
Review Article
Austin J Biosens & Bioelectron. 2015;1(1): 1003.
Advancement of Fluorescent Methods for Detection of Nitric Oxide
New SY*
1School of Environmental and Biological Engineering, Nanjing University of Science & Technology, China
2Chemistry Department, University of South Florida, USA
*Corresponding author: Jinming Kong, School of Environmental and Biological Engineering, Nanjing University of Science & Technology, 210094 Nanjing, PR China.
Xueji Zhang, Chemistry Department, College of Arts and Sciences, University of South Florida, East Fowler Ave, Tampa, Florida 33620-4202, USA.
Received: September 30, 2014; Accepted: January 29, 2015; Published: February 02, 2015
Abstract
Biologically Nitric Oxide (NO) is an important inorganic compound involved in numerous signaling pathways, which has promoted the demand for analytical methods for detection of NO. Fluorescent sensing, more effective than any other detection methods, enables monitoring NO in physiological environments such as cell and blood. Fluorescent probes based detection methods have wide applications in selective and sensitive monitoring of NO production in vivo. In this review, we highlight the novel fluorescent NO sensors developed in recent years, including fabrication, analytical characteristics and biological applications.
Keywords: Nitric oxide; Fluorescent probes; Photo induced electron transfer; Reactive nitrogen and oxygen species
Abbreviations
NO: Nitric Oxide; DAFs: Diaminofluoresceins; DANs: Diaminonaphthalenes; DARs: Diaminorhodamines; SWNT: Single- Walled Carbon Nanotube; QDs: Quantum Dots; PET: Photo induced Electron Transfer; FRET: Fluorescence Resonance Energy Transfer; RNOS: Reactive Nitrogen And Oxygen Species; RH: Rhodamine B Hydrazide; RB: Rhodamine B; RBSe: Rhodamine B Selenolactone; DHA: Dehydroascorbic Acid; AA: Ascorbic Acid; iNOS: inducible NO Synthase; IFN-γ: Interferon-γ; LPS: Lipopolysaccharide; SN: Seminaphthofluorescein; CS: Chitosan; DMS: Dimethyl Sulfate; H3TCA: Tricarboxytriphenylamine; PMOFs: Porous Metal-Organic Frameworks; NIR: Near-Infrared; HEX-DMA: 1,6-hexanedioldimethacrylate; PMMA: Poly (methyl methacrylate); mHP: Modified Hyperbranched Polyether; NTPED: N-(3-(Trimethoxysilyl) propyl) ethylenediamine; CA: Cellulose Acetate; DTC: N-(dithiocarbaxy) sarcosine
Introduction
Although NO is well known as an environmental pollutant generated from incomplete combustion of molecules containing nitrogen, it is also important in human body at the concentration ranging from sub-nanomolar to micro molar levels. Furchgott, Ignarro and Murad reported in 1987 that NO is the endotheliumderived relaxation factor and they ultimately shared the Nobel Prize in Physiology in 1998 [1]. Hereafter, many researchers have continued to explore the unknown domains of NO. NO is now understood to be active in several physiological events taking place in cardiovascular, immune, nervous systems as well as pathological processes, [2] for example, arteriolosclerosis [3] and hypertension [4] are connected with underproduction of NO while cancer [5] and diabetes [6] are related to its overproduction. NO is biosynthesized endogenously by nitric oxide Synthase which is a heme-containing enzyme and catalyzes L-arginine to L-citrulline. It is widespread in mammals, plants, bacteria and invertebrates [7]. NO released exogenously has been found to result in various biological responses such as platelet activation decrease [8] and microbial viability reduction [9]. NO diffuses rapidly with an average lifetime from milliseconds to seconds.
Great attention for NO and its biological roles have prompted the development of analytical techniques for its detection and quantification. The effect of NO depends on its widely varying concentration in human body, ranging from sub-nanomolar to micro molar levels. NO can react with oxygen, thiol, heme and so on, thus it has short half-life which is typically less than 10 seconds in biological environment [10]. Consequently, methods with adequate sensitivity and high affectivity are required in NO detection. Moreover, high selectivity for NO over interfering species is also necessary due to the complexity of biological systems.
The majority of NO detection approaches can be classified into electrochemical and spectroscopic methods. Most electrochemical NO detection methods involve electro reduction, direct electro oxidation and catalytic electro oxidation [11]. Spectroscopy methods involve either indirect detection of byproducts (i.e. Griess reaction, chemiluminescence) or direct detection of adducts (i.e. absorbance, electron paramagnetic resonance spectroscopy, fluorescence) [12,13]. However, most of these methods suffer from problems with selectivity and sensitivity as well as disadvantages for NO detection in vivo as a result of the toxicity and the complex processes. By contrast, fluorescent method which is commonly used for intracellular detection of NO shows many excellent characteristics such as lowcost, high selectivity and sensitivity. Fluorescent probes can respond in direct and selective manners to NO. As such, they provide a valuable approach to explore the generation, accumulation and translocation of NO in biology with both spatial and temporal resolution [14].
The majority of NO detection approaches can be classified into electrochemical and spectroscopic methods. Most electrochemical NO detection methods involve electro reduction, direct electro oxidation and catalytic electro oxidation [11]. Spectroscopy methods involve either indirect detection of byproducts (i.e. Griess reaction, chemiluminescence) or direct detection of adducts (i.e. absorbance, electron paramagnetic resonance spectroscopy, fluorescence) [12,13]. However, most of these methods suffer from problems with selectivity and sensitivity as well as disadvantages for NO detection in vivo as a result of the toxicity and the complex processes. By contrast, fluorescent method which is commonly used for intracellular detection of NO shows many excellent characteristics such as lowcost, high selectivity and sensitivity. Fluorescent probes can respond in direct and selective manners to NO. As such, they provide a valuable approach to explore the generation, accumulation and translocation of NO in biology with both spatial and temporal resolution [14]. the past decades, numerous fluorescent probes for effective DNA detection have been explored and the commonly used probes can be classified into organic probes (i.e., Diaminofluoresceins (DAFs), [17-21] Diaminonaphthalenes (DANs), [22-24] Diaminorhodamines (DARs), [25] DAMBO-PH. [26]), metal complex-based probes (i.e., copper, [27,28] ferrum, [29] cobalt, [30] ruthenium, [31] dirhodium [32] complexes), Single-Walled Carbon Nanotube (SWNT) -based probes [33] and Quantum Dots (QDs) -based probes [33]. These fluorescent probes have excellent selectivity, high sensitivity and low toxicity for NO sensing in vivo [34-38]. The team led by Lippard has focused on several kinds of these fluorescent probes and reviewed all the commonly used probes before [31,33,39]. This review highlights the novel fluorescent NO sensors in recent years.
