
Research Article
Austin J Biosens & Bioelectron. 2016; 2(1): 1017.
Multi Targets Detecting Based on Glucose Monitoring Platform
Xiulan Zhang¹ and Jamie Hu¹*
¹Changzhou HT Biotechnology Inc. China
²Shanghai Bioscan Inc, China
*Corresponding author: Jamie Hu, Biosensorist, Shanghai Bioscan Inc. Room 302, Gumei Business Centre, 1905 Longming Road, Shanghai 201101, China
Received: February 22, 2016; Accepted: April 04, 2016; Published: April 27, 2016
Abstract
The enzyme sensor field has grown greatly since 1962. Today’s biosensor market is dominated by glucose electrodes which are used for the rapid detection of blood sugar levels by diabetes sufferers. In this paper we take a comprehensive review on the inception, growth and development of the enzyme sensor field from a commercial viewpoint. The current status of the technology is evaluated and future trends that multi targets enzyme sensor i.e. cholesterol, lactate and ethanol enzyme sensors in this dynamic and fast moving field are also discussed.
Keywords: Biosensor; Enzyme; Screen-Printing; Electrochemistry; Amperometry; Glucose; Cholesterol; Lactate; Ethanol
Abbreviations
GOx: Glucose Oxidise; ChOx: Cholesterol Oxidise; ChEt: Cholesterol Esterase; LOx: Lactate Oxidise; AlOx: Alcohol Oxidize; GDH: Glucose Dehydrogenase; LDH: Lactate Dehydrogenase; HEC: Hydroxyethyl Cellulose; BSA: Bovine Serum Albumin; POD: Horseradish Peroxidase; SPEs: Screen-printed electrodes; Med-: Mediator (oxidation state); Med+: Mediator (reduction state); CV: Cyclic Voltammetry
Introduction
One of the main challenges facing the bio-analytical chemist is the development of different methods that respond to the growing need to perform rapid tests. These methods must be sensitive and accurate, and able to determine various substances with different properties in living samples. Lab on chip is a great idea but it is difficult to fabricate and expensive for a simple test. Recent years, electrochemical techniques have been much considered as routine methods due to their high sensitivity and selectivity, portable fieldbased size and low-cost. For example, glucose meter and glucose sensor is the most successful monitoring system for diabetic products, which account for approximately 95% of the current Chinese market for biosensors, and have been estimated at approximately $120–150 million. The reasons why the blood glucose market was particularly receptive are numerous, but the biggest factor is the prevalence of diabetes in China. 140 million adults with diabetes were confirmed and a further 230 million adults were also found with pre-diabetes (120 million men and 110 million women). These results indicate that diabetes has become a major public health problem in China, and national strategies aimed at preventing, detecting, and treating diabetes are urgently needed. In the absence of a cure for diabetes, home blood glucose monitoring will need to continue, and the current commercial dominance of mediated electrochemical biosensors will not be easily replaced. Since diabetic patients need to control their blood glucose levels carefully, the importance of self-monitoring of blood glucose has been widely recognized as an effective method of measuring blood sugar not only in clinics but also at home and in the working place.
Cardiovascular diseases in people are increasing day by day and cardiac arrest is a major cause of death in universal consensus. There are several causes for this but one of the most important reasons is hypercholesterolemia i.e. the increased concentration of cholesterol in the blood [1,2]. The development of efficient rapid analytical methods is important. HPLC [3], gas–liquid chromatography [4,5] methods used for the determination of total cholesterol offer sensitivity and selectivity but are neither suitable for rapid nor cost effective detection.
Enzymatic procedures have practically replaced the chemical methods based on the classical Libermann–Burchard reaction, used traditionally for free and total cholesterol determination [6]. Owing to the advantages of simplicity, rapidness and cost effectivity, a few cholesterol biosensors have been developed which are based on Cholesterol Oxidase (ChOx) and Cholesterol Esterase (ChEt) [7-14], , Cytochrome P450scc [15], fiber-optic biosensor [16,6] and acoustic wave [17]. Lactate levels in human blood or tissues are correlated to the presence of several diseases such as tissue hypoxia, cardiogenic or endotoxic shocks, respiratory failure and systemic disorders derived from neoplasic diseases, liver and renal failures or diabetes mellitus.
