
Research Article
Austin J Biosens & Bioelectron. 2016; 2(2): 1022
Analsis of the Efficiency of Fiber Optic Sos-Type Biosensor Work at the Different Ways of the Sensitive Layer Formation
Starodub NF* and Taran MV
Department of Molecular Biology, National University of Life and Environmental Sciences of Ukraine, Ukraine
*Corresponding author: Starodub NF, Department of Molecular Biology, Microbiology and Biosafety of National University of Life and Environmental Sciences of Ukraine, Heroyiv Oborony st., 15, Kyiv-03041, Ukraine
Received: August 16, 2016; Accepted: October 10, 2016; Published: October 13, 2016
Abstract
It was analyzed the efficiency of different ways for the functionalization of optrodes by the referent cells at the determination of the gene toxicity of a different chemical substances. As biosensor structure it was taken fiber optical sensor and as sensitive elements it was used the bacterial SOS system. The functionalization of fiber optics was fulfilled through including referent cells into the special sol–gel mixture, entrapping in alginate gel, or in photopolymerizable membranes, or in the box from the cellophane films. It was stated that most available for the obtained the most relevant results are the approaches with the application of the sol-gel mixture and cellophane film box. Moreover, the last has number advantages, in particular, in respect of simplicity, not necessary in additional materials and possibility registration of very fast response. Unfortunately, including the referent cells into photopolymerisable membranes and alginate gel have demonstrated much less efficiently of the generated signal, apparently due to additional impact on the referent cells and through the possibility of a the presence of calcium binding structures in the medium being analyzed.
Keywords: Gene toxicity; Control; Functionalization of optrodes; Optimal conditions
Abbreviations
TMOS: Tetramethylorthosilicate; LB: Liquid Microbial Growth Medium; DMS: Dimethylsulfate; MC: Nmitomycin C; Et: Ethanol
Introduction
Now it is proposed number variants of biosensors for the control of the genotoxicity of the different types of environmental objects. As a rule they include the cells with two closely combined blocks of genes which: a) control reparative processes during the DNA damage and b) coding some fluorescent protein. It was described such SOS systems on the basis of the transformed E. coli, Staphylococcus aureus, Bacillus subtilis, Rickettsia typhi and S. typhimurium TA1535, cells of fish and some mammalians with the inclusion of genes coded enzymes (β-galactosidase, alkaline phosphatase and others), chemiluminescent or green fluorescent proteins and others serve as tester system [1,2]. Early [3] we proposed simple SOS-type biosensor based on the fiber optics worked in differential regime and allowed the control of such environmental objects which are chemical nature.
The one of very important problem which arouses at the creation of any biosensors is the optimization of the integration of the biological selective structures with the transducer surface. Especially it is appeared at the application of the different types of cells. As a rule for this purpose the number of organic and polymeric materials is recommended for application [4-6].
The recombinant bacteria were incorporated in soft gels such as agar, agarose, polyacrylamide or calcium and strontium alginates and sol-gel [7-9]. Moreover, it was used the encapsulation of cells by a dialysis membrane [10] and that based on a glycerol–acryl vinyl acetate copolymer latex [11].
Eearly [12,13] have demonstrated very high efficiency of the photopolymerizable membranes for the immobilization of the number enzymes on the transducer surface. Moreover, it was appeared the information about the application of the similar approaches for the including cells into such membranes [14].
The main problems at the immobilization of the genetic engineering of bacteria for the expression of the reporting enzymes in the response to physiological stress conditions are connected with the soft hydrogel supports, biodegradation susceptibility, diffusion limitation due to the thick films involved, low physical deformation resistance and the instability of the alginates in calcium-poor solutions and in the presence of calcium chelates. In this article we would like to give the experimental demonstration of the efficiency of the number of approaches for the immobilization of cells at the creation of the biosensor based on the fiber optics at the control of the genotoxicity effects of the number of the toxic agents.
Materials and Methods
The fibre optics biosensor on the basis of cells combined the SOS system, indicative of DNA-damaging agents, as a receptor component and the bioluminescence system as a rapid reporter one was constructer as described in [3]. This device works in the differential regime which allows registering the comparative level of the chemiluminescence between the presence of the analysed substance in the measuring cell and distillate water instant its (control probe).
For the immobilization of the prepared cell culture it was used several approaches. One of them was fulfilled according to [7] and was based on the application of the special sol–gel mixture which was obtained by the mixing 2 ml of TMOS (Sigma-Aldrich) with 1 ml of distilled water and 0,25 ml of 0,1 M HCl. The mixture was sonicated for 10 min to ensure uniformity and left on the 24 h at 4 oC. Suspension (1 ml with concentration of 108 cells/ml) in LB was thoroughly mixed with 0.5 ml of the sol–gel solution and then it was kept in the presence of optrodes during 10 min. After that optrodes were removed and dried for 5 min under room temperature. At last optrodes were washed with phosphate buffer and then by LB medium, both at pH 7, and kept in the special measuring cell in phosphate buffer.
Second approach for the cell immobilization on the optrodes was fulfilled according to [12]. In this case cells were entrapped in alginate gel by mixing sodium alginate solution (1%) with cells in the ratio 1:1. The mixture was deposited on the optrodes and then calcium chloride solution (1%) was applied on the top of it. The cell sensor was left in a refrigerator at humid atmosphere for 30 min. Formed membrane was washed by calcium chloride (10 mM) buffer at pH 7.3 to remove free cells from the membrane surface.