Fluorescent methods for detection of NO
In the past few years, the fluorescent methods for detection of NO are mainly based on Photo induced Electron Transfer (PET), Fluorescence Resonance Energy Transfer (FRET) and fluorescence response to ring-opened or ring-closed reaction. On the other hand, the fluorescent methods can also be classified into organic probesbased methods, metal complex probes-based methods, SWNT probesbased methods and QD probes-based methods. These fluorescent methods have excellent selectivity and sensitivity for NO and are available for different applications. In addition, these new-developed methods are more advantageous than the methods commonly used before in various aspects.
Organic probes-based methods
Numerous fluorescent methods for NO detection employ the probes with electron-rich o-phenylenediamine fraction. The introduction of o-phenylenediamine fraction in a fluorophore results in PET from the lone-pair electrons of amine to the fluorophore to quench the fluorescence. Conversion of the o-phenylenediamine to electron-poor aryltriazole in the presence of NO decreases the energy of lone-pair electrons and turns off the PET to restore fluorescence [39].
A typical example is the method employing Lyso-NINO, a lysosome-specific and two-photon fluorescent probe which has lower cytotoxicity, excellent lysosomal localization, high selectivity and sensitivity for monitoring endogenous and exogenous NO in lysosomes of macrophage cells [40]. Lyso-NINO based on PET is integration of lysosome-targeting (aminoethyl)morpholine, two-photon fluorophore naphthalimide and NO-capturing o-phenylenediamine. o-Phenylenediamine is employed not only as NO-captor but also as a fluorescence quencher for naphthalimide. In the lysosomal pH ranging from 4.5 to 5.5, Lyso-NINO exhibits weak fluorescence while the reaction conduct Lyso-NINO-T of Lyso-NINO with NO shows strong fluorescence (Figure 1). In addition, the photo properties of Lyso-NINO are not interfered by the byproducts of NO in lysosomes. Flow cytometry can be used for quantitative analysis of endogenous NO and iNOS inducers can effectively increase endogenous NO. Another example is a quinoline derivative QNO, a two-photon fluorescent probe composed of a glycinamide linker, an o-phenylenediamine and a quinoline derivative [41]. It also has characteristics such as good photo stability, low cytotoxicity and pH insensitivity. QNO itself exhibit very weak fluorescence because of PET. A triazine fraction is formed after the rapid reaction with NO, the PET is inhibited and the fluorescence is restored without any shift in wavelength (Figure 2). QNO exhibits high selectivity for NO over other biologically Reactive Nitrogen and Oxygen Species (RNOS). DANPBO-H [42] and DANPBO-M [43] are also o-phenylenediamine-based probes. The incorporation of two tetrahydronaphthalene with the pyrrole moiety of BODIPY [44] has extended the excitation and emission wavelengths as well as increased the lipophilicity. The probes have excellent intracellular retention because of their strong lipophilicity. Even under the irradiation of xenon lamp over 24h, the fluorescence intensity of the probes remains unchangeable. Their good photo stability is ensured by that the probes have no heavy atoms and withdrawing groups. DANPBOs themselves have very weak fluorescence at pH above 4, however, they react with NO to generate triazoles DANPBO-Ts with high fluorescence. Other biologically RNOS have no obvious interference with NO detection. A similar probe BOPB [45] has been also reported to be utilized for NO detection (Figure 3). Such kind of probes with o-phenylenediamine fraction react with NO indirectly, thus these methods are possibly irreversible and inaccurate.
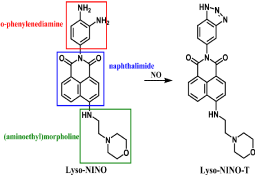
Figure 1: Chemical structure of Lyso-NINO and reaction between Lyso-NINO and NO.
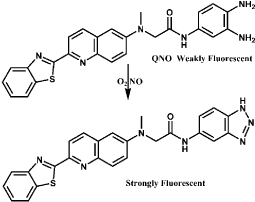
Figure 2: Chemical structure of QNO and the fluorescence response to NO.
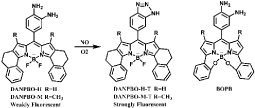
Figure 3: Chemical structures of DANPBOs and BOPB as well as reaction of DANPBOs with NO.
Another kind of NO detection methods apply rhodamine-based probes, in which ring-closed rhodamine moiety is non-fluorescent while the fluorescence is restored when the ring is opened in the presence of NO [46]. For example, Rhodamine B Hydrazide (RH) is a colorless and non-fluorescent probe for exogenous and endogenous NO detection [47]. NO reacts with its oxidized product NO2 to generate N2O3 and then N2O3 nitrosylates the amino group of RH to form diazonium group. An azide intermediate is generated and converted into Rhodamine B (RB) due to the ring-opened reaction (Figure 4). The probe exhibits excellent stability under pH above 4 and shows high selectivity to NO over other biologically RNOS. The fast reaction of Rhodamine B Selenolactone (RBSe) with NO is also ring-opened reaction [48]. RBSe itself has very weak fluorescence, while pink color and strong fluorescence are immediately generated with the addition of NO (Figure 5). The fluorescence intensity is hardly affected by pH ranging from 6.8 to 7.8, thus RBSe is suitable for detection of NO in physiological environment. Furthermore, though RBSe can react with Ag (I) and Hg(II), the concentration of the two ions in normal physiological environment do not interfere the detection of NO.
