
Review Article
Austin J Biosens & Bioelectron. 2015;1(3): 1011.
Molecularly Imprinted Polymers (Mips) for Bioanalytical Sensors: Strategies for Incorporation of Mips into Sensing Platforms
Marloes Peeters*
Queen Mary University of London, School of Biological and Chemical Sciences, UK
*Corresponding author: Marloes Peeters, Queen Mary University of London, School of Biological and Chemical Sciences, Mile End Road, E1 4NS London, United Kingdom
Received: February 26, 2015; Accepted: April 21, 2015; Published: May 11, 2015
Abstract
Molecularly Imprinted Polymers (MIPs) are synthetic receptors which have very beneficial properties compared to natural antibodies; they are robust, low-cost, have a high specificity, and can even detect their target molecules in complex matrices. MIPs for bioanalytical sensors are seemingly a perfect fit, but their use is limited due to the challenging incorporation of these receptors into sensing devices. In this review, various functionalization strategies will be discussed depending on the polymerization techniques that are employed and the morphology that is required for the sensor system. Furthermore, an outlook is given into using graphene based systems as sensor platforms since they could enhance the binding capacity of the MIPs.
Keywords: Molecularly Imprinted Polymers (MIPs); Biosensors; Polymerization techniques
Abbreviations
ATRP: Atom-Transfer Radical-Polymerization; GO: Graphene Oxide; HPLC: High Performance Liquid Chromatography; MIPs: Molecularly Imprinted Polymers; NMP: Nitroxide Mediated Polymerization; PVC: Polyvinylchloride; PDMS: Polydimethylsiloxane; RAFT: Reversible Addition-Fragmentation Chain Transfer
Introduction
Molecularly Imprinted Polymers are synthetic receptors containing recognition sites with a predetermined selectivity for various substances, ranging from ions, to neurotransmitters, proteins, and even whole cells [1-4]. Their specificty and selectivity towards their target molecule is similar to natural antibodies, but MIPs are superior in terms of their long-sterm stability, chemical inertness, and their ability to withstand extremes of pH and temperature [5-7]. In this review, we will focus on different functionalization strategies for MIPs targeted for small molecules since for the detection of larger molecules, in general surface imprinting techniques have the preference [8,9]. First, a two step process is discussed in which first the MIP particles are polymerized first and then attached to an electrode surface via a different procedure. Second, direct polymerization of the MIP particles is reviewed, which seems more straightforward but also significantly complicates the polymerization process. Finally, in recent years there has been a growing interest into graphene and graphene oxide and this material, becaue of its high surface area, could potentially improve the binding capacity of the imprints.
MIP functionalization onto sensor surface via a two step process
Bulk polymerization: micron sized particles: The most common method to produce MIPs to date is by bulk polymerization. While this might not seem the most elegant approach, it can be widely applied and offers a straight-forward synthesis [10,11]. Following this approach, all the components including monomers, template, crosslinker molecules, and initiator are dissolved into an appropriate porogen. The mixture is then polymerized by exposure to UV radiation or heat and a rigid block is obtained, which afterwards is processed by grinding and sieving [12]. The resulting material consists of micron sized particles which have irregular shapes with heterogenic parts due to the lack of control during the reaction [13]. The latter is not necessarily a drawback, because particles up to sizes of 25 micron can be directly packed into separation columns. Anderson et al. [14] demonstrated that a column equipped with MIP particles in the High-Performance Liquid Chromatography (HPLC) mode could not only separate similar structures of carbobenzoxy-aspartic acid, but could also discriminate between their enantiomeric forms. Huang et al. [15] followed a similar procedure and showed the separation of enantiomers and diastereomers of cinchona alkaloids by using a molecularly imprinted monolithic stationary phase. For sensing purposes, the functionalization strategy is less straightforward and also depends on the type of read-out technique that is employed.
