
Review Article
Austin J Biosens & Bioelectron. 2015;1(2): 1009.
Hemin/G-Quadruplex Dnazyme for Electrochemical Biosensing
Yanping Gao*, Lin Liu and Ning Xia*
College of Chemistry and Chemical Engineering, Anyang Normal University, China
*Corresponding author: Ning Xia, College of Chemistry and Chemical Engineering, Anyang Normal University, Anyang, Henan 455000, People’s Republic of China
*Corresponding author: Yanping Gao, College of Chemistry and Chemical Engineering, Anyang Normal University, Anyang, Henan 455000, People’s Republic of China
Received: February 20, 2015; Accepted: March 29, 2015; Published: March 31, 2015
Abstract
Guanine quadruplex (G-quadruplex) can bind tightly with hemin to form the hemin/G-quadruplex that displays robust peroxidase activity. Acting as a peroxidase-mimicking DNAzyme, hemin/G-quadruplex has been considered as a promising artificial enzyme for biosensing because of its low cost, high thermal stability as well as easy preparation and modification in the laboratory. Recently, hemin/G-quadruplex–based electrochemical biosensors have been successfully used for the detection of proteins, DNA, metal ions and small molecules. In this short review, we highlighted the recent advances of the design of strategies for the fabrication of hemin/G-quadruplex–based electrochemical biosensors.
Keywords: Electrochemical biosensors; Guanine quadruplex; Hemin; DNAzyme
Abbreviations
G-quadruplex: Guanine quadruplex; HRP: Horseradish Peroxidase; GOx: Glucose Oxidase; PSA: Prostate Specific Antigen; FR: Folate Receptor; Fc: Ferrocene; MB: Methylene Blue; RCA: Rolling Circle Amplification; HCR: Hybridization Chain Reaction; EXPAR: Exponential Amplification Reaction; miRNA: microRNA; AuNPs: Gold Nanoparticles; Pt@PdNWs: Pt@Pd Nanowires; PdNPs: Pd Nanoparticles; rGO: reduced Gaphene Oxide; SWPN.b: Pebrine disease related Spore Wall Protein of Nosemabombycis; ADH: Alcohol Dehydrogenase; TBA: Thrombin Binding Aptamer; HPtCoNCs: PtCo Nanochains; FeTe NRs: iron Telluride Nanorods; PtNTs: Platinum Nanotubes; GDH: Glucose Dehydrogenase
Introduction
In recent years, enzyme-amplified electrochemical biosensors have attracted considerable attention and have emerged as viable alternatives to conventional spectrophotometric enzyme affinity assays for detection of trace amounts of biomarkers in biological studies, clinical diagnostics, and treatment. Commonly, peroxidase and phosphatase are the used enzyme labels in connection to electrochemical monitoring of the biocatalytic reaction product. The enzymatic reaction may follow two strategies: one in which the current is the electro catalytic response of a redox couple serving as a substrate to a redox enzyme label and another in which an electrochemically active product of the enzyme label is detected. Guanine-rich nucleic acid sequence can fold into Guanine quadruplex (G-quadruplex) that is found to complex tightly with hemin to form the hemin/G-quadruplex. Under physiological conditions, the hemin/G-quadruplex displays robust peroxidase activity. Recently, the hemin/G-quadruplex peroxidase-mimicking DNAzyme has been considered as a promising artificial enzyme for biosensing due to its low cost, high stability and simple preparation procedure in the laboratory. In this short review, we highlighted the recent advances of the design of strategies for the fabrication of hemin/G-quadruplex– based electrochemical biosensors.