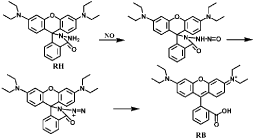
Figure 4: Sensing mechanism of RH with NO.
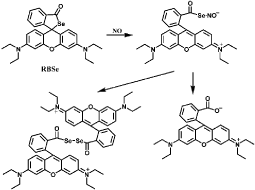
Figure 5: Chemical structure of RBSe and fluorescence response to NO.
Cou-Rho-NO is designed based on a FRET dyad, coumarin and rhodamine [49]. A piperazine moiety between coumarin and rhodamine is the linker to avoid fluorescence quenching in aqueous environment. In addition, an o-phenylenediamine moiety is the reaction site for NO. The FRET does not occur when the rhodamine acceptor is ring-closed in the absence of NO. In other words, when Cou-Rho-NO reacts with NO, the FRET is generated and the fluorescence intensity of the rhodamine moiety increases (Figure 6). Cou-Rho-NO reacts with NO under the solution of pH 3-10. Cou- Rho-NO probe is suitable for fluorescent imaging of endogenously intracellular NO. Furthermore, though Dehydroascorbic Acid (DHA) and Ascorbic Acid (AA) interfere with detection of NO in o-phenylenediamine-based probes, Cou-Rho-NO with o-phenylenediamine moiety essentially does not show ratiometric response to DHA or AA. In addition, a probe FP-H2O2-NO with a boronate-based moiety attached on the coumarin side of Cou-Rho- NO has been reported to be applied for simultaneous detection of NO and H2O2 which are both important biomolecules in signal transduction and oxidative pathways [50].
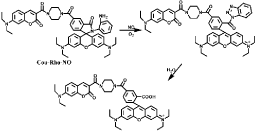
Figure 6: Reaction of Cou-Rho-NO with NO and the occurrence of FRET.
Especially, a method is developed based on N-nitrosation and formation of diazo ring. NO550 is a particular easily synthesized probe which presents selective and sensitive response to NO with the occurrence of red-shifted signal and the generation of diazo ring system (AZO550, Figure 7). NO550 reacts with NO rapidly in neutral or even alkalescence solution. The cyano and dimethylamino groups allow internal charge transfer on photo excitation. The ring closure reaction occurs at the para to the dimethylamino group because of steric hindrance and the stability of six-membered ring [51]. The color of NO550 solution changes from light-yellow to deep-red and the fluorescence intensity enhances with the increased concentration of NO. NO550 can get across cell membranes but cannot cross nuclear membranes thus it is suitable for both extra- and intracellular NO detection. Good characteristics of NO550 such as high specificity and sensitivity, low-dependence of pH and facile synthesis make it an excellent fluorescent probe for NO detection.
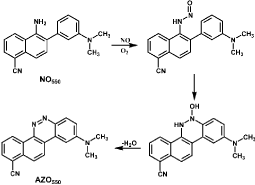
Figure 7: Reaction of NO550 with NO and the generation of AZO550.
Cu2+/Cu(II) complex probes-based methods
A widespread method for NO fluorescent detection is based on metal complex scaffold, such as cobalt, ruthenium, dirhodium complexes. However, these probes exhibit water-incompatibility and low sensitivity for in vivo applications. Cu (II) complexes have been developed widely to overcome these limitations. In the system, fluorescent ligand carrying a secondary amine is quenched on coordination to paramagnetic Cu(II) center, while NO reduces the paramagnetic Cu(II) to antimagnetic Cu+/Cu(II) with concomitant nitrosation (Nitrosation is a process of converting organic compounds into nitro so derivatives, i.e. compounds containing the R-NO functionality) and deprotonation of the secondary amine, which decreases the energy of lone-pair electrons on the nitrogen atom as well as turns off PET in the N-nitrosated product [39]. The majority of metal complexes are only used in organic solvents because the fluorescent ligands can be replaced by H2O molecules from the metal center in aqueous environments and the fluorescence can also restore even in the absence of NO. Though other radicals such as NO2 and H2O2 interfere with the detection, such probes exhibit high selectivity for NO detection over other biologically RNOS. Unlike the o-phenylenediamine-based probes, oxygen is not required when employing Cu (II) complex-based probes, which is beneficial for NO imaging in hypoxic environments. In addition, such probes can be conjugated to Fe3O4 nanoparticles to form magnetism-targeted probes [52].
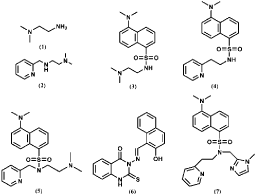
Figure 8: Structure of the N-donor ligands of Cu (II) complexes for NO detection.
Several Cu(II) complexes of N-donor ligands have been reported to be highly selective and sensitive for sensing NO in a physiologically relevant media (Figure 8) [53-55]. Furthermore, Cu (bpq)(OAc) (H2O) is utilized for direct detection of NO in which N-(8-quinolyl) pyridine-2-carboxamide) (Hbpq) is the ligand for Cu(II) [56]. The Cu (II) is stabilized by interaction with carbonyl oxygen atoms to form a dimeric compound. The fluorescence of the probe is quenched by Cu(II) acetate while the fluorescence is increased with the reduction of Cu(II) to Cu(II) in the presence of NO. The fluorescence quenching and increasing processes are reversible, in other words, the fluorescence is quenched again when NO is released (Figure 9). In addition, the fluorescence of a naphthalene-sulfonaminoquinoline based Cu (II) complex Cu (NSQ)2 is completely quenched and the addition of NO can restore the fluorescence [57] (Figure 10). Cu(NSQ)2 can also exhibit excellent fluorescence responding to NO in organic thin film such as polycaprolactone based film. CuQNE with naphthalimide fractions as the fluorophore is a highly selective and sensitive probe for intracellular NO detection [58] (Figure 11). CuQNE keeps a stable fluorescence under pH 6-10, facilitating NO detection in physiological conditions.
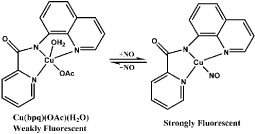
Figure 9: Fluorescence response of Cu(bpq)(OAc)(H2O) to NO.