Tan et al. [16] suspended MIP powders obtained by bulk polymerization into a mixture of the solvent tetrahydrofuran and Polyvinylchloride (PVC) powder. The resulting fluid was applied onto a Ag-electrode and rotated at a certain speed. After evaporation of the solvent in air, a MIP coating was formed which could detect concentrations of 25 nM L-nicotine in double-distilled water with microgravimetric read-out. A similar approach was followed Thoelen et al. [17], but instead of spincoating the MIP powers and a polymer together, first a thin layer (~200 nM) of a conjugated polymer was spincoated onto the electrodes. Then, the MIP particles were transferred onto the surface using a poly (dimethylsiloxane) PDMS stamp. Subsequently, the substrate is heated above the glass transition temperature of the polymer layer, allowing the MIP particles to sink into the layer. After cooling down, the particles are trapped into the matrix and can be used for sensing purposes (Figure 1). This technique can be compared to the conformation of an iceberg in the water, hence can be classified as the “iceberg model”.
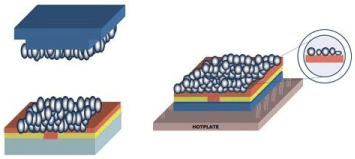
Figure 1: Immobilization method for the MIPs based on the so called “ice-berg
method”. First, a PDMS stamp with MIP particles is applied to the surface and
subsequently, the particles will sink into the polymer film layer due to heating
of the adhesive layer above the glass transition temperature [17].
In this case, also MIPs targeted for L-nicotine were employed and with impedance spectroscopy as read-out technique the attained detection limit was in the low nanomolar regime. This technique can be transferred towards MIPs for other template molecules [18,19], but also the solvent used during the spincoating is altered. For instance, for gravimetric and optical read-out techique there is no necessity for a conjugated polymer and commercially available PVC can be used [20]. The bulk polymerization process has some distinct drawbacks; there is no control over the polymerization or over the binding sites, the grinding process is labour intense, the yield is poor because much material is lost during the sieving, and scale up might be troublesome [21,22]. Therefore, other polymerization methods have been studied which will result in smaller and more regular beads.
Other polymerization techniques: towards sub micron sized particles: Suspension polymerization with liquid perfluorocarbon as the dispersing phase results can be used to obtain homogeneous beads. By adusting the amount of stabilizing polymer present, the diameter of the particles can be varied between 5 and 50 micrometer. While there are no heterogeneous binding sites in the material present as compared to MIPs obtained by bulk polymerization process, no significant improvement was found when performing sensing experiments [23]. Another issue is that the particles are still large, and to optimize this another technique has to be employed. Precipitation polymerization allowed to scale down and Ye et al. [24] demonstrated with radioligand binding analysis that the MIP microspheres have a higher for their target molecules than MIP powders which were obtained by traditional grinding and sieving procedures . Their method is highly efficient with a high yield and can be transferred to other template molecules, however precipitation polymerization requires a high amount of solvent and of the template and is therefore not cost efficient. Emulsion polymerization, the formation of small beads in an oil-water phase which is stabilized by a surfactant, could potentially overcome these issues. There are different approaches, for instance the group of Whitcombe followed a procedure in which the imprint molecule is part of the surfactant and therefore all binding sites were present at the surface [25]. This resulted into excellent specifity towards the target molecule and to particle sizes below 100 nm. All of the described polymerization techniques, however, do not establish full control over the formation of the binding sites. Controlled radical polymerization techniques, such as atom transfer radical polymerization [26], Nitroxide-Mediated Polymerization (NMP) [27], Reversible Addition-Fragmentation Chain-Transfer (RAFT) [28], and iniferter-mediated polymerization [29], have fast activation-deactivation cycles and allow to control the growth and termination of the polymerization. In this manner, new MIP structures can be designed, especially because with a living system it is possible to construct multiple successive polymer layers. However, with crosslinked system such as MIPs this becomes more complicated and, moreover, especially for ATRP and NMP not all the monomers used for MIP synthesis are compatible with these techniques. In the following paragraph, an example will be given of ATRP prepared MIPs as this system is extremely suitable for direct growing onto the surface. In the case of NMP, reports in literature are sparse but MIPs have beendesigned for cholesterol-imprinted polymers and they displayed a higher binding affinity compared to MIPs prepared by traditional radical polymerization [27]. Likewise, for the iniferter technique few examples can be found, however Pérez-Moral and Mayes reported on the synthesis of particles with a polystyrene core and different complex polymer shells which could recognize its target molecule, the drug propranolol. The RAFT polymerization technique is more versatile and is used widely. One of the issues in using MIPs for biosensors is water compatability; to achieve this, the surface has to be hydrophilic and this involves post-modification of the MIPs [30]. With RAFT, narrowly dispersed water-compatible MIP microspheres can be obtained via a one-pot method which is a significant improvement [31]. While great improvements have been made in the area of size, and control over the binding sites, still polymer particles or beads are obtained after this procedure and functionalization onto sensor surfaces requires a two step process. Therefore, for sensing applications it is also very interesting to look at direct polymerization onto the surface of the substrate.