Hemin/G-quadruplex–based electrochemical biosensors
Willner’s group, for the first time, demonstrated that the hemin/ G-quadruplex Horseradish Peroxidase (HRP)-mimicking DNAzyme on electrode exhibited bioelectrocatalytic functions toward the electro catalyzed reduction of H2O2 [1]. The electro catalytic properties of the DNAzyme were then used to develop electrochemical sensing platforms for analyzing the activities of enzymes and their substrates, DNA-sensors and aptasensors. The simplest methodology was to attach Glucose Oxidase (GOx) to the electrode surface through a nucleic acid sequence able to form a G-quadruplex structure in the presence of hemin (Figure 1) [1]. The GOx mediated the glucose oxidation to gluconic acid and H2O2 and the resulting H2O2 was analyzed through its electro catalyzed reduction by the DNAzyme. The success of the initial efforts provides good motivation for further manipulation of kinds of hemin/G-quadruplex–based electrochemical biosensors for detection of DNA [1], proteins (e.g. thrombin, recombinant human IFN-g) [2,3] and metal ions [4,5]. The most common strategy consists on modification of the gold electrode by a hairpin nucleic acid oligonucleotides that contains both a sequence capable to form G-quadruplex and an aptamer able to specifically bind the analyte. In the presence of the analyte and hemin, the hairpin structures were opened, the analyte bound to the aptamer part, and hemin/G-quadruplex structures were formed on the electrode surface. Furthermore, sandwich-like electrochemical biosensors with single hemin/G-quadruplex DNAzyme or hemin/Gquadruplex DNAzyme wires containing many units of hemin/Gquadruplex DNAzyme as the signal tags have also been developed for detection of Prostate Specific Antigen (PSA) and telomere [6], cisplatin (a cytotoxic and antineoplastic metallodrug) [7] as well as Folate Receptor (FR) [8]. In the DNAzyme electrochemical assays, redox reporters such as Ferrocene (Fc) and Methylene Blue (MB) could be used as the electron-transfer mediators for the DNAzyme to improve the sensitivity [9-11].
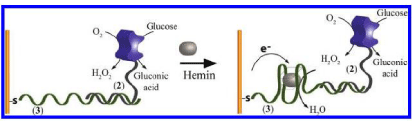
Figure 1: Sensing of glucose through the bioelectrocatalyzed reduction of
H2O2 generated by the GOx-mediated oxidation of glucose using a HRPmimicking
DNAzyme dybridized to a nucleic acid-functionalized GOx (From
reference [1], with permission. Copyright E 2014 American Chemical Society).
Although the hemin/G-quadruplex–based electrochemical biosensors have been constructed with high simplicity and good reproducibility, single signal amplification by DNAzyme is not sufficient for detecting the ultra-low concentration of analytes. Thus, a number of research groups have been exploring various methods to improve the sensitivity by dual-amplification, such as multi-enzyme cooperation systems, redox cycling reactions and/ or nanomaterials. Among kinds of multi-enzyme cooperation systems, signal amplification by hemin/G-quadruplex DNAzyme plus Rolling Circle Amplification (RCA) [12], isothermal amplification [13], Hybridization Chain Reaction (HCR) [14] and the isothermal Exponential Amplification Reaction (EXPAR) [15] attracted researchers’ considerable attention to design sensitive detection methods for assay of targets. Typically, Yu et al. reported an electrochemical biosensor based on the arched probe mediated isothermal EXPAR (Figure 2) [15]. The arched probe consisted of two strands (Strand 1 and Strand 2), which were partially complementary at both ends. Strand 2 prevented the hybridization of primer with Strand 1 and Strand 1 minimized the formation of any nonspecific hemin-containing G-quadruplex from Strand 2. The recognition site of the nicking endonuclease was located in the loop region of Strand 2 and was unsuitable to bind with the enzyme. Target miRNA was complementary to the 5' stem region and part of the loop region of Strand 1. The separation of one hybridized domain through the formation of a target-substrate complex led to the thermal melting of the remaining duplex. After cleavage of the arched probe, the free Strand 2 bound to hemin to form the hemin/G-quadruplex DNAzyme on the surface of the electrode. On the other hand, Strand 1 hybridized with the target was released to the solution and initiated a series of cyclic chain amplification reactions. Once the engaging primer annealed with the complementary region of Strand 1, the polymerase initiated the polymerization, which regenerated the target miRNA and synthesized a DNA duplex. As a result, the displaced miRNA was free to bind to another probe and triggered a new cycle for recycling the target and forming a DNA duplex as well. The DNA duplex generated above activated the recognition site of the nicking enzyme. After the nicking endonucleases nicked at the DNA duplex, the polymerization started and the primer part got extended plots of electrode modification at different stages.
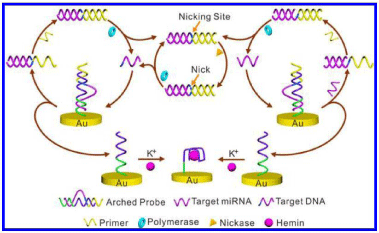
Figure 2: Schematic illustration of the arched probe mediated EXPAR
strategy based on polymerase and nicking endonucleases for the miRNA
assay (from reference [15], with permission. Copyright E 2014 American
Chemical Society).
