (CDCl3, 300 MHz): d 6.75 (d, 2H, J = 8.4 Hz, Ar-H), 6.78 (d, 1H, J = 8.4 Hz, Ar-H), 6.96 (d, 2H, J = 7.8 Hz, Ar-H), 7.08 (d, 2H, J = 7.8 Hz, Ar-H), 7.32 (t, 2H, J = 8.0 Hz, Ar-H), 7.62 (q, 3H, J = 7.5 Hz, Ar-H), 7.74 (d, 3H, J = 7.2 Hz, Ar-H), 8.11 (q, 1H, J = 8.2 Hz, Ar-H), 8.25 (s, 2H, Ar-OH), 8.28 (d, 2H, J = 7.8 Hz, Ar-H). 13C NMR: (CDCl3, 75 MHz): d 82.8, 110.5, 116.5, 117.8, 118.5, 119.6, 124.4, 125.1, 125.6, 126.5, 128.3, 128.8, 128.9, 129.5, 130.2, 130.3, 130.4, 130.6, 133.6, 134.6, 135.6, 149.7, 150.2, 150.6, 150.8, 152.7, 164.5, 169.3. Anal. Calcd for C35H20INO5: C, 63.55; H, 3.05; N, 2.12. Found: C, 63.55; H, 3.05; N, 2.12.
Physicochemical and spectral data for 3',6'-dihydroxy-4-phenyl-2-(p-tolyl)-8H-spiro[furo[3,4-g]quinoline-6,9'-xanthen]-8-one (4e): A mixture of 4-Methylbenzaldehyde (1e, 0.12 g, 1.0 mmol), 4-Aminofluorescein (2, 0.34 g, 1.0 mmol), Phenylacetylene (3, 0.10 g, 1.0 mmol) and CuCl (0.07 g, 0.3 mmol) in 10 mL of THF afforded compound 4e as an pale yellow solid (0.36 g, 66%); [a]D31 + 62.8 (c 0.1, CHCl3); ¹H NMR: (CDCl3, 300 MHz): d 2.34 (s, 3H, -CH3), 6.75 (d, 2H, J = 8.4 Hz, Ar-H), 6.77 (d, 1H, J = 8.4 Hz, Ar-H), 6.96 (d, 2H, J = 7.8 Hz, Ar-H), 7.08 (t, 2H, J = 7.8 Hz, Ar-H), 7.38 (t, 2H, J = 8.0 Hz, Ar-H), 7.56 (q, 3H, J = 7.5 Hz, Ar-H), 7.72 (d, 3H, J = 6.8 Hz, Ar-H), 8.08 (d, 1H, J = 8.5 Hz, Ar-H), 8.23 (s, 2H, Ar-OH), 8.28 (d, 2H, J = 7.8 Hz, Ar-H). 13C NMR: (CDCl3, 75 MHz): d 20.1, 82.5, 110.4, 116.7, 117.9, 118.6, 119.6, 124.5, 125.7, 125.7, 126.1, 128.2, 128.8, 128.9, 129.3, 130.2, 130.3, 130.4, 130.9, 133.2, 134.1, 135.4, 149.6, 150.2, 150.6, 150.8, 152.5, 164.2, 169.2. Anal. Calcd for C36H23NO5: C, 78.68; H, 4.22; N, 2.55. Found: C, 78.66; H, 4.24; N, 2.53.
Ligand-Based Target Prediction and Molecular Docking Studies
The 3D structure of the molecule is drawn using the Discovery Studio software and was subjected to target protein prediction studies using D3 Similarity in the D3Targets-2019-nCoV server [23]. The Server uses two-dimensional and three-dimensional similarity of known molecular structure to predict target proteins for the desire/ query compounds. Further docking was performed using the D3Docking server which used Autodock Vina [24] to study the efficiency of the molecule for interaction with the 3C-like protease of COVID 19. K36 and O6K were used as control for the study.