Direct MIP functionalization onto sensor surfaces
MIPs can be synthesized in situ at an electrode surface via electropolymerization.First attempts were made on gold with phenolic monomers, but the layers were thick and uncovered areas had to blocked with other molecules in order to prevent non-specific binding [32,33]. Significant improvements have been made in the field, allowing to deposit films at precise spot of the sensor surface with even a complex geometry [34]. The additional benefit is that the thickness and density can be easily regulated by changing the voltage. The electrodes are proven to be stable, responses are reproducible, and the selectivity is high, but often detection limits are only in the order of micron/micromolar range which is often not within the physiologically relevant regime [35]. There are various strategies to lower this detection limit, one solution could be to use a two-step process. Lenain et al. [36] first developed sub micron spheres by emulsion polymerization and then coupled it to electrode surfaces via electropolymerization, enabling the trace detection of metergoline in driniking water. Hybrid architectures can also be obtained by combining molecularly imprinted polymers with enzymes or a self-assembled monolayer. Yarman et al. demonstrated for the first time the integration of an enzyme with a MIP layer in a sensor configuration which is new step towards characterizing and detecting electroactive proteins such as cytochrome c [37]. For non-conducting surfaces, surface patterns can be created by photopolymerization [38] or grafting techniques can be employed.Photo polymerization, like electropolymerization, allows precise protocol over the shape, spatial resolution and size of the patterns, and can also allow to deposit different MIPs onto a surface so parallelization is achieved. Guillon et al. [39] described a simple method with a low-cost of the cheap fabrication, with the possibility of mass production and turning sensors into portable devices. Controlled techniques can also be employed to directly graft MIPs onto surfaces. For instance, silica can be immobilized with an initiator and then the MIP can be directly grafted onto the silica by surface-initated ATRP [40]. The resulting MIPs showed a high binding affinity for 17β-estradiol and could even be used for the trace determination in complicated beef samples.
Graphene and graphene oxide MIP hybrids: an outlook to enhancing the binding capacity of MIP sensors
It is interesting to consider grafting MIPs onto graphene or graphene oxide as they posses a high-surface-to-volume ratio, possess unique mechanical and electrical properties, and they can be selectively deposited onto other electrode surfaces [41,42]. Because of the enhancement of the surface area, it is easy to remove the template and binding capacity is increased. Mao et al. [43] used free radical techniques and simply dispersed graphene sheets with template and functional monomers into an organic solvent in order to obtain a MIP for dopamine. More sophisticated methods can be found when Graphene Oxide (GO) is used, as the oxyen functionality allows facile chemical modification of the surface. MIP-GO hybrids have been synthesized by both ATRP and RAFT polymerization. Chang et al. succesfully developed a MIP for 2,4-dichlorophenol, but prepation time was long and copper catalyst removal is complicated, which could have a negative effect when working with neurotransmitters or living cells [44]. A first MIP onto GO by RAFT polymerization was described by Li et al. [45], who used its potential use in nano electromechanical devices [45]. Peeters et al. developed a novel synthesis method, tremendously cutting back preparation time, but in order to transfer the GO particles onto the sensor surface an additional functionalization step was required [46]. If this procedure could be performed onto GO which is selectively developed onto surfaces, this could be of significant interest for bioanalytical sensing applications.
Conclusion
There are many polymerization techniques for MIPs, not to mention there are also various functionalization strategies. It is not an easy task to select the optimal method and this also depends entirely on the morpohology that is required for the sensor application. In Table 1, dection limis are summarized for the different polymerization techniques and different surface functionalization strategies.