As an essential aspect of bioanalysis, signal amplification by employing nanomaterials and hemin/G-quadruplex DNAzyme has been successfully achieved for sensitive detection of biorecognition events. Typically, gold Nanoparticles (AuNPs) have been widely used in diagnostics and detection because of their unique characteristics, such as high surface-to-volume ratio, high surface energy, ability to decrease proteins-metal particles distance, and the functioning as electron conducting pathways between prosthetic groups and the electrode surface. Many groups reported the hemin/Gquadruplex– based electrochemical biosensors with AuNPs as the carriers to load large amount of signal labels [16-18] and/or as the electrode materials [14,19-27] to increase the electrode surface and enhance the biocompatibility, electro catalysis and redox property of electrode. Because of the extraordinary physicochemical and structural properties of carbon nanomaterials, such as grapheme and carbon nanotubes, these exciting new materials have quickly sparked tremendous interests in electrochemical biosensors. The hemin/G-quadruplex functionalized graphene and carbon nanotubes nanocomposites have been successfully utilized as the signal labels for electrochemical biosensing [28-32]. More interestingly, recently, a few of novel strategies for designing of hemin/G-quadruplex electrochemical biosensors took advantage of the DNAzyme acting both as a NADH oxidase assisting the oxidation of NADH to NAD+ together with the generation of H2O2 in the presence of dissolved O2 as well as a HRP-mimicking DNAzyme to bioelectrocatalyze the reduction of the produced H2O2 [22,33]. Typically, Yuan’s group was the first to construct an electrochemical aptasensors for thrombin detection with the hemin/G-quadruplex acting as an NADH oxidase and HRP-mimicking DNAzyme simultaneously in 2012 [22]. Lately, by using autonomously assembled hemin/G-quadruplex DNAzyme nanowires [23], hemin/G-quadruplex functionalized Pt@Pd nanowires (Pt@PdNWs) as well as thionine and hemin/G-quadruplex functionalized Pd Nanoparticles (PdNPs)-reduced Gaphene Oxide (rGO) (denoted as PdNPs-rGO) [29] and dendrimer-rGO [30] for signal amplification, they reported several pseudobienzyme electrochemical biosensors for the detection of thrombin, Hg2+ and Pebrine disease related Spore Wall Protein of Nosemabombycis (SWPN.b). The results indicated that the signal amplification efficiency of a bienzyme-catalyzed amplification system was obviously superior to that of a monoenzyme-catalyzed amplification system. For this consideration, Yuan’s group further reported several hemin/G-quadruplex triple-enzyme-catalyzed amplification systems [26,34-37]. For example, with the amplification of Alcohol Dehydrogenase (ADH)-Pt–Pd nanowires bionanocomposite and the hemin/G-quadruplex structure acting as NADH oxidase and HRPmimicking DNAzyme simultaneously, they developed a pseudo triple-enzyme cascade electrocatalytic electrochemical aptasensors for thrombin detection (Figure 3) [26]. With the addition of ethanol to the electrolyte, the ADH immobilized on the Pt–Pd nanowires catalyzed ethanol to acetaldehyde accompanied by NAD+ being converted to NADH. Then the hemin/G-quadruplex firstly served as NADH oxidase, converting the produced NADH to NAD+ with the concomitant local formation of high concentration of H2O2. Subsequently, the hemin/G-quadruplex acted as HRP-mimicking DNAzyme, bioelectrocatalyzing the produced H2O2 At the same time, the Pt–Pd nanowires employed in the strategy not only provided a large surface area for immobilizing Thrombin Binding Aptamer (TBA) and ADH, but also served as HRP-mimicking DNAzyme which rapidly bioelectrocatalyzed the reduction of the produced H2O2 Thus, such a pseudo triple-enzyme cascade electrochemical aptasensor could greatly promote the electron transfer of hemin and resulted in the dramatic enhancement of electrochemical signal. Also, with the exciting properties of gaphene, they reported a hemin/G-quadruplex triple-enzyme-catalyzed amplification system with ADH– and hemin/G-quadruplex–functionalized rGO-AuNPs nanocomposites as the carriers [34]. Moreover, based on the Hollow PtCo Nanochains (HPtCoNCs) functionalized by bi-enzyme-HRP mimicking DNAzyme and GOx [35] and Fe3O4–Au nanocomposites functionalized by GOx and hemin/G-quadruplex [37] as the signal enhancers, Yuan’s group also developed two highly sensitive electrochemical thrombin aptasensors. Furthermore, they fabricated an ultrasensitive sandwich-type electrochemical aptasensor forthrombin based on a triplex signal amplification strategy of hemin/ G-quadruplex, blocking reagent-HRP and iron Telluride Nanorods (FeTe NRs) [36]. With porous Platinum Nanotubes (PtNTs) labeled with hemin/G-quadruplex and Glucose Dehydrogenase (GDH) as signal labels, Sun et al. presented a sensitive electrochemical aptasensor for thrombin detection [38]. In the presence of glucose and NAD+, GDH catalyzed the oxidation of glucose with the production of NADH. Then, hemin/G-quadruplex as NADH oxidase catalyzed the oxidation of NADH to in situ generate H2O2 Based on the corporate electro catalysis of PtNTs and hemin/G-quadruplex toward H2O2, the electrochemical signal was significantly amplified.