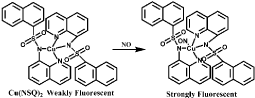
Figure 10: Chemical structure of Cu(NSQ)2 and its fluorescence response to NO.
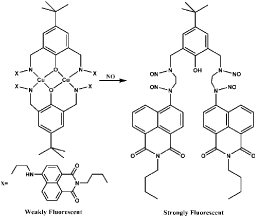
Figure 11: Chemical structure of CuQNE and the reaction of CuQNE with NO.
Cu(II) complexes based on ring-opened reaction of rhodamine moiety are commonly used probes for NO fluorescent detection. For example, CuRBT with a ring-closed rhodamine-containing tridentate N-donor is a Cu(II) complex-based probe for intracellular NO monitoring [59]. Tris (2-aminoethyl) amine moiety as the efficient chelator of Cu(II) is incorporated to rhodamine B moiety. Initially, NO coordination to Cu(II) followed by formation of NO+, then NO+ incorporates to the nitrogen of amide to open the spirolactam and Cu (II) is reduced to Cu(II), resulting in nitrosation and fluorescence response (Figure 12). Cu(II) in CuRBT is easily reduced to Cu(II) in the pH 6.5-9.0 only with a little variation of fluorescence intensity. RBTP- Cu(II) and RB-Py-Cu(II) are both similar probes for intracellular NO imaging [60] (Figure 13).
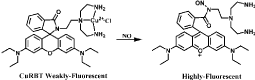
Figure 12: Chemical structure of CuRBT and reaction of CuRBT with NO.

Figure 13: Fluorescent response of RB-TP- Cu(II) and RB-Py- Cu(II) to NO.
CuFL is widely used for NO sensing, however, it easily diffuses out of cells under continual media perfusion. To improve the detection ability, several analogue based on CuFL are developed recently. Cu2 (FL2A) and Cu2(FL2E) are generated by combining the ligands FL2A and FL2E with two equivalents of CuCl2 [61-64] (Figure 14). Cu2 (FL2E) employs ester extending for cell-trapping and is suitable for detection of endogenous NO in live cells. Cu2 (FL2A) is membrane-impermeable because the negative charge of carboxyl ate prevents it from entering live cell (Figure 10). Inducible NO synthase (iNOS) such as Interferon-γ (IFN-γ) and Lipopolysaccharide (LPS) can significantly increase fluorescence. In addition, such probes are more sensitive for NO over Zn(II) and can be used to monitor NO production in olfactory bulb by fluorescence imaging. Cu (II) complex of SNFL used for NO sensing is also analogue of CuFL1 [65]. Cu(II) is bound at an 8-aminoquinaldine unit and a Seminaphthofluorescein (SN) moiety is extended to it. Fluorescence intensity increases significantly under anaerobic conditions due to the generation of fluorescent nitrosamine product (Figure 15). Such probes emit at longer wavelengths comparing to CuFL thus it is available for multidye imaging and deeper imaging. In addition, the lower wavelength excitation decreases cells and tissues damage. Though the SN and similar probes have been reported to be applied for detection of Zn(II), CuSNFL is more sensitive to NO. CuFL-CS NS diazeniumdiolates are synthesized by incorporating FL with Chitosan (CS) through electrostatic self-assembly, and then reacts with pressurized NO and Dimethyl Sulfate (DMS). The resultant FLCS NS diazeniumdiolates are protected by methyl groups and react with CuCl2. CuFL-CS NS diazeniumdiolates themselves are spherical and non-fluorescent due to the paramagnetic Cu(II) as well as the PET [66]. Under physiological conditions, the protecting methyl groups can segregate and release NO, some of which react with the CuFL fraction and reduce Cu(II) to Cu(II) (Figure 16). Consequently, such stable fluorescent probes can be utilized to monitor the released NO based on the fluorescence increase caused by NO.
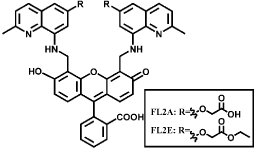
Figure 14: Chemical structures of FL2A and FL2E.
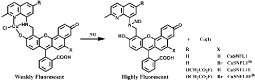
Figure 15: Chemical structure of CuSNFL family and fluorescence response to NO.
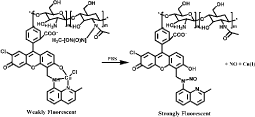
Figure 16: NO release and detection with CuFL-CS NS diazeniumdiolates.
Especially, Cu-TCA (H3TCA=tricarboxytriphenylamine) has a three-dimensional porous structure steadied by the well-established Cu2(O2CR)4 units (Figure 17) [67]. The Porous Metal-Organic Frameworks (PMOFs) can deliver gaseous NO, confirming their applications for gas storage and detection. Cu(II) quenches the fluorescence of trephenylamine and Cu-TCA only presents very weak fluorescence, however, the distinctly fluorescent enhancement is provided in the presence of NO. This probe can be used in the biological NO imaging in living cells. Another similar PMOFs Eu- TCA based on the hydrothermal reaction between europium nitrate and H3TCA has applications on ratio metric detection of NO.
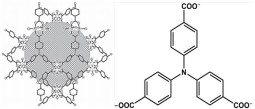
Figure 17: Schematic view of Cu-TCA crystal packing and the chemical structure of TCA. (Reprinted from reference 67. Copyright 2012 Advanced Functional Materials).