Results and Discussion
Synthesis of Quinolines
Aldehydes (1a-e) and 4-Aminofluorescein (2) reacts with phenylacetylene (3) in the presence of CuCl as a catalyst in THF solvent and results in 66-73% yield of the respective quinoline (4a-e) as shown in (Scheme 1). The ¹H NMR spectra of the quinoline exhibit signals for the aromatic ring of the quinoline core structure in the region of 8.22-6.98 ppm. However, the aromatic carbons in the 13C NMR spectrum corresponding to the quinoline core structure resonate in the region of 158.1-110.2 ppm, which provides evidence for the quinoline over the possible fluorescein-amine. All these observations provide clear support for the formation of the 2,4-disubstituted quinoline. Structure of reaction time and product yields are given in (Table 1).
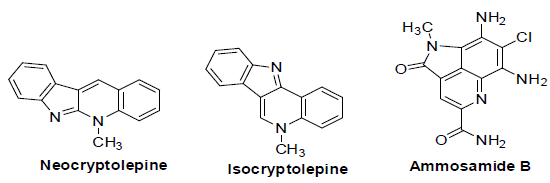
Scheme 1: Synthesis of quinolines.
S. No
R
Time (h)
Yield (%)
1
Cl (4a)
8
73
2
Br (4b)
8
70
3
F (4c)
8
67
4
I (4d)
8
68
5
Me (4e)
8
66
Table 1: Structure of reaction time and product yields.
The fi rst A³ coupling approach for quinoline synthesis was described by Rajasekar et al. in the presence of catalyst CuCl in 2014 [24]. The A³ coupled product propargylamine 5 underwent a Cu-mediated allenyl isomerization (6), followed by a intramolecular cyclization and dehydrogenative oxidation to accomplish quinoline 4a-e are shown in (Scheme 2).
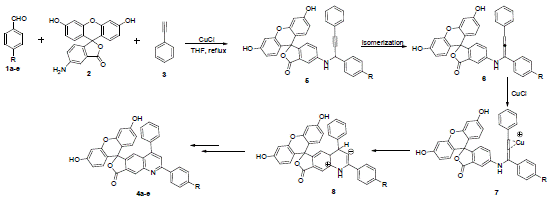
Scheme 2: First A³ coupling approach for quinoline synthesis.
Target Prediction using D3 Similarity
D3Similarity is based on the two-dimensional and three-dimensional similarity of molecular structure. The target proteins are predicted based on the active compounds already available from experimental studies, followed by virtual screening via 2D and 3D similarities. The compounds showed a similarity index of 20.74 to CBMicro_032111 which is an inhibitor of 3C-like protease (Table 2). COVID 19 protein targets were primaried in the present study and the docking were performed.
Compound
Pubchem CID
Mol ID
Target Name
2867165
CBMicro_032111
20.74
69.64
29.77
virus: 3C-like protease
4b
2867165
CBMicro_032111
20.98
69.48
30.19
virus: 3C-like protease
4c
70673796
CHEMBL2235440
20.82
61.8
33.69
virus: 3C-like protease
4d
2867165
CBMicro_032111
20.74
69.64
29.77
virus: 3C-like protease
4e
70673796
CHEMBL2235440
21.57
61.98
0.348
virus: 3C-like protease
4a
66839793
2-(3,4-Dihydroxyphenyl)-6-(3,7-dimethylocta-2,6-dienyl)-5,7-dihydroxy-2,3-dihydrochromen-4-one
22.01
61.98
0.3221
virus: Papain-like protease
4b
66839793
2-(3,4-Dihydroxyphenyl)-6-(3,7-dimethylocta-2,6-dienyl)-5,7-dihydroxy-2,3-dihydrochromen-4-one
22.01
68.34
0.3221
virus: Papain-like protease
4c
66839793
2-(3,4-Dihydroxyphenyl)-6-(3,7-dimethylocta-2,6-dienyl)-5,7-dihydroxy-2,3-dihydrochromen-4-one
22.71
68.35
0.3209
virus: Papain-like protease
4d
66839793
2-(3,4-Dihydroxyphenyl)-6-(3,7-dimethylocta-2,6-dienyl)-5,7-dihydroxy-2,3-dihydrochromen-4-one
21.91
70.76
0.3209
virus: Papain-like protease
4e
66839793
2-(3,4-Dihydroxyphenyl)-6-(3,7-dimethylocta-2,6-dienyl)-5,7-dihydroxy-2,3-dihydrochromen-4-one
22.63
68.26
0.332
virus: Papain-like protease
Table 2: COVID 19 protein Target prediction of compounds using 3D similarity search.