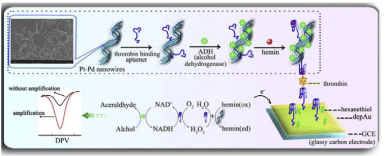
Figure 3: The schematic diagrams of fabricated pseudo triple-enzyme
electrochemical aptasensor based on the electrochemical signal amplification
of Pt–Pd nanowires and hemin/G-quadruplex (From reference [26], with
permission. Copyright E 2014 Elsevier Science B.V.).
Conclusion
Hemin/G-quadruplex DNAzyme is a promising artificial enzyme in the design of electrochemical biosensors because of its outstanding advantages over natural enzymes, such as low cost, simple preparation procedure and high thermal stability. Over the past years, hemin/G-quadruplex DNAzyme has been a constant source of inspiration for chemists in their efforts to develop hemin/ G-quadruplex–based electrochemical biosensors in view of the advantages of electrochemical techniques. Although the applications of hemin/G-quadruplex DNAzyme are still at the basic research level, the rapid progress in the field and the multidisciplinary applications of these biocatalytic materials hold great promises for additional developments.
References
- Pelossof G, Tel-Vered R, Elbaz J, Willner I. Amplified biosensing using the horseradish peroxidase-mimicking DNAzyme as an electrocatalyst. Anal Chem. 2010; 82: 4396-4402.
- Shen B, Wang Q, Zhu D, Luo J, Cheng G, He P, et al. G-quadruplex-based DNAzymes aptasensor for the amplified electrochemical detection of thrombin. Electroanalysis. 2010; 24: 2985-2990.
- Zhang H, Jiang B, Xiang Y, Chai Y, Yuan R. Label-free and amplified electrochemical detection of cytokine based on hairpin aptamer and catalytic DNAzyme. Analyst. 2012; 137: 1020-1023.
- Pelossof G, Tel-Vered R, Willner I. Amplified surface plasmon resonance and electrochemical detection of Pb2+ ions using the Pb2+-dependent DNAzyme and hemin/G-quadruplex as a label. Anal Chem. 2012; 84: 3703-3709.
- Zhang Z, Yin J, Wu Z, Yu R. Electrocatalytic assay of mercury(II) ions using a bifunctional oligonucleotide signal probe. Anal Chim Acta. 2013; 762: 47-53.
- Liu J, Lu CY, Zhou H, Xu JJ, Wang ZH, Chen HY. A dual-functional electrochemical biosensor for the detection of prostate specific antigen and telomerase activity. Chem Commun (Camb). 2013; 49: 6602-6604.
- Wang G, He X, Chen L, Zhu Y, Zhang X, Wang L. Conformational switch for cisplatin with hemin/G-quadruplex DNAzyme supersandwich structure. Biosens Bioelectron. 2013; 50: 210-216.
- Wang G, He X, Wang L, Zhang X. A folate receptor electrochemical sensor based on terminal protection and supersandwich DNAzyme amplification. Biosens Bioelectron. 2013; 42: 337-341.