Thus, the development of highly sensitive and reliable lactate monitoring methods are of great interest in clinical diagnostics [18,19], food technology [20], beverage and fermentation industries [21] and clinical medicine [22,23]. Various procedures have been proposed for the direct measurement and monitoring of lactic acid levels in very different samples. The fundamentals, advantages and disadvantages of some of these methodologies have been summarized and compared in a recent review [24]. Among them, biosensor is one of the most widely investigated devices for two fundamental reasons: (1) the immobilization of an enzyme on the electrode surface may be done in an easy and reproducible way; and (2) the specificity of enzymes allows analytical measurements to be performed directly on the sample, regardless of their complexity, reducing the analysis time. Biosensors based on L-lactate Oxidase (LOx) and L-lactate Dehydrogenase (LDH) has been widely used for the determination of l-lactate [25-27] and allows the determination of hydrogen peroxide generated in the enzymatic reaction [28].
The measurement of ethanol plays an important role in the quality control of alcoholic beverages during the fermentation process. It is also necessary to have rigorous analytical methods for ethanol in beverages for tax regulation purposes. Meanwhile, there are a large number of alcoholics in China which ignore the traffic regulations and life safety. The quick test of blood alcohol is important in this country trying to give a warning to those people after drink too much.
A variety of methods and strategies had been reported for the determination of ethanol including: gas chromatography [29], liquid chromatography detection [30], refractometry [31], spectraphotometry [32] based on the detection of NADH. These methods are relatively expensive, complex to perform and time consuming; therefore, alternative approaches are desirable. We had a solution in this work that an amperometric biosensor might offer such an alternative [33] as this device can be fabricated at low cost and any lay person could use it easily.
As these four substances mentioned above need to be tested in great amount daily, we described the development and fabrication of four enzyme electrodes (glucose, cholesterol, lactate and ethanol) integrated into a single strip by using screen-printing technology. The multi-targets test strip can be used to detect four substances in one drop of blood sample based on amperometric principle. This method offers the possibility of mass-production of biosensors at easy way and very cost effective. There have many investigations and studies for the four kinds of biosensors respectively, but the rest three products are rare commercially available in the market.
Technologies
Since the 1990s, screen-printing technology, adapted from the microelectronics industry, has offered high-volume production of extremely inexpensive, and yet highly reproducible and reliable single-use sensors; a technique which holds great promise for onsite and point of care test. Therefore, the use of screen printing technology in the serial production of disposable low-cost electrodes for the electrochemical determination of a wide range of substances is currently undergoing widespread growth [34,35].
Screen-Printed Electrodes (SPEs) are devices that are produced by printing different inks on various types of plastic or ceramic substrates. Plastic material is commonly used as it is easy to cut into different shape. Polyester screens are generally used for printing with patterns designed by the analyst in accordance with the analytical purpose in mind. The composition of the various inks used for printing on the electrodes determines the selectivity and sensitivity required for each analysis. Alternatively, a wide variety of devices of this type are commercially available. The great versatility presented by the SPEs lies in the wide range of ways in which the electrodes may be modified. The composition of the printing inks may be altered by the addition of very different substances such as metals, enzymes, polymers, complexion agents, etc. On the other hand, the possibility also exists of modifying the manufactured electrodes by means of depositing various substances on the surface of the electrodes such as metal films, polymers, enzymes and mediators, etc. [36,37].
Enzymes
Enzymes were the first biocatalysts used in biosensors and remain by far the most commonly employed. Clark and Lyons [38] were the pioneers who showed that an enzyme could be integrated into an electrode, thus making a biosensor for the determination of glucose. Since then, enzymes have been extensively used in biosensor construction [39]. Disposable biosensors based on enzyme immobilization on SPEs have been widely used for the analysis of several analytes.