Docking and Interaction of Synthesized Componds against 3C-like Protease
Docking studies were carried out between the protein and the ligand in D3Docking server. The docking studies were performed against the dimer and monomer of 3C-like protease. CYS A:145, HIS A:163, HIS A:164, GLU A:166, HIS A:41, MET A:49, TYR A:54, PHE A:140, LEU A:141, ASN A:142, GLY A:143, SER A:144, MET A:165, HIS A:172, ASP A:187, GLN A:189 are the active site amino acid of the monomer of 3C-like protease, similarly THR A:26, HIS A:41, MET A:49, PHE A:140, LEU A:141, ASN A:142, GLY A:143, SER A:144, CYS A:145, HIS A:163, HIS A:164, MET A:165, GLU A:166, HIS A:172, ASP A:187, GLN A:189 are the active site residues of dimer of 3C-like protease. The docking score for 4b and 4c of -8.2 kcal/mol and -8.1 kcal/mol respectively for monomer of 3C-like protease, and -8.88 kcal/mol and -8.9 kcal/mol respectively for dimer of 3C-like protease (Table 3). The molecular interactions shows 4b and 4c has 2 hydrogen bonds each comparitivly less when compared to K36 (8) and O6K (5) for 3C-like protease monomer (Figure 2). The in teraction of 4b and 4c with 3C-like protease dimer also showed 3 and 4 hydrogen bonds when compared to K36 and O6K which showed 6 hydrogen bond. Similar hydrophobic interactions were observed in all the interactions (Figure 3).
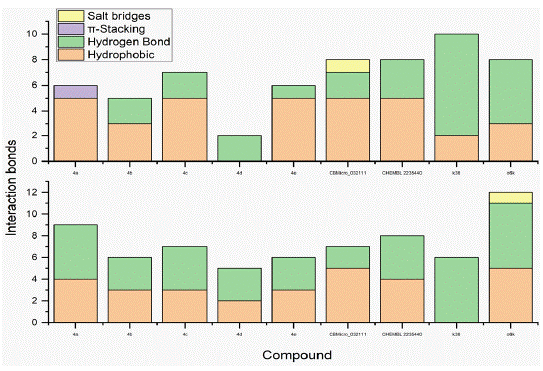
Figure 2: Number of interactions (Hydrogen bond, Hydrophobic interaction, Pi-staking and salt bridges) present in the 3C-like protease and ligand complex.
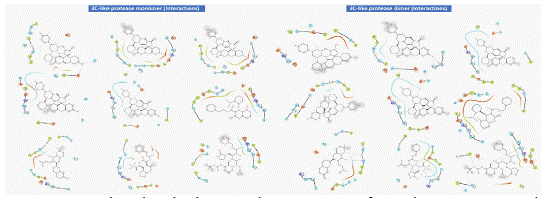
Figure 3: Molecular docking and interaction of 3C Like protease and 2-(4-chlorophenyl)-3’,6’-dihydroxy-4-phenyl-8H-spiro[furo[3,4-g]quinoline-6,9’-xanthen]-8-one (4). (A) Aminoacid interaction in the active site between Dimer 3C like protease and synthesized molecule. (B) Aminoacid interaction in the active site between Monomer 3C like protease and synthesized molecule.