- Tang J, Hou L, Tang D, Zhang B, Zhou J, Chen G. Hemin/G-quadruplex-based DNAzyme concatamers as electrocatalysts and biolabels for amplified electrochemical immunosensing of IgG1. Chem Commun (Camb). 2012; 48: 8180-8182.
- Kaneko N, Horii K, Kato S, Akitomi J, Waga I. High-throughput quantitative screening of peroxidase-mimicking DNAzymes on a microarray by using electrochemical detection. Anal Chem. 2013; 85: 5430-5435.
- Wang G, Chen L, Zhu Y, Wang L, Zhang X. Adenosine triphosphate sensing by electro catalysis with DNAzyme. Electroanalysis. 2014; 26: 312-318.
- Wang Q, Yang C, Xiang Y, Yuan R, Chai Y. Dual amplified and ultrasensitive electrochemical detection of mutant DNA Biomarkers based on nuclease-assisted target recycling and rolling circle amplifications. Biosens Bioelectron. 2014; 55: 266-271.
- Zhang X, Wu D, Liu Z, Cai S, Zhao Y, Chen M, et al. An ultrasensitive label-free electrochemical biosensor for microRNA-21 detection based on a 2'-O-methyl modified DNAzyme and duplex-specific nuclease assisted target recycling. Chem Commun (Camb). 2014; 50: 12375-12377.
- Xie S, Chai Y, Yuan Y, Bai L, Yuan R. A novel electrochemical aptasensor for highly sensitive detection of thrombin based on the autonomous assembly of hemin/G-quadruplex horseradish peroxidase-mimicking DNAzyme nanowires. Anal Chim Acta. 2014; 832: 51-57.
- Yu Y, Chen Z, Shi L, Yang F, Pan J, Zhang B, et al. Ultrasensitive electrochemical detection of MicroRNA based on an arched probe mediated isothermal exponential amplification. Anal Chem. 2014; 86: 8200-8205.
- Zhou J, Lai W, Zhuang J, Tang J, Tang D. Nanogold-functionalized DNAzyme concatamers with redox-active intercalators for quadruple signal amplification of electrochemical immunoassay. ACS Appl Mater Interfaces. 2013; 5: 2773-2781.
- Xu M, Zhuang J, Chen X, Chen G, Tang D. A difunctional DNA-AuNP dendrimer coupling DNAzyme with intercalators for femtomolar detection of nucleic acids. Chem Commun (Camb). 2013; 49: 7304-7306.
- Wang G, Chen L, Zhu Y, He X, Xu G, Zhang X. Development of an electrochemical sensor based on the catalysis of ferrocene actuated hemin/G-quadruplex enzyme for the detection of potassium ions. Biosens Bioelectron. 2014; 61: 410-416.
- Jiang B, Wang M, Li C, Xie J. Label-free and amplified aptasensor for thrombin detection based on background reduction and direct electron transfer of hemin. Biosens Bioelectron. 2013; 43: 289-292.
- Meng X, Zhou Y, Liang Q, Qu X, Yang Q, Yin H, et al. Electrochemical determination of microRNA-21 based on bio bar code and hemin/G-quadruplet DNAenzyme. Analyst. 2013; 138: 3409-3415.
- Zhang J, Chai Y, Yuan R, Yuan Y, Bai L, Xie S, et al. A novel electrochemical aptasensor for thrombin detection based on the hybridization chain reaction with hemin/G-quadruplex DNAzyme-signal amplification. Analyst. 2013; 138: 4558-4564.
- Yuan Y, Yuan R, Chai Y, Zhuo Y, Ye X, Gan X, et al. Hemin/G-quadruplex simultaneously acts as NADH oxidase and HRP-mimicking DNAzyme for simple, sensitive pseudobienzyme electrochemical detection of thrombin. Chem Commun (Camb). 2012; 48: 4621-4623.
- Yuan Y, Gao M, Liu G, Chai Y, Wei S, Yuan R. Sensitive pseudobienzyme electrocatalytic DNA biosensor for mercury(II) ion by using the autonomously assembled hemin/G-quadruplex DNAzyme nanowires for signal amplification. Anal Chim Acta. 2014; 811: 23-28.