Enzyme inks are made of different oxidase with binder, stabilizer, mediator and surfactant etc. Glucose Oxidase (GOD), Cholesterol Oxidase (ChOD), Lactate Oxidase (LOD) and Alcoholic Oxidase (AlOD) enzyme inks were printed separately in different working areas on a single strip. Figure 1 shows an enzyme ink product and Table 1 lists the normal ingredient of an enzyme ink.
Figure 1: Enzyme ink.
Enzyme
Binder
Stabilizer
Mediator
Surfactant
Buffer
GOx, ChOx, LOx, AlOx
HEC
DCM5
BSA
Animal glue
Sodium glutamate
Ferrocene, ferricyanide,
ferrocyanide, thionine, phenothiazine
Triton X-100
Sodium cholate
Tween 80
Phosphate buffer
Table 1: Ingredient of enzyme inks.
Mediators
A typical electrochemical reaction for a mediated oxidation proceeds in three steps. The enzyme takes part in first redox reaction with the substrate and is then re-oxidized by a mediator. Finally the mediator is re-oxidized by the electrode. In this paper, a variety of reversible electron acceptors have been studied as mediators for redox enzymes. The electron transfer mediator has to satisfy the following requirements: (i) the redox potential of the mediator should be small enough to avoid interfering electrochemical reactions, (ii) both oxidized and reduced forms of mediator should be stable enough and (iii) the second-order rate constant for reaction between mediator and enzyme should be high enough to minimize competition with oxygen [40]. Consequently, the choice of the mediator is critical to achieve high sensitivity and selectivity.
Various chemicals can be used as electron transfer mediators for redox enzymes when immobilized on an electrode surface. Ferrocene and its derivatives, ferri/ferrocyanide, complexes of transition metals such as osmium and ruthenium as well as redox organic dyes are widely used redox mediators for oxidases. Ferri/ferrocyanide is one of the most commonly used and efficient soluble inorganic mediators [41-44].
In this paper, several mediators such as ferrocene, ferri/ ferrocyanide, thionine, and phenothiazine et al. have been studied for different redox enzymes (GOx, ChOx, LOx, AlOx) and the best mediator for different biosensors was chosen after rigorous investigation. The enzyme reactions and the electron transfer processes are showed as below.
(a) Glucose + Med- + GOx→ Gluconicacid + Med+ + H2O2
H2O2 → 2H+ + 2e−
(b) Cholesterol +O2 +H2O+CHOX→cholest-4-en-3-one + H2O2
H2O2 +H++ 2 Med++POD→2 Med-+2K++2H2O
2K++2 Med-+2e-→2 Med+
(c) Lactate + Med- + LOx→ Pyruvate + Med+ + H2O2
H2O2 → 2H+ + 2e−
(d) CH2CH2OH + Med- + AlOX →CH2CHO + Med+ + H2O2
H2O2 → 2H+ + 2e−
Fabrications
Screen printing inks: In this paper, a flat semi-automatic printer is used to print working and reference electrode on surface of a plastic support, and then insulation ink layer is printed forming a working and reference reaction area and leave the area open in order to print enzyme ink covering on it. It will lead a good reproducibility for detection and decrease the noise happening in enzyme reaction. After printing, printed working and reference electrode should be dried in a conveyor oven at 75. Then the insulation ink is printed on the finished base working and reference electrode, the UV generator is used to dry the ink in seconds. The enzyme ink printing is the last stage of the processing; the ink should be printed in well controlled conditions such as temperature and humidity. The processing of the enzyme ink presents a great advantage: it avoids problems of enzyme denaturation as it is not necessary to employ organic solvents, and the curing temperature is therefore minder. It enables the production of test strip to be increased to half-million pieces per day, which leads to a considerable reduction in operation cost. This processing technology for large-scale production of enzyme sensor has been successfully commercialized in local industries [45].