SWNT probes-based methods
Semiconducting SWNT-based methods are developed recently for intracellular NO sensing. SWNT has several unique advantages such as Near-Infrared (NIR) fluorescence, which enables photo bleaching resistance and detection in deeper tissues. SWNT is usually encased in phenylated derivative of o-phenylenediamine-functionalized dextran which is capable of adsorption onto SWNT for NO sensing [68]. However, it exhibits disadvantage of fluorescence bleaching after reaction with NO. Recently, a novel probe AT15-SWNT based on specific sequence of d(AT)15 oligonucleotides attaching on SWNT has been developed [69]. Though SWNT combined with other DNA sequences show fluorescence enhancement or quenching from analytes such as NADH, dopamine, riboflavin and L-ascorbic acid, d(AT)15 uniquely imparts SWNT with high selectivity and sensitivity for NO. The bases of AT15-SWNT stack on SWNT sidewall through p-p stacking while the phosphate and deoxyribose backbone extends away from SWNT, allowing SWNT to remain colloidal and stable (Figure 18). Fluorescence decrease is generated when the AT15-SWNT is exposed to NO, indicating the dynamics of NO adsorption through SWNT exciton quenching. The electron transfer from SWNT to noncovalently adsorbed NO results in the fluorescence quenching. The wrapping AT15 is responsible for the selectivity of the probe because certain odor molecules can be recognized by the sequence-specific DNA attached on SWNT through electronic resistance change. The adsorption rate as well as the quenched fluorescence intensity is linearly proportional to the concentration of NO.
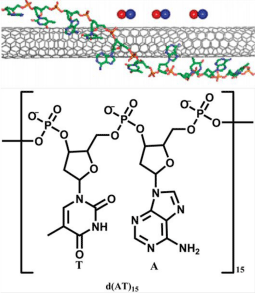
Figure 18: Schematic structure of AT15-SWNT and the unit of d (AT)15. (Reprinted from reference 69. Copyright 2011 American Chemical Society.)
QDs probes-based methods
QDs are fluorescent semiconductor nanocrystals with several unique properties, such as one hundred times stronger fluorescence than organic dyes, photo bleaching resistance and stable fluorescence even exposed to long-time lighting. The QDsbased methods utilizing conjugation of CdSe-ZnS nanocrystals and tris (dithiocarbamato) iron (III) have been reported before for NO sensing [70]. Recently, QDs adsorbed or conjugated on polymers have been developed. QD adsorbed on polymethacrylate surface has been applied for time-resolved fluorescence detection of NO. For instance, 1,6-Hexanedioldimethacrylate (HEX-DMA) is used for the synthesis of polymethacrylate film [71]. The fluorescence is quenched with the increasing concentration of NO. Penetration of QDs into the polymethacrylate flims is very scarce, facilitating the contact of QDs with NO. Another kind of QD-based probe QDs-poly (methyl methacrylate) (PMMA) nanocomposites are synthesized through in situ bulk polymerization [72]. The optical properties of this probe can be tuned and stabilized by varying the molar ratio of QDs to PMMA. The fluorescence intensity does not change obviously when oxygen is introduced because most of QDs are enveloped by PMMA so that the oxygen cannot interact directly with the surface of QDs. In addition, the modified Hyper Branched Polyether (mHP) as the supporting material conjugated QDs is a spherical probe employed for NO donating and real-time detecting [73]. The HP is synthesized by cation ring-opening polymerization then the HP is modified with N-(3-(Trimethoxysilyl) propyl) ethylenediamine (NTPED) to obtain amino groups. The mHP reacts with NO to generate mHP-NO nanospheres containing diazeniumdiolates as NO donors and then conjugated with QDs. The QDs-mHP-NO release NO in physiological environment and the NO molecules diffuse to the surface of nanospheres as well as take advantage of the lone pair electrons of Cd, resulting in the decrease of fluorescence (Figure 19). Apart from these probes, Cellulose Acetate (CA) [74] and Chitosan (CS) [75] can also be utilized to embed QDs for NO detection (Figure 20). However, most of such probes present no selectivity for discrimination of biologically RNOS.
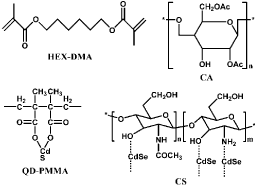
Figure 19: Chemical structure of HEX-DMA, QD-PMMA, CA and CS.
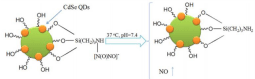
Figure 20: NO release and detection mechanism of QDs-mHP-NO nanopheres. (Reprinted from reference 73. Copyright 2014 Materials Letters.)
To improve the selectivity over other biologically RNOS, QDs modified with ferric ammonium N-(Dithiocarbaxy) sarcosine (DTC) complexes have been designed [76]. The carboxyl of DTC is covalently bound with the amino polymer on the surface of QDs through condensation reaction. The fluorescence of QDs-Fe (III)(DTC)3 is weak as a result of the FRET from QDs to Fe(III)(DTC)3 complexes. NO can displace the DTC in Fe(III)(DTC)3 and reduce Fe(III) to Fe(II), resulting in the obstruction of FRET and greatly enhancing the fluorescence of QDs-Fe(III)(DTC)3 (Figure 21). The specific detection is not interfered with other biologically RNOS. QDs-Fe(III) (DTC)3 is highly resistant to photo bleaching. In addition, though with the presence of Cu(II), Hg(II) and Cd(II) decrease the sensitivity of QDs-Fe(III)(DTC)3 because they quench the fluorescence induced by NO, these metal cations can be removed by simple pretreatment.
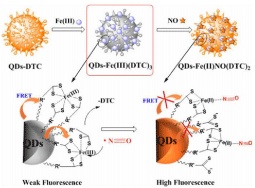
Figure 21: Schematic illustration of method for fluorescent NO detection with QDs-Fe (III)(DTC)3. (Reprinted from reference 76. Copyright 2014 Analytical Chemistry).
Conclusion and Future Perspectives
In this review, the recent advancement of fluorescent methods for the detection of NO based on organic probes, metal complex probes, SWNT probes and QDs probes have been discussed. Specifically, the use of clinical NO measurement as diagnostic and prognostic indicators necessitates inexpensive and convenient devices. Fluorescent NO sensors, more than any other type of NO measurement technique, are well suited to fill this role. In addition to their ease of fabrication and miniaturization, the instrumentation required to perform sensitive measurements is both affordable and portable.