- Wang Q, Song Y, Chai Y, Pan G, Li T, Yuan Y, et al. Electrochemical immunosensor for detecting the sporewall protein of Nosemabombycis based on the amplification of hemin/G-quadruplex DNAzyme concatamers functionalized Pt@Pd nanowires. Biosens Bioelectron. 2014; 60: 118-123.
- Yuan Y, Chai Y, Yuan R, Zhuo Y, Gan X. An ultrasensitive electrochemical aptasensor with autonomous assembly of hemin-G-quadruplex DNAzyme nanowires for pseudo triple-enzyme cascade electrocatalytic amplification. Chem Commun (Camb). 2013; 49: 7328-7330.
- Zheng Y, Chai Y, Yuan Y, Yuan R. A pseudo triple-enzyme electrochemical aptasensor based on the amplification of Pt-Pd nanowires and hemin/G-quadruplex. Anal Chim Acta. 2014; 834: 45-50.
- Peng K, Zhao H, Yuan Y, Yuan R, Wu X. Mediator-free triple-enzyme cascade electrocatalytic aptasensor with exonuclease-assisted target recycling and hybridization chain reaction amplification. Biosens Bioelectron. 2014; 55: 366-371.
- Li Y, Deng J, Fang L, Yu K, Huang H, Jiang L, et al. A novel electrochemical DNA biosensor based on HRP-mimicking hemin/G-quadruplex wrapped GOx nanocomposites as tag for detection of Escherichia coli O157:H7. Biosens Bioelectron. 2015; 63: 1-6.
- Xie S, Chai Y, Yuan R, Bai L, Yuan Y, Wang Y. A dual-amplification aptasensor for highly sensitive detection of thrombin based on the functionalized graphene-Pd nanoparticles composites and the hemin/G-quadruplex. Anal Chim Acta. 2012; 755: 46-53.
- Yuan Y, Liu G, Yuan R, Chai Y, Gan X, Bai L. Dendrimer functionalized reduced graphene oxide as nanocarrier for sensitive pseudobienzyme electrochemical aptasensor. Biosens Bioelectron. 2013; 42: 474-480.
- Zhang J, Chai Y, Yuan R, Yuan Y, Bai L, Xie S. A highly sensitive electrochemical aptasensor for thrombin detection using functionalized mesoporous silica@multiwalled carbon nanotubes as signal tags and DNAzyme signal amplification. Analyst. 2013; 138: 6938-6945.
- Yuan Y, Gou X, Yuan R, Chai Y, Zhuo Y, Mao L, et al. Electrochemical aptasensor based on the dual-amplification of G-quadruplex horseradish peroxidase-mimicking DNAzyme and blocking reagent-horseradish peroxidase. Biosens Bioelectron. 2011; 26: 4236-4240.
- Golub E, Freeman R, Willner I. A hemin/G-quadruplex acts as an NADH oxidase and NADH peroxidase mimicking DNAzyme. Angew Chem Int Ed Engl. 2011; 50: 11710-11714.
- Yi H, Xu W, Yuan Y, Bai L, Wu Y, Chai Y, et al. A pseudo triple-enzyme cascade amplified aptasensor for thrombin detection based on hemin/G-quadruplex as signal label. Biosens Bioelectron. 2014; 54: 415-420.
- Bai L, Yuan R, Chai Y, Yuan Y, Zhuo Y, Mao L. Bi-enzyme functionlized hollow PtCo nanochains as labels for an electrochemical aptasensor. Biosens Bioelectron. 2011; 26: 4331-4336.
- Jiang L, Yuan R, Chai Y, Yuan Y, Bai L, Wang Y. An ultrasensitive electrochemical aptasensor for thrombin based on the triplex-amplification of hemin/G-quadruplex horseradish peroxidase-mimicking DNAzyme and horseradish peroxidase decorated FeTe nanorods. Analyst. 2013; 138: 1497-1503.
- Jing P, Xu W, Yi H, Wu Y, Bai L, Yuan R. An amplified electrochemical aptasensor for thrombin detection based on pseudobienzymic Fe3O4-Au nanocomposites and electroactive hemin/G-quadruplex as signal enhancers. Analyst. 2014; 139: 1756-1761.
- Sun A, Qi Q, Wang X, Bie P. Porous platinum nanotubes labeled with hemin/G-quadruplex based electrochemical aptasensor for sensitive thrombin analysis via the cascade signal amplification. Biosens Bioelectron. 2014; 57: 16-21.