In this paper, carbon inks were printed both as working and reference electrode for glucose biosensor, but for cholesterol/lactate/ ethanol biosensor, the carbon material is only used as working electrode. Ag/AgCl ink was chosen as a reference electrode for cholesterol/lactate/ethanol biosensors to improve the sensitivity of electrodes.
Assembling and cutting procedures: After semifinal electrode products are fabricated, double bonding, single bonding and hydrophilic membrane materials were assembled on the electrode substrate to form a sample inlet spacer in which blood sample could be automatically suck in quantitatively by capillarity. Also the conductive part of the strip is sealed leaving the strip contact opening. An automatic cutting machine is used to cut the assembled strips into single one which were stored into a strip vial with desiccant. It can be stable for about 18 months without opening and 3 months after first opening. Figure 2 shows the 4 in 1 strips produced from the company.
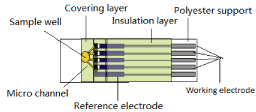
Figure 2: Four in one test strip.
Result and Discussion
A bio-potentiostat was used for cyclic voltammetry and chronoamprometric measurements.
Figure 3 shows the result of glucose CV curve. Figure 4 shows the response current of glucose biosensor. Figure 5 shows the linear range of glucose biosensor.
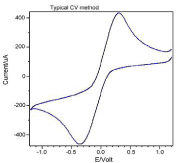
Figure 3: Cyclic voltammograms for glucose sensor in 50 mM ferri/
ferrocyanide and 100 mM KCl support electrolyte. Scan rate at 100 mV/s.
Ercon carbon ink.

Figure 4: Response current of the glucose sensor against time.
Glucose concentration: (a) 2.8mM, (b) 5.6mM,(c) 11.1mM, (d) 16.6mM, (e)
22.2mM.
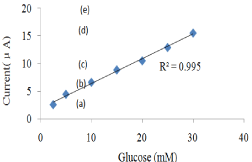
Figure 5: Linear range for different glucose concentration.
Figure 6 shows the result of cholesterol CV curve. Figure 7 shows the response current of cholesterol biosensor. Figure 8 shows the linear range of cholesterol biosensor.
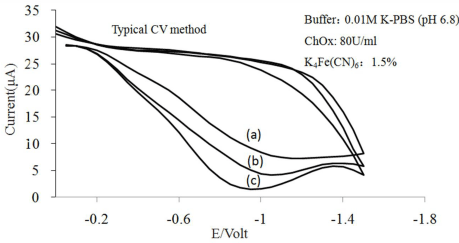
Figure 6: Cyclic voltammograms for different cholesterol concentration of
cholesterol sensor.
Scan rate at 100 mV/s. (a) Blank, (b) 7.76mM, (c) 12.94mM.
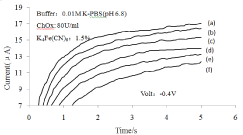
Figure 7: Response current of the cholesterol sensor against time.
Cholesterol concentration: (a)Blank, (b)2.59mM (c) 5.18mM, (d) 7.76mM, (e)
10.35mM, (f) 12.94mM.
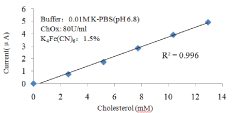
Figure 8: Linear range for different cholesterol concentration.
Figure 9 shows the response current of lactate biosensor. Figure 10 shows the linear range of lactate biosensor.
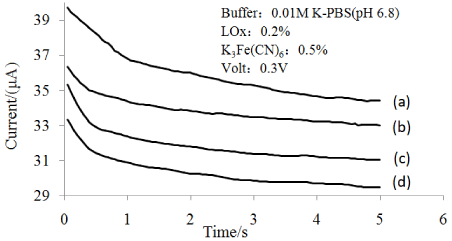
Figure 9: Response current of the lactate sensor against time. Lactate
concentration (a) 2mM, (b) 4mM, (c) 6mM, (d) 8mM.