Two step functionalization
Surface functionalization method
Transducer
Target
Detection limit
Ref
bulk polymerization
“iceberg method”
microgravimetry
L-nicotine
25 nM
Tan et. al [16]
bulk polymerization
“iceberg method”
impedance spectroscopy
L-nicotine
~1 nM
Thoelen et. al [17]
precipitation polymerization
direct packing
radioligand binding assay
caffeine, theophylline
~50-100 nM
Ye et al. [24]
emulsion polymerization
direct packing
HPLC
cholesterol
~mM range
Pérez et al. [25]
Direct functionalization onto surface
electropolymerization
-
capacitive sensor
phenylalanine
~mM range
Panasyuk et al. [33]
cyclic voltammetry deposition
-
differential pulse voltammetry
paracetamol
5 μM
Özcan et al. [34]
emulsion polymerization (electrografting)
-
HPLC
metergoline
~1 μM
Lenain et al. [36]
surface-initiated ATRP
-
HPLC
17β-estradiol
~nM
Gong et al. [40]
MIP graphene/graphene oxide hybrids
free radical polymerization
absorption onto electrode
chronoamperometry
dopamine
100 μM
Mao et al. [43]
ATRP
dispersion of particles, measuring resulting solution
HPLC/ UV-Vis
2,4,-dichlorophenol
~μM
Chang et al. [44]
surface initiated RAFT polymerization
“iceberg method”
heat-transfer method
histamine
25 nM
Peeters et al. [46]
Table 1: Summary of the obtained dection limits according to different polymerization techniques and different functionalization strategies.
From Table 1 is directly clear that the detection limit of the target is not only determined by its polymerization technique or functionalization strategy, but also the transducer that is used is of great significance. However, it has to be noted that direct surface functionalization allows a better control over the imprint structure and significantly reduces preparation time; therefore, it seems the most promising option for future perspectives. Besides this observation, it also directly becomes clear that while there are only recents attempts of forming hybrid structures of MIPs with graphene or graphene oxide, their detection limits are already comparable to more traditional sensors. This means that the beneficial properties of graphene, such as large surface area and good electrical and thermical conductivity, could also offer benefits for future MIP sensing and more research has to be conducted to construct sensor platforms with even lower detection limits and a more straightforward functionalization procedure.
References
- Mosbach K. Molecular imprinting. Trends Biochem Sci. 1994; 19: 9-14.
- Ng SM, Narayanaswamy R. Fluorescence sensor using a molecularly imprinted polymer as a recognition receptor for the detection of aluminium ions in aqueous media. Anal Bioanal Chem. 2006; 386: 1235-1244.
- Mayes AG, Mosbach K. Molecularly imprinted polymers: useful materials for analytical chemistry? TrAC Trends Anal Chem. 1997; 16: 321-332.
- Bers K, Eersels K, van Grinsven B, Daemen M, Bogie JF, Hendriks JJ, et al. Heat-transfer resistance measurement (HTM)-based cell detection at trace levels using a progressive enrichment approach with highly selective cell-binding surface imprints. Langmuir. 2014; 1: 3631-3639.
- Rampey AM, Umpleby RJ, Rushton GT II, Iseman JC, Shah RN, Shimizu KD. Characterization of the imprint effect and the influence of imprinting conditions on affinity, capacity, and heterogeneity in molecularly imprinted polymers using the Freundlich isotherm-affinity distribution analysis. Anal Chem. 2004; 76: 1123-1133.
- Svenson J, Nicholls IA. On the thermal and chemical stability of molecularly imprinted polymers. Anal Chim Acta. 2001; 435: 19-24.
- Spivak DA. Optimization, evaluation, and characterization of molecularly imprinted polymers. Adv Drug Deliv Rev. 2005; 57: 1779-1794.
- Hayden O, Dickert FL. Selective microorganism detection with cell surface imprinted polymers. Adv Mater. 2001; 13: 1480-1483.