However, considerable challenges remain in synthesizing ideal probes for sensitive detection or in vivo imaging because of the low concentration and short lifetime of NO in physiological environment. More emphasis should be placed on probes exhibiting highly selective to NO and the interaction mechanisms. Furthermore, other factors should be considered in the design of probes for biological systems, such as good solubility, low toxicity, high photo stability, good cellpermeability and high interference-resistance. Probes with NIR fluorescence have a great potential for in vivo NO imaging because of low background and high penetrability. These probes will be significant for understanding the physiological and pathological functions of NO in living systems. In addition, though a continued focus on improving the analytical performance of fluorescent NO sensors is important, future research also must address and improve the ability of NO sensors to resist biofouling for more reliable use in vivo. Indeed, sensors biofouling often results in diminished analytical performance and poor reproducibility. Strategies for improving biocompatibility include passive protection of the sensor through the use of sensor membranes that resist biofouling and polymers that actively release antifouling agents. A most promising approach for reducing biofouling of implantable sensors is based on NOrelease from sensor membranes. Clearly, such a strategy would be problematic for NO sensors.
References
- Ignarro LJ, Buga GM, Wood KS, Byrns RE, Chaudhuri G. Endothelium-derived relaxing factor produced and released from artery and vein is nitric oxide. Proc Natl Acad Sci USA. 1987; 84: 9265-9269.
- Fukumura D, Kashiwagi S, Jain RK. The role of nitric oxide in tumour progression. Nat Rev Cancer. 2006; 6: 521-534.
- Weis M, Kledal TN, Lin KY, Panchal SN, Gao SZ, Valantine HA, et al. Cytomegalovirus infection impairs the nitric oxide synthase pathway: role of asymmetric dimethylarginine in transplant arteriosclerosis. Circulation. 2004; 109: 500-505.
- Johnson RA, Freeman RH. Sustained hypertension in the rat induced by chronic blockade of nitric oxide production. Am J Hypertens. 1992; 5: 919-922.
- Hussain SP, Hofseth LJ, Harris CC. Radical causes of cancer. Nat Rev Cancer. 2003; 3: 276-285.
- Pieper GM. Review of alterations in endothelial nitric oxide production in diabetes: protective role of arginine on endothelial dysfunction. Hypertension. 1998; 31: 1047-1060.
- Nathan C, Xie QW. Nitric oxide synthases: roles, tolls, and controls. Cell. 1994; 78: 915-918.
- Riccio DA, Dobmeier KP, Hetrick EM, Privett BJ, Paul HS, Schoenfisch MH. Nitric oxide-releasing S-nitrosothiol-modified xerogels. Biomaterials. 2009; 30: 4494-4502.
- Hetrick EM, Shin JH, Stasko NA, Johnson CB, Wespe DA, Holmuhamedov E, et al. Bactericidal efficacy of nitric oxide-releasing silica nanoparticles. ACS Nano. 2008; 2: 235-246.
- Thomas DD, Ridnour LA, Isenberg JS, Flores-Santana W, Switzer CH, Donzelli S, et al. The chemical biology of nitric oxide: implications in cellular signaling. Free Radic Biol Med. 2008; 45: 18-31.
- Privett BJ, Shin JH, Schoenfisch MH. Electrochemical nitric oxide sensors for physiological measurements. Chem Soc Rev. 2010; 39: 1925-1935.
- Sun J, Zhang XJ, Broderick M, Fein H. Measurement of nitric oxide production in biological systems by using Griess reaction assay. Sensors. 2003; 3: 276-284.
- Hetrick EM, Schoenfisch MH. Analytical chemistry of nitric oxide. Annu Rev Anal Chem (Palo Alto Calif). 2009; 2: 409-433.
- Huang KJ, Zhang M, Xie WZ, Zhang HS, Feng YQ, Wang H. Sensitive determination of nitric oxide in some rat tissues using polymer monolith microextraction coupled to high-performance liquid chromatography with fluorescence detection. Anal. Bioanal. Chem. 2007; 388: 939-946.
- Neill SJ, Desikan R, Hancock JT. Nitric oxide signalling in plants. New Phytol. 2003; 159: 11-35.
- Desikan R, Cheung MK, Bright J, Henson D, Hancock JT, Neill SJ. ABA, hydrogen peroxide and nitric oxide signalling in stomatal guard cells. J Exp Bot. 2004; 55: 205-212.
- Kojima H, Nakatsubo N, Kikuchi K, Kawahara S, Kirino Y, Nagoshi H, et al. Detection and imaging of nitric oxide with novel fluorescent indicators: diaminofluoresceins. Anal Chem. 1998; 70: 2446-2453.
- Nakatsubo N, Kojima H, Kikuchi K, Nagoshi H, Hirata Y, Maeda D, et al. Direct evidence of nitric oxide production from bovine aortic endothelial cells using new fluorescence indicators: diaminofluoresceins. FEBS Lett. 1998; 427: 263-266.
- Itoh Y, Ma FH, Hoshi H, Oka M, Noda K, Ukai Y, et al. Determination and bioimaging method for nitric oxide in biological specimens by diaminofluorescein fluorometry. Anal Biochem. 2000; 287: 203-209.
- Leikert JF, Räthel TR, Müller C, Vollmar AM, Dirsch VM. Reliable in vitro measurement of nitric oxide released from endothelial cells using low concentrations of the fluorescent probe 4,5-diaminofluorescein. FEBS Lett. 2001; 506: 131-134.
- Mur LA, Mandon J, Cristescu SM, Harren FJ, Prats E. Methods of nitric oxide detection in plants: a commentary. Plant Sci. 2011; 181: 509-519.
- Woitzik J, Abromeit N, Schaefer F. Measurement of nitric oxide metabolites in brain microdialysates by a sensitive fluorometric high-performance liquid chromatography assay. Anal Biochem. 2001; 289: 10-17.
- Gharavi N, El-Kadi AO. Measurement of nitric oxide in murine Hepatoma Hepa1c1c7 cells by reversed phase HPLC with fluorescence detection. J Pharm Pharm Sci. 2003; 6: 302-307.
- Wanga M, Xu ZC, Wang X, Cui JN. A fluorescent and colorimetric chemosensor for nitric oxide based on 1,8-naphthalimide. DYES PIGMENTS. 2013; 96: 333-337.