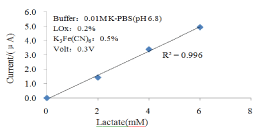
Figure 10: Linear range for different lactate concentration.
Table 2 shows the linear ranges and the sensitivities of the glucose/cholesterol/lactate biosensors. The glucose biosensor has a linear range from 1mM to 33.3mM. The cholesterol biosensor has a detection range from 2.59 mM to 12.94 mM and the lactate biosensor can detected from 0.5mM to 6mM which covers the whole clinical criteria of human blood cholesterol and lactate level, but the sensitivity need to be further improved.
Linear range
Sensitivity
Glucose biosensor
1~33.3mM
430nA/mM
Cholesterol biosensor
2.59~12.94 mM
351nA/mM
Lactate biosensor
0.5 to 6mM
650nA/mM
Table 2: The linear ranges and the sensitivities of the glucose/cholesterol/lactate biosensors.
Selectivity
Enzyme specificity is a key property which can be exploited in biosensor technology. Compared with chemical catalysts, enzymes demonstrate a significantly greater level of substrate specificity, primarily because of the constraints placed on the substrate molecule by the active site environment. This fact involves factors such as molecular size, stereochemistry, polarity, functional groups and relative bond energies.
GOD has higher substrate specificity than PQQ-GDH, because the later can react with maltose, galactose and maltotriose. Table 3 the enzyme properties from the manufacturer show GOD having better substrate specificity. Table 4 and 5 show the substrate specificity of cholesterol oxidase and alcohol oxidase from manufacturer. Although some chemical substances can react with ChOx or AlOx as a substrate, they are very low concentration in human blood so we can detect targets without big interference.
Substrate(0.1M)
Relative activity
D-Glucose
100
2-Dexy- D-glucose
16.2
Glucono-1,5-lactone
0.06
Galactose
3.10
Mannose
2.10
Fructose
0.24
Xylose
0.93
Maltose
0.69
Table 3: Substrate specificity of glucose oxidase from the manufacturer.
Substrate(0.1mM)
Relative activity
Cholesterol
100
Pregenolone
53
β-Cholestanol
63
Stigmasterol
33
Ergosterol
37
Lanosterol
2
Dehydroiso-androsterone
23
Table 4: Substrate specificity of cholesterol oxidase from manufacturer.
Substrate(0.1mM)
Relative activity (%)
Ethanol
79.3
n-Propanol
46.3
Iso- Propanol
25.8
n-Butanol
39.6
From aldehyde
48.2
Table 5: Substrate Specificity of Alcohol oxidase from manufacturer.
Applications
Multi-targets electrode like, glucose, cholesterol, lactate and alcohol can be integrated and fabricated into a single strip by screenprinting technology. All of targets mentioned above have similar electrochemical determination principle which can be detected based on the amperometry assay. Not only so, most of elderly patient with chronic diseases needs to test these chemicals every day, They are suffered from needle puncture for sampling blood, if we can integrate these four test targets into single strip (4 in 1), it will be a large market because these is no commercial multi-targets enzyme electrode product right now and this novel four-in-one super test strip will be very helpful to the people suffer from diabetes, hyperlipidemia, cardiovascular diseases and alcoholics.
Future perspective
Geriatric diseases such as diabetes, hypertension, hyperlipidemia and cardiovascular are becoming epidemic diseases to affect the life of seniors. People suffered from these diseases are looking forward to have a smart device which uses tiny sample blood and reads multiple data back in seconds [46]. This four in one test strip obviously satisfies with the requirement.