Compound
3C-like protease monomer
3C-like protease dimer
3C-like protease monomer (Interactions)
3C-like protease dimer (Interactions)
4a
-6.6
-6.8
Hydrophobic 41A HIS 3.28
Hydrophobic 47A GLU 3.97
Hydrophobic 141A LEU 3.81
Hydrophobic 166A GLU 3.9
Hydrophobic 187A ASP 3.71
p-Stacking 41A HIS .86Hydrophobic 25A THR 2.82
Hydrophobic 27A LEU 3.47
Hydrophobic 166A GLU 3.92
Hydrophobic 168A PRO 2.94
Hydrogen Bond 1B SER 2.89
Hydrogen Bond 47A GLU 2.95
Hydrogen Bond 166A GLU 2.25
Hydrogen Bond 166A GLU 1.8
Hydrogen Bond 189A GLN 2.714b
-8.2
-8.8
Hydrophobic 140A PHE 3.81
Hydrophobic 141A LEU 3.87
Hydrophobic 166A GLU 3.3
Hydrogen Bond 189A GLN 2.64
Hydrogen Bond 192A GLN 3.19Hydrophobic 140A PHE 3.8
Hydrophobic 165A MET 3.87
Hydrophobic 166A GLU 3.46
Hydrogen Bond 188A ARG 1.7
Hydrogen Bond 189A GLN 2.61
Hydrogen Bond 192A GLN 2.754c
-8.1
-8.9
Hydrophobic 41A HIS 3.55
Hydrophobic 47A GLU 3.31
Hydrophobic 167A LEU 3.36
Hydrophobic 168A PRO 3.76
Hydrophobic 192A GLN 3.36
Hydrogen Bond 47AGLU 2.31
Hydrogen Bond 166A GLU 3.24Hydrophobic 140A PHE .75
Hydrophobic 165A MET 3.83
Hydrophobic 166A GLU 3.42
Hydrogen Bond 186A VAL 2.07
Hydrogen Bond 188A ARG 2.4
Hydrogen Bond 189A GLN 2.67
Hydrogen Bond 192A GLN 2.684d
-8.1
-5.8
Hydrophobic 140A PHE 3.8
Hydrophobic 166A GLU 3.35Hydrophobic 47A GLU 3.32
Hydrophobic 168A PRO 3.27
Hydrogen Bond 1B SER 2.02
Hydrogen Bond 47A GLU 3.16
Hydrogen Bond 142A ASN 3.074e
-7.4
-7.9
Hydrophobic 41A HIS 3.57
Hydrophobic 47A GLU 3.27
Hydrophobic 167A LEU 3.56
Hydrophobic 168A PRO 3.64
Hydrophobic 192A GLN 3.37
Hydrogen Bond 47A GLU 2.39Hydrophobic 41A HIS 3.79
Hydrophobic 47A GLU 3.34
Hydrophobic 192A GLN 3.49
Hydrogen Bond 1B SER 2
Hydrogen Bond 1B SER 2.34
Hydrogen Bond 47A GLU 2.73CBMicro_
032111 (Control)-7
-7.2
Hydrophobic 47A GLU 3.17
Hydrophobic 165A MET 3.92
Hydrophobic 166A GLU 3.68
Hydrophobic 189A GLN 3.88
Hydrophobic 192A GLN 3.95
Hydrogen Bond 189A GLN 3.08
Hydrogen Bond 192A GLN 2.4
Salt Bridges 41A HIS 5.3Hydrophobic 47A GLU 3.16
Hydrophobic 140A PHE 3.9
Hydrophobic 166A GLU 3.68
Hydrophobic 189A GLN 3.86
Hydrophobic 192A GLN 3.92
Hydrogen Bond 189A GLN 3.11
Hydrogen Bond 192A GLN 2.39CHEMBL
2235440 (Control)-8
-8
Hydrophobic 25A THR 3.76
Hydrophobic 27A LEU 3.83
Hydrophobic 140A PHE 3.56
Hydrophobic 165A MET 3.45
Hydrophobic 166A GLU 3.44
Hydrogen Bond 142A ASN 2.26
Hydrogen Bond 143A GLY 2.61
Hydrogen Bond 189A GLN 2.48Hydrophobic 25A THR 3.76
Hydrophobic 27A LEU 3.94
Hydrophobic 140A PHE 3.65
Hydrophobic 166A GLU 3.62
Hydrogen Bond 142A ASN 2.23
Hydrogen Bond 143A GLY 2.75
Hydrogen Bond 189A GLN 2.57
Hydrogen Bond 190A THR 3.15K36 (Control)
-6.8
-7.2
Hydrophobic 166A GLU 3.8
Hydrophobic 168A PRO 3.84
Hydrogen Bond 166A GLU 2.05
Hydrogen Bond 166A GLU 2.36
Hydrogen Bond 166A GLU 2.23
Hydrogen Bond 188A ARG 2.45
Hydrogen Bond 189A GLN 2.7
Hydrogen Bond 190A THR 2.17
Hydrogen Bond 190A THR 3.28
Hydrogen Bond 192A GLN 2.32Hydrogen Bond 166A GLU 2.6
Hydrogen Bond166A GLU 2.14
Hydrogen Bond 189A GLN 2.15
Hydrogen Bond 190A THR 2.22
Hydrogen Bond 190A THR 2.56
Hydrogen Bond 192A GLN 2.34O6K (Control)
-6.9
-7.3
Hydrophobic 27A LEU 3.82
Hydrophobic 166A GLU 3.71
Hydrophobic 168A PRO 3.39
Hydrogen Bond 47A GLU 2.17
Hydrogen Bond 187A ASP 3.2