- Dickert FL, Hayden G. Bioimprinting of polymers and sol-gel phases. Selective detection of yeasts with imprinted polymers. Anal Chem. 2002; 74: 1302-1306.
- Haupt K. Molecularly imprinted polymers: the next generation. Anal Chem. 2003; 75: 376-383.
- O'Mahony J, Wei S, Molinelli A, Mizaikoff B. Imprinted polymeric materials. Insight into the nature of prepolymerization complexes of quercetin imprinted polymers. Anal Chem. 2006; 78: 6187-6190.
- Arshady R, Mosbach K. Synthesis of substrate-selective polymers by host-guest polymerization. Macromol Chem Phys. 1981; 182: 687-692.
- Gao N, Niu QJ, Du RK. Preparation and recognition performance of cytisine alkaloid-imprinted material prepared using novel surface molecular imprinting technique. J Sep Sci. 2010; 33:1338-1348.
- Andersson LI, Mosbach K. Enantiomeric resolution on molecularly imprinted polymers prepared with only non-covalent and non-ionic interactions. J Chromatogr. 1990; 516: 313-322.
- Huang X, Zou H, Chen X, Luo Q, Kong L. Molecularly imprinted monolithic stationary phases for liquid chromatographic separation of enantiomers and diastereomers. J Chromatogr A. 2003; 984: 273-282.
- Tan Y, Yin J, Liang C, Peng H, Nie L, Yao S. A study of a new TSM bio-mimetic sensor using a molecularly imprinted polymer coating and its application for the determination of nicotine in human serum and urine. Bioelectrochemistry. 2001; 53: 141-148.
- Thoelen R, Vansweevelt R, Duchateau J, Horemans F, D'Haen J, Lutsen L, et al. A MIP-based impedimetric sensor for the detection of low-MW molecules. Biosens Bioelectron. 2008; 23: 913-918.
- Peeters M, Troost FJ, van Grinsven B, Horemans F, Alenus J, Murib MS, et al. MIP-based biomimetic sensor for the electronic detection of serotonin in human blood plasma. Sens Actuators B: Chem. 2012; 171- 602-610.
- Peeters M, Troost FJ, Mingels RHG, Welsch T, van Grinsven B, Vranken T, et al. Impedimetric detection of histamine in bowel fluids using synthetic receptors with pH-optimized binding characteristics. Anal Chem. 2013; 85: 1475-1483.
- Alenus J, Ethirajan A, Horemans F, Weustenraed A, Csipai P, Peeters M, et al. Molecularly imprinted polymers as synthetic receptors for the QCM-D-based detection of L-nicotine in diluted saliva and urine samples. Anal Bioanal Chem. 2013; 405: 6479-6487.
- Haupt K, Mosbach K. Molecularly imprinted polymers and their use in biomimetic sensors. Chem Rev. 2000; 100: 2495-2504.
- Maier NM, Lindner W. Chiral recognition applications of molecularly imprinted polymers: a critical review. Anal Bioanal Chem. 2007; 389: 377-397.
- Mayes AG, Mosbach K. Molecularly imprinted polymer beads: suspension polymerization using a liquid perfluorocarbon as the dispersing phase. Anal Chem. 1996; 68: 3769-3774.
- Ye L, Weiss R, Mosbach K. Synthesis and characterization of molecularly imprinted microspheres. Macromolecules. 2000; 33: 8239-8245.
- Pérez N, Whitcombe MJ, Vulfson EN. Molecularly imprinted nanoparticles prepared by core-shell emulsion polymerization. J Apl Pol Sci. 2000; 77; 1851-1859.
- Matyjaszewski K, Xia J. Atom transfer radical polymerization. Chem Rev. 2001; 101: 2921-2990.
- Boonpangrak S, Whitcombe MJ, Prachayasittikul V, Mosbach K, Ye L. Preparation of molecularly imprinted polymers using nitroxide-mediated living radical polymerization. Biosens Bioelectron. 2006; 22: 349-354.
- Chiefari J, Chong YK, Ercole F, Krstina J, Jeffery J, Tam PTL, et al. Living free-radical polymerization by reversible addition-fragmentation chain transfer: The RAFT process. Macromolecules. 1998; 31: 5559-5562.