Furthermore, most of people over the world have a long history of drinking, people like drinking for various reasons and leading to certain diseases. Although administration has issued different traffic management regulations against drunk driving for years, people still want to know their blood alcohol level after drink with luck psychology. The quick test of blood alcohol based on this monitoring platform is trying to give a warning to those people after drink too much. In this paper, screen-printing technology was used to fabricate a “four-in-one” integrated electrochemical biosensor to measure blood glucose, total cholesterol, blood lactate and blood alcohol in one shoot. For patients, it may be a better way to detect these targets by one piece of “four-in-one” super test strip and only need 1-5μL blood sample, 5-10 seconds time needed.
Until now, we have good results from glucose, cholesterol and lactate from the single strip. We have made a breakthrough progress about the screen-printing technology for four targets and figured out a scheme for these four electrodes. All of this shows that we can print at least four kinds of enzyme in different areas on one strip to create four current responses in separated circuit without interference. It is really expected that the “four-in-one” super test strip will be in mass produced and commercialized in near future.
Conclusion
The 4 in1 test strip is compatible with commercially available platform of glucose testing system. This improvement substantially increases the range of options for four analytes based on the principle of electrochemical detection for applications in resource-constrained environments.
This electrochemical system has several potential advantages.
(i) It is portable reliable, and inexpensive. (ii) It takes advantage of the sophisticated engineering already embedded in commercially available, inexpensive glucose testing platform. (iii) It can be adapted to analytes other than glucose. (iv) The electrochemistry is insensitive to local conditions, and to certain types of contaminants (suspended solids) present in samples. (v) It can be interfaced with a cell phone (either by human reporting of the data or by photography of the LCD display or, in principle, by a coded interface). It can also be used in home patient care with telephone or internet communications. (vi) It can also be in principally adapted to a range of different types of assays (amperometry, as here chronoamperometry, cyclic voltammetry, anodic stripping voltammetry, and others) This 4 in 1 test strip with four useful characteristics for applications in resourcelimited environments. (i) It is well suited to mass production at low cost using screen-printing technology. It can, therefore, be manufactured almost everywhere, and easily adapted to local use. (ii) Its fundamental design is sufficiently simple that this design can be modified to generate test strips that fit into different analytes. (iii) It is based on a system of plastic-based support which is easily cut into different shape. (iv) It has sufficient flexibility to manufacture in mass, a half million test strips can be produced in single day which is very cost effective with four different functionalities—concentration of analytes by the technology At this stage of development, the 4 in 1 strip that we have fabricated are slightly less accurate than single used commercial test strips, possibly due to the manual procedures we used in their fabrication; this accuracy will certainly improve with further engineering and attention to manufacturing detail specially in the processing in four kinds of enzymes deposit on the same surface of a single strip. We are aware that production process of the strip might be sensitive to temperature and humidity. A systematic study needs to be carried out to achieve a better understanding on this issue; this problem, however, can be fixed by improved engineering, such as well controlled humidity and temperature conditions in clean room for test strip production.
References
- Zicha J, Kunes J, Devynck MA. Abnormalities of membrane function and lipid metabolism in hypertension: a review. Am J Hypertens. 1999; 12: 315-331.
- Foster R, Cassidy J, O'Donoghue E. Electrochemical diagnostic strip device for total cholesterol and its subfraction. Electroanalysis. 2000; 12: 716–721.
- Wong WW, Hachey DL, Clarke LL, Zhang S, Llaurador M, Pond WG. An improved HPLC method to purify erythrocyte cholesterol for estimation of in vivo cholesterol synthesis using the deuterium method. Appl Radiat Isot. 1994; 45: 529-533.
- Blomhoff JP. Serum cholesterol determination by gas-liquid chromatography. Clin Chim Acta. 1973; 43: 257-265.
- Agullo E, Susna B. Gas–liquid chromatographic determination of total free cholesterol in egg pastas. Food Res Int. 1996; 29: 77–80.
- Marazuela MD, Cuesta B, Moreno-Bondi MC, Quejido A. Free cholesterol fiber-optic biosensor for serum samples with simplex optimization. Biosens Bioelectron. 1997; 12: 233-240.