Hydrogen Bond 189A GLN 2.42
Hydrogen Bond 189A GLN 2.14
Hydrogen Bond 192A GLN 3.49Hydrophobic 25A THR 3.68
Hydrophobic 27A LEU 3.64
Hydrophobic 47A GLU 3.83
Hydrophobic 168A PRO 3.43
Hydrophobic 189A GLN 3.85
Hydrogen Bond 143A GLY 2.99
Hydrogen Bond 166A GLU 3.06
Hydrogen Bond 187A ASP 3.19
Hydrogen Bond 189A GLN 2.18
Hydrogen Bond 190A THR 2.33
Hydrogen Bond 192A GLN 2.3
Salt Bridges 47A GLU 5.04
Table 3: Hydrogen bond and hydrophobic interaction between compounds and 3C-like protease.
Conclusions
In conclusion, we have reported the synthesis of a novel class of quinoline. The most noteworthy aspect of this research is the development of an efficient and general route for the synthesis of the quinoline through a one-pot synthesis from aminofluorescein, benzaldehyde and phenylacetylene. This methodology of a one-pot three-component reaction in the synthesis of quinoline with substitution at the C-2 and C-4 positions appears attractive for the combinatorial synthesis of a quinoline library. Quinolines are potential antiviral molecules and in the present study, an attempt to fi nd its potential against COVID 19 is been assed using in silico docking studies. This resulted in identifying the synthesized compound to be a potential inhibitor molecule against the 3C like protease both as monomer and dimer. Further in vitro and in vivo studies can help in fi nding the efficacy of the synthesized molecule as a potential antiviral agent.
Acknowledgement
M. R. acknowledges fi nancial support from the DBT, New Delhi. The authors thank ‘Sathyabama Institute of Science and Technology, Chennai, Tamilnadu, India’ for providing infrastructure and instrumentation support to execute the research work.
References
- Kouznetsov VV, Vargas Méndez LY, Puerto Galvis CE, Ortiz Villamizar MC. The direct C–H alkenylation of quinoline N -oxides as a suitable strategy for the synthesis of promising antiparasitic drugs. New J Chem. 2020; 44: 12–19.
- Weyesa A, Mulugeta E. Recent advances in the synthesis of biologically and pharmaceutically active quinoline and its analogues: a review. RSC Adv. 2020; 10: 20784–20793.
- Mekheimer RA, Al-Sheikh MA, Medrasi HY, Sadek KU. Advancements in the synthesis of fused tetracyclic quinoline derivatives. RSC Adv. 2020; 10: 19867–19935.
- Lam KH, Lee KKH, Gambari R, Kok SHL, Kok TW, Chan ASC, et al. Anti-tumour and pharmacokinetics study of 2-Formyl-8-hydroxy-quinolinium chloride as Galipea longiflora alkaloid analogue. Phytomedicine. 2014; 21: 877-82.
- Vincent MJ, Bergeron E, Benjannet S, Erickson BR, Rollin PE, et al. Chloroquine is a potent inhibitor of SARS coronavirus infection and spread. Virol J. 2005; 2: 69.
- Lam KH, Gambari R, Lee KKH, Chen YX, Kok SHL, Wong RSM, et al. Preparation of 8-hydroxyquinoline derivatives as potential antibiotics against Staphylococcus aureus. Bioorg Med Chem Lett. 2014; 24: 367–370.
- Vandekerckhove S, Tran HG, Desmet T, D’Hooghe M. Evaluation of (4-aminobutyloxy) quinolines as a novel class of antifungal agents. Bioorganic Med Chem Lett. 2013; 23: 4641-3.
- Ratheesh M, Sindhu G, Helen A. Anti-inflammatory effect of quinoline alkaloid skimmianine isolated from Ruta graveolens L. Inflamm Res. 2013; 62: 367-76.