- Kojima H, Hirotani M, Nakatsubo N, Kikuchi K, Urano Y, Higuchi T, et al. Bioimaging of nitric oxide with fluorescent indicators based on the rhodamine chromophore. Anal Chem. 2001; 73: 1967-1973.
- Gabe Y, Urano Y, Kikuchi K, Kojima H, Nagano T. Highly sensitive fluorescence probes for nitric oxide based on boron dipyrromethene chromophoresRational design of potentially useful bioimaging fluorescence probe. J. Am. Chem. Soc. 2004; 126: 3357-3367.
- Lim MH, Wong BA, Pitcock WH, Mokshagundam D, Baik MH, Lippard SJ. Direct nitric oxide detection in aqueous solution by copper(II) fluorescein complexes. J Am Chem Soc. 2006; 128: 14364-14373.
- McQuade LE, Lippard SJ. Fluorescence-based nitric oxide sensing by Cu(II) complexes that can be trapped in living cells. Inorg Chem. 2010; 49: 7464-7471.
- Gomes A, Fernandes E, Lima JL. Use of fluorescence probes for detection of reactive nitrogen species: a review. J Fluoresc. 2006; 16: 119-139.
- Nagano T, Yoshimura T. Bioimaging of nitric oxide. Chem Rev. 2002; 102: 1235-1270.
- McQuade LE, Lippard SJ. Fluorescent probes to investigate nitric oxide and other reactive nitrogen species in biology (truncated form: fluorescent probes of reactive nitrogen species). Curr Opin Chem Biol. 2010; 14: 43-49.
- Hilderbrand SA, Lim MH, Lippard SJ. Dirhodium tetracarboxylate scaffolds as reversible fluorescence-based nitric oxide sensors. J Am Chem Soc. 2004; 126: 4972-4978.
- Chen X, Tian X, Shin I, Yoon J. Fluorescent and luminescent probes for detection of reactive oxygen and nitrogen species. Chem Soc Rev. 2011; 40: 4783-4804.
- Gomes A, Fernandes E, Lima JL. Fluorescence probes used for detection of reactive oxygen species. J Biochem Biophys Methods. 2005; 65: 45-80.
- Bryan NS, Grisham MB. Methods to detect nitric oxide and its metabolites in biological samples. Free Radic Biol Med. 2007; 43: 645-657.
- Wardman P. Fluorescent and luminescent probes for measurement of oxidative and nitrosative species in cells and tissues: progress, pitfalls, and prospects. Free Radic Biol Med. 2007; 43: 995-1022.
- Miller EW, Chang CJ. Fluorescent probes for nitric oxide and hydrogen peroxide in cell signaling. Curr Opin Chem Biol. 2007; 11: 620-625.
- Kalyanaraman B, Darley-Usmar V, Davies KJ, Dennery PA, Forman HJ, Grisham MB, et al. Measuring reactive oxygen and nitrogen species with fluorescent probes: challenges and limitations. Free Radic Biol Med. 2012; 52: 1-6.
- Pluth MD, Tomat E, Lippard SJ. Biochemistry of mobile zinc and nitric oxide revealed by fluorescent sensors. Annu Rev Biochem. 2011; 80: 333-355.
- Yu H, Xiao Y, Jin L. A lysosome-targetable and two-photon fluorescent probe for monitoring endogenous and exogenous nitric oxide in living cells. J Am Chem Soc. 2012; 134: 17486-17489.
- Dong X, Heo CH, Chen S, Kim HM, Liu Z. Quinoline-based two-photon fluorescent probe for nitric oxide in live cells and tissues. Anal Chem. 2014; 86: 308-311.
- Zhang HX, Chen JB, Guo XF, Wang H, Zhang HS. Highly sensitive determination of nitric oxide in biologic samples by a near-infrared BODIPY-based fluorescent probe coupled with high-performance liquid chromatography. Talanta. 2013; 116: 335-342.
- Zhang HX, Chen JB, Guo XF, Wang H, Zhang HS. Highly sensitive low-background fluorescent probes for imaging of nitric oxide in cells and tissues. Anal Chem. 2014; 86: 3115-3123.
- Gabe Y, Ueno T, Urano Y, Kojima H, Nagano T. Tunable design strategy for fluorescence probes based on 4-substituted BODIPY chromophore: improvement of highly sensitive fluorescence probe for nitric oxide. Anal. Bioanal. Chem. 2006; 386: 621-626.
- Chen JB, Zhang HX, Guo XF, Wang H, Zhang HS. Novel B,O-chelated fluorescent probe for nitric oxide imaging in Raw 264.7 macrophages and onion tissues. Anal Chim Acta. 2013; 800: 77-86.
- Zheng H, Shang GQ, Yang SY, Gao X, Xu JG. Fluorogenic and chromogenic rhodamine spirolactam based probe for nitric oxide by spiro ring opening reaction. Org Lett. 2008; 10: 2357-2360.
- Wu CM, Chen YH, Dayananda K, Shiue TW, Hung CH, Liaw WF, et al. Sensitivity evaluation of rhodamine B hydrazide towards nitric oxide and its application for macrophage cells imaging. Anal Chim Acta. 2011; 708: 141-148.
- Sun C, Shi W, Song Y, Chen W, Ma H. An unprecedented strategy for selective and sensitive fluorescence detection of nitric oxide based on its reaction with a selenide. Chem Commun (Camb). 2011; 47: 8638-8640.
- Yuan L, Lin W, Xie Y, Chen B, Song J. Development of a ratiometric fluorescent sensor for ratiometric imaging of endogenously produced nitric oxide in macrophage cells. Chem Commun (Camb). 2011; 47: 9372-9374.
- Yuan L, Lin W, Xie Y, Chen B, Zhu S. Single fluorescent probe responds to H2O2, NO, and H2O2/NO with three different sets of fluorescence signals. J Am Chem Soc. 2012; 134: 1305-1315.
- Yang Y, Seidlits SK, Adams MM, Lynch VM, Schmidt CE, Anslyn EV, et al. A highly selective low-background fluorescent imaging agent for nitric oxide. J Am Chem Soc. 2010; 132: 13114-13116.