- Li G, Liao JM, Hu GQ, Ma NZ, Wu PJ. Study of carbon nanotube modified biosensor for monitoring total cholesterol in blood. Biosens Bioelectron. 2005; 20: 2140-2144.
- Singh S, Chaubey A, Malhotra BD. Amperometric cholesterol biosensor based on immobilized cholesterol esteresa and cholesterol oxidase on conducting polypyrrole films. Anal Chim Acta. 2004; 502: 229–234.
- Tan X, Li M, Cai P, Luo L, Zou X. An amperometric cholesterol biosensor based on multiwalled carbon nanotubes and organically modified sol-gel/chitosan hybrid composite film. Anal Biochem. 2005; 337: 111-120.
- Vidal JC, Espuelas J, Garcia-Ruiz E, Castillo JR. Amperometric cholesterol biosensors based on the electropolymerization of pyrrole and the electrocatalytic effect of Prussian-Blue layers helped with self-assembled monolayers. Talanta. 2004; 64: 655-664.
- Brahim S, Nariresingh D, Guiseppi_Elie A. Amperometric determination of cholesterol in serum using a biosensor of cholesterol oxidase contained within a polypyrrole–hydrogel membrane. Anal Chim Acta. 2001; 448: 27-36.
- Vidal JC, Espuelas J, Castillo JR. Amperometric cholesterol biosensor based on an immobilized monolayer of flavin adenine dinucleotide cofactor. Anal Biochem. 2004; 333: 88-98.
- Ram MK, Bertoncello P, Ding H, Paddeu S, Nicolini C. Cholesterol biosensors prepared by layer-by-layer technique. Biosens Bioelectron. 2001; 16: 849-856.
- Situmorang M, Alexander PW, Hibbert DB. Flow injection potentiometry for enzymatic assay of cholesterol with a tungsten electrode sensor. Talanta. 1999; 49: 639-649.
- Shumyantseva V, Deluca G, Bulko T, Carrara S, Nicolini C, Usanov SA, et al. Cholesterol amperometric biosensor based on cytochrome P450scc. Biosens Bioelectron. 2004; 19: 971-976.
- Kim SH, Nam SM, Byun GS, Yun SY, Hong S. Determination of cholesterol by a diode laser/fiber optic colorometric spectrometer. Bull Korean Chem Soc. 2000; 21: 389–392.
- Martin SP, Lamb DJ, Lynch JM, Reddy SM. Enzyme-based determination of cholesterol using quartz crystal acoustic wave sensor. Anal Chim Acta. 2003; 487: 91-100.
- Burmeister JJ, Palmer M, Gerhardt GA. L-lactate measures in brain tissue with ceramic-based multisite microelectrodes. Biosens Bioelectron. 2005; 20: 1772-1779.
- Patel NG, Erlenkotter A, Cammann K, Chemnitius GC. Fabrication and characterization of disposable type lactate oxidase sensors for dairy products and clinical analysis. Sens Actuators B: Chem. 2000; 67: 134–141.
- Luca SD, Florescu M, Ghica ME, Lupu A, Palleschi G, Brett CM, et al. Carbon film electrodes for oxidase-based enzyme sensors in food analysis. Talanta. 2005; 68: 171-178.
- Zaydan R, Dion M, Boujtita M. Development of a new method, based on a bioreactor coupled with an L-lactate biosensor, toward the determination of a nonspecific inhibition of L-lactic acid production during milk fermentation. J Agric Food Chem. 2004; 52: 8-14.
- Volpe G, Moscone D, Compagnone D, Palleschi G. In vivo continuous monitoring of L-lactate coupling subcutaneous microdialysis and an electrochemical biocell. Sens. Actuators B: Chem. 1995; 24: 138-141.
- Hart JP, Crew A, Crouch E, Honeychurch KC, Pemberton RM. Some Recent Designs and Developments of Screen‐Printed Carbon Electrochemical Sensors/Biosensors for Biomedical, Environmental, and Industrial Analyses. Anal Lett. 2004; 37: 789–793.