- Kouznetsov V, Vargas Mendez L, Milena Leal S, Mora Cruz U, Andres Coronado C, et al., 2008. Target-Oriented Synthesis of Antiparasitic 2-Hetaryl Substituted Quinolines Based on Imino Diels-Alder Reactions. Lett Drug Des Discov. 2008; 4: 293-296.
- Rolain JM, Colson P, Raoult D. Recycling of chloroquine and its hydroxyl analogue to face bacterial, fungal and viral infections in the 21st century. Int J Antimicrob Agents. 2007; 30: 297-308.
- Zheng X, Wang L, Wang B, Miao K, Xiang K, et al. Discovery of Piperazinylquinoline Derivatives as Novel Respiratory Syncytial Virus Fusion Inhibitors. ACS Med Chem Lett. 2016; 7: 558-62.
- Talamas FX, Abbot SC, Anand S, Brameld KA, Carter DS, Chen J, et al. Discovery of N-[4-[6-tert-butyl-5-methoxy-8-(6-methoxy-2-oxo-1 H -pyridin-3-yl)-3-quinolyl]phenyl]methanesulfonamide (RG7109), a potent inhibitor of the hepatitis C virus NS5B polymerase. J Med Chem. 2014; 57: 1914-1931.
- Goodell JR, Puig-Basagoiti F, Forshey BM, Shi PY, Ferguson DM. Identifi cation of compounds with anti-West Nile virus activity. J Med Chem. 2006.
- Huang SH, Lien JC, Chen CJ, Liu YC, Wang CY, et al. Antiviral activity of a novel compound cw-33 against Japanese encephalitis virus through inhibiting intracellular calcium overload. Int J Mol Sci. 2016; 17: 1386.
- Barbosa-Lima G, Moraes AM, Araújo A da S, da Silva ET, Freitas CS, et al. 2,8-bis(trifluoromethyl)quinoline analogs show improved anti-Zika virus activity, compared to mefloquine. Eur J Med Chem. 2017; 127: 334-340.
- Kim J, Kim S, Kim D, Chang S. Ru-Catalyzed Deoxygenative Regioselective C8–H Arylation of Quinoline N -Oxides. J Org Chem. 2019; 84: 13150–13158.
- You G, Xi D, Sun J, Hao L, Xia C. Transition-metal- and oxidant-free three-component reaction of quinoline: N -oxides, sodium metabisulfi te and aryldiazonium tetrafluoroborates via a dual radical coupling process. Org Biomol Chem. 2019; 17: 9479-9488.
- Upadhyay A, Chandrakar P, Gupta S, Parmar N, Singh SK, et al. Synthesis, Biological Evaluation, Structure–Activity Relationship, and Mechanism of Action Studies of Quinoline–Metronidazole Derivatives Against Experimental Visceral Leishmaniasis. J Med Chem. 2019; 62: 5655–5671.
- Tarnow P, Zordick C, Bottke A, Fischer B, Kuhne F, et al. Characterization of Quinoline Yellow Dyes As Transient Aryl Hydrocarbon Receptor Agonists. Chem Res Toxicol. 2020; 33: 742–750.
- Ko MS, Kurapati S, Jo Y, Cho B, Cho DG. Tuned Al3+ selectivity and p-extended properties of di-2-picolylamine-substituted quinoline-based tolan. Tetrahedron Lett. 2020; 61: 151808.
- Vuong H, Stentzel MR, Klumpp DA. Superacid-promoted synthesis of quinoline derivatives. Tetrahedron Lett. 2020; 61: 151630.
- Rajasekar M, Mohan Das T. Synthesis and antioxidant properties of novel fluorescein-based quinoline glycoconjugates. J Carbohydr Chem. 2014; 137-151.
- Shi Y, Zhang X, Mu K, Peng C, Zhu Z, Wang X, et al. D3Targets-2019-nCoV: a webserver for predicting drug targets and for multi-target and multi-site based virtual screening against COVID-19. Acta Pharm. Sin B. 2020; 10: 1239-1248.
- Trott O, Olson AJ. AutoDock Vina: improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J Comput Chem. 2010; 31: 455–61.