- Gui R, Jiang Y, Huang S. Self-assembly synthesis of magneticfluorescein derivatives for Cu(II)-assisted OFF-ONfluorescence probe of nitric oxide. Mater. Lett. 2014; 132: 436-439.
- Kumar P, Kalita A, Mondal B. Nitric oxide sensors based on copper(II) complexes of N-donor ligands. Inorg. Chim. Acta. 2013; 404: 88-96.
- Anand T, Sivaraman G, Chellappa D. Quinazoline copper(II) ensemble as turn-on fluorescence sensor for cysteine and chemodosimeter for NO. J. Photoch. Photobio. A. 2014; 281: 47-52.
- Mondal B, Kumar P, Ghosh P, Kalita A. Fluorescence-based detection of nitric oxide in aqueous and methanol media using a copper(II) complex. Chem Commun (Camb). 2011; 47: 2964-2966.
- Meghdadi S, Amirnasr M, Amiri A, Mobarakeh ZM, Azarkamanzad Z. Benign synthesis of N-(8-quinolyl)pyridine-2-carboxamide) ligand (Hbpq), and its Ni(II) and Cu(II) complexes: A fluorescent probe for direct detection of nitric oxide in acetonitrile solution based on Hbpq copper(II) acetate interaction. CR Chim. 2014; 17: 477-483.
- Yu M, Wang W, Zhang N. Convenient and selective "off-on" detection nitric oxide in solution and thin film with quinoline based fluorescence sensor. Spectrochim Acta A Mol Biomol Spectrosc. 2014; 126: 329-332.
- Hu X, Zhang X, Song H, He C, Bao Y, Tang Q, et al. A novel copper (?) complex-based fluorescence probe for nitric oxide detecting and imaging. Tetrahedron. 2012; 68: 8371-8375.
- Hu X, Wang J, Zhu X, Dong D, Zhang X, Wu S, et al. A copper(II) rhodamine complex with a tripodal ligand as a highly selective fluorescence imaging agent for nitric oxide. Chem Commun (Camb). 2011; 47: 11507-11509.
- Meng Q, Zhang Y, Hou D, Xin G, Li T, He C, et al. Fluorimetric and colorimetric detection of nitric oxide in living cells by rhodamine derivatives assisted by Cu2+. Tetrahedron. 2013; 69: 636-641.
- McQuade LE, Ma J, Lowe G, Ghatpande A, Gelperin A, Lippard SJ. Visualization of nitric oxide production in the mouse main olfactory bulb by a cell-trappable copper(II) fluorescent probe. Proc Natl Acad Sci USA. 2010; 107: 8525-8530.
- McQuade LE, Lippard SJ. Fluorescence-based nitric oxide sensing by Cu(II) complexes that can be trapped in living cells. Inorg Chem. 2010; 49: 7464-7471.
- Pluth MD, McQuade LE, Lippard SJ. Cell-trappable fluorescent probes for nitric oxide visualization in living cells. Org Lett. 2010; 12: 2318-2321.
- Sartoretto JL, Kalwa H, Romero N, Michel T. In vivo imaging of nitric oxide and hydrogen peroxide in cardiac myocytes. Methods Enzymol. 2013; 528: 61-78.
- Pluth MD, Chan MR, McQuade LE, Lippard SJ. Seminaphthofluorescein-based fluorescent probes for imaging nitric oxide in live cells. Inorg Chem. 2011; 50: 9385-9392.
- Tan L, Wan A, Li H. Fluorescent chitosan complex nanosphere diazeniumdiolates as donors and sensitive real-time probes of nitric oxide. Analyst. 2013; 138: 879-886.
- Wu P, Wang J, He C, Zhang X, Wang Y, Liu T, et al. Luminescent metal-organic frameworks for selectively sensing nitric oxide in an aqueous solution and in living cells. Adv. Funct. Mater. 2012; 22: 1698-1703.
- Kim JH, Heller DA, Jin H, Barone PW, Song C, Zhang J, et al. The rational design of nitric oxide selectivity in single-walled carbon nanotube near-infrared fluorescence sensors for biological detection. Nat Chem. 2009; 1: 473-481.
- Zhang J, Boghossian AA, Barone PW, Rwei A, Kim JH, Lin D, et al. Single molecule detection of nitric oxide enabled by d(AT)15 DNA adsorbed to near infrared fluorescent single-walled carbon nanotubes. J Am Chem Soc. 2011; 133: 567-581.
- Wang S, Han MY, Huang D. Nitric oxide switches on the photoluminescence of molecularly engineered quantum dots. J Am Chem Soc. 2009; 131: 11692-11694.
- Fabrega Vt, Izquierdo MA, Burguete MI, Galindo F, Luis SV. Quantum dot-polymethacrylate composites for the analysis of NOx by fluorescence spectroscopy. Inorg. Chim. Acta. 2012; 381: 212-217.
- Ding L, Li T, Zhong Y, Fan C, Huang J. Synthesis and characterization of a novel nitric oxide fluorescent probe CdS-PMMA nanocomposite via in-situ bulk polymerization. Mater Sci Eng C Mater Biol Appl. 2014; 35: 29-35.
- Liu S, Jin L, Chronakis IS, Li X, Ge M. Hyperbranched polyether hybrid nanospheres with CdSe quantum dots incorporated for selective detection of nitric oxide. Mater. Lett. 2014; 123: 104-106.
- Ding L, Fan C, Zhong Y, Li T, Huang J. A sensitive optic fiber sensor based on CdSe QDs fluorophore for nitric oxide detection. Sens. Actuators. B. 2013; 185: 70-76.
- Tan L, Wan A, Li H, Zhang H, Lu Q. Biocompatible quantum dots chitosan nanocomposites for fluorescence detection of nitric oxide. Mater. Chem. Phys. 2012; 134: 562-566.
- Sun J, Yan Y, Sun M, Yu H, Zhang K, Huang D, et al. Fluorescence turn-on detection of gaseous nitric oxide using ferric dithiocarbamate complex functionalized quantum dots. Anal Chem. 2014; 86: 5628-5632.