- Suman, Ashok Kumar. Sens Transducers J. 2008; 96: 18–31.
- Palmisano F, De Benedetto GE, Zambonin CG. Lactate Amperometric Biosensor Based on an Electrosynthesized Bilayer Film with Covalently Immobilized Enzyme. Analyst. 1997; 122: 365–369.
- Parra A, Casero E, Vazquez L, Pariente F, Lorenzo E. Design and characterization of a lactate biosensor based on immobilized lactate oxidase onto gold surfaces. Anal Chim Acta. 2006; 555: 308–315.
- Jena BK, Raj CR. Electroanalysis. 2007; 7: 816–822.
- Perdomo J, Sundermeier C, Hinkers H, Martinez MO, Seifert W, Knoll M. Biosens Bioelectron. 1999; 14: 27–32.
- Clarkson SP, Onnrod IHL, Sharpe FR. Determination of ethanol in beer by direct injection gas chromatography. A comparison of 6 identical systems. J Inst Brew. 1995; 101: 191-193.
- Liden H, Vijayakumar AR, Gorton L, Marko Varga G. Rapid alcohol determination in plasma and urine by column liquid chromatography with biosensor detection. J Pharm Biomed Anal. 1998; 17: 1111–1128.
- William S. Official Methods of Analysis of the Association of Official Analytical Chemists, 14th edition. Association of Official Analytical Chemists, Inc. Washington DC. 1984.
- Dubowski KM. Alcohol Analysis: Clinical laboratory aspects. Part II. Laboratory Management. 1982.
- Turner APF. Biosensors: realities and aspirations. Annali di. 1997; 87: 255–260.
- Renedo OD, Alonso-Lomillo MA, Martínez MJ. Recent developments in the field of screen-printed electrodes and their related applications. Talanta. 2007; 73: 202-219.
- Wang J. Decentralized electrochemical monitoring of trace metals: from disposable strips to remote electrodes. Plenary lecture. Analyst. 1994; 119: 763-766.
- Hart JP, Crew A, Crouch E, Honeychurch KC, Pemberton RM. Anal Lett. 2004; 37: 789.
- Eggings BR. Chemical Sensors and Biosensors, John Wiley and Sons. Chichester. 2002.
- Clark LC, Lyons C. Electrode systems for continuous monitoring in cardiovascular surgery. Ann N Y Acad Sci. 1962; 102: 29-45.
- Kauffmann JM, Guilbault GG. Enzyme electrode biosensors: theory and applications. Methods Biochem Anal. 1992; 36: 63-113.
- Cardosi M, Turner A. The Realization of Electron Transfer from Biological Molecules to Electrodes. Oxford University Press, New York. 1987.
- Dubinin AG, Li F, Li Y, Yu J. A solid-state immobilized enzyme polymer membrane microelectrode for measuring lactate-ion concentration. Bioelectrochemistry and Bioenergetics. 1991; 25: 131-135.
- Jaffari SA, Turner APF. Novel hexacyanoferrate(III) modified graphite disc electrodes and their application in enzyme electrodes—Part I. Biosensors and Bioelectronics. 1997; 12: 1–9.
- Shul'ga AA, Koudelka-Hep M, de Rooij NF, Netchiporouk LI. Glucose-sensitive enzyme field effect transistor using potassium ferricyanide as an oxidizing substrate. Anal Chem. 1994; 66: 205-210.
- Sharma Min, Sharma Mam. Journal of International Academic Research for Multidisciplinary. 2015; 3: 329–343.
- Hu J, Zhang XE. Impact of epidemic rates of diabetes on the Chinese blood glucose testing market. J Diabetes Sci Technol. 2011; 5: 1294-1299.
- Carrara S, Ghoreishizadeh S, Olivo J, Taurino I, Baj-Rossi C, Cavallini A, et al. Fully integrated biochip platforms for advanced healthcare. Sensors (Basel). 2012; 12: 11013-11060.