- Otsu T, Yoshida M. Role of initiator-transfer agent-terminator (iniferter) in radical polymerizations: Polymer design by organic disulfides as iniferters. Makromol Chem Rap Commun. 1982; 3: 127-132.
- Pérez-Moral N, Mayes AG. Molecularly imprinted multi-layer core-shell nanoparticles – a surface grafting approach. Macromol Rap Commun. 2007; 28: 2170-2175.
- Pan G, Zhang Y, Ma Y, Li C, Zhang H. Efficient one-pot synthesis of water-compatible molecularly imprinted polymer microspheres by facile RAFT precipitation polymerization. Angew Chem Int Ed Engl. 2011; 50: 11731-11734.
- Cheng Z, Wang E, Yang X. Capacitive detection of glucose using molecularly imprinted polymers. Biosens Bioelectron. 2001; 16: 179-185.
- Panasyuk TL, Mirsky VM, Piletsky SA, Wolfbeis OS. Electropolymerized molecularly imprinted polymers as receptors layers in capacitive chemical sensors. Anal Chem. 1999; 71: 4609-4613.
- Özcan L, Sahin Y. Determiniation of a paracetamol based on electropolymerized-molecularly imprinted polypyrrole modified pencil graphite electrode. Sens Act B: Chem. 2007; 127: 362-369.
- Wang Q, Paim LL, Zhang X, Wang S, Stradiotto NS. An electrochemical sensor for reducing sugars based on a glassy carbon electrode modified with electropolmyerized molecularly imprinted poly-o-phenyldiamine film. Electroanalysis. 2014; 26: 1612-1622.
- Lenain P, De Saeger S, Mattiasson B, HedstrÖm M. Affinity sensor based on immobilized molecular imprinted synthetic recognition elements. Biosens Bioelectron. 2015; 69C: 34-39.
- Yarman A, Dechtrirat D, Bosserdt M, Jetzschmann KJ, Gajovic-Eichelmann N, Scheller FW. Cytochrome c-derived hybrid systems based on molecularly imprinted polymers. Electroanalysis. 2015; 27: 1-15.
- Fuchs Y, Soppera O, Haupt K. Photopolymerization and photostructuring of molecularly imprinted polymers for sensor applications--a review. Anal Chim Acta. 2012; 717: 7-20.
- Guillon S, Lemaire R, Linares AV, Haupt K, Ayela C. Single step patterning of molecularly imprinted polymers for large scale fabrication of microbiochips. Lab Chip. 2009; 9: 2987-2991.
- Gong Y, Niu Y, Gong X, Ma M, Ren X, Zhu W, et al. Preparation of 17β-estradiol-imprinted material by surface-initiated atom transfer radical polymerization and its application. J Sep Sci. 2015; 38: 1254-1261.
- Park S, Ruoff RS. Chemical methods for the production of graphenes. Nat Nanotechnol. 2009; 4: 217-224.
- Burg BR, Lutolf F, Schneider J, Schirmer NC, Schwamb T, Poulikakos D. High-yield dielectrophoretic assembly of two-dimensional graphene nanostructures. Appl Phys Lett. 2009; 94: 053110.
- Mao Y, Bao Y, Gan S, Li F, Niu L. Electrochemical sensor for dopamine based on a novel graphene-molecular imprinted polymers composite recognition element. Biosens Bioelectron. 2011; 28: 291-297.
- Chang L, Wu S, Chen S. Preparation of a graphene oxide-molecularly imprinted polymer composites via atom transfer radical polymerization. J Mater Sci. 2011; 46: 2024-2029.
- Li C, Han J, Chang RY, Benewicz BC. Versatile method to prepare RAFT agent anchored substrates and the preparation of PMMA grafted nanoparticles. Macromol. 2006; 39: 3175-3183. sdew
- Peeters M, Kobben S, Jiménez-Monroy KL, Modesto L, Kraus M, Vandenryt T, et al. Thermal detection of histamine with a graphene oxide based molecularly imprinted polymer platform prepared by reversible addition-fragmentation chain transfer polymerization. Sens Actuat B: Chem. 2014; 203: 527-535.