The third approach was based on the application of the photopolymerisable membranes as it was described early [6,13] with little modifications which concerned by the next.
At last it was used the approach based on the application of the cellophane films. It was preliminary boiled in the distillate water during 15 min. Then, the small cylinders with the diameter about 5-6 mm were formed from the cellophane films. These cylinders were filled by the prepared suspension in LB medium at the concentration of 107-108 cell/mL and equipped with optrodes. Such complex of the optrode with cell suspension was introduced into the measuring cell of the biosensor system [14].
In all cases optrodes were careful cleaned according to recommendation [7] I was made successively by a 1:1:1 acetone: ethanol: chloroform mixture, soap solution, piranha solution (H2SO4:H2O2 4:1) solution, distilled water and 1M NaOH. Then, the clean glass pieces were dried for an hour in a 80°C oven.
After the incubation of the optrodes or the complex of the optrode with cell suspension in the measuring cell filled by the solution to be analysed during some time (from 10 to 90 min) at the room temperature the light emission was measured. The signal was presented in the units relative to the control value.
As a toxic element for the testing system it was used DMS, MC and Et with the concentrations in range of 5 μM to 0.5 mM, 10 nM to 1 μM and 0,5-4%, respectively. All these reagents were from Sigma- Aldridge (USA).
Results and Discussion
In the experiments it was used the maximal possible concentrations of chemical agents: 0.5 mM, 1 μM and 3% for DMS, MC and Et, respectively [3].
At first it was carried out experiments to obtain kinetic of the induced luminescence in case of the application of the classic special sol–gel mixture for cell immobilization (Figure 1a). For the DMS and MC the registered luminescence was appeared trough about 10 min and achieved maximal level during 150-180 min. After that time the level of the luminescent signal was stay on the some level or become to decrease though some time (no faster then 180-240 min and its depended on analysed substance). Of course, due to the relatively long time of the achievement of the maximal level of the signal, there is possible to do express control through the much less time of exposition, for example during 20 min. In case of the application of Et the appearance of the luminescence was revealed through 15 min and its level was decreased after the exposition in 50 min.
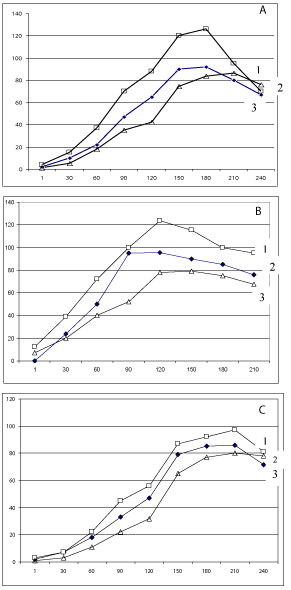
Figure 1: Dynamics of changes of the chemiluminescence level of biosensor
after adding the DMS (1) MC (2) and Et (3) to the measuring cell. Ordinate -
relative units of the chemiluminescent level and Abscise - time of measuring
in min. Way of functionalization of optrodes by SOS cell culture in: sol-gel (a),
cylinders with the cellophane film (b) and photopolymerizable membrane (c).
The very fast signal was registered if the cylinders with the cellophane film were used (Figure 1b). So, in case of the analysis of MC, DMS and Et it was achieved maximal level during 60, 90 and 45 min, respectively. If it was used the ways with the application of the photopolymerisable membranes or alginate gels the results were much less effective in the comparison with that obtained by others above mentioned ways (Figure 1c). The main reason of such situation may be connected with the number of factors: 1) difficulties in the penetration through barrier of more dense membrane; 2) additional effect of physicochemical factors on the referent cell during membrane formation and 3) non-controlled influence of calcium binding structures in solution to be analysed. The possibility of impact of the last factor it was demonstrated by experiments with the application of calmodulin as calcium binding structure in solution to be analysed. It was demonstrated that the presence calmodulin in the concentration of 10-3 M decreases maximal signal of biosensor about on 10% if it was prepared through optrode functionalization by alginate gel. The some effect but much less was registered in the case of the application of photopolymerisable membrane. At the sometime such effect was not registered in the case when the optrodes were introduced into the cylinders with the cellophane film contained SOS cell culture with the solution to be analysed.
Conclusion
There is possible to conclude on the basis of the obtained results that the most available and effective ways for the functionalization of the transducer surface in form of fibre optics is direct their interaction with the referent cells in box from the cellophane film. It is very simple way and allowed very fast biosensor response. This approach allows the results similar to that when functionalization is realised through the application of sol-gel technology. But the last is more complicate and demands some additional reagents and analytical procedures. In the respect of two others way which were used there is necessary to underline that they may be used too but they provide more uncomfortable situation, namely, delaying sensor response and dependence of it from the presence of some calcium binding structures in solution to be analysed.
Acknowledgement
This work has been supported by the NATO Science for Peace and Security Program, grant of “Nanostructured Materials for the Catalytic Abatement of Chemical Warfare Agents” (NanoContraChem), number of 984481.
References
- Elad T, Lee J, Gu MB, Belkin Sh. Microbial Cell Arrays. Adv Biochem Engin/ Biotechnol. 2010; 117: 85–108.
- Starodub NF. Genotoxiticy: modern instrumental approaches for its control in environmental objects. J Biosens Bioelectron. 2015; 6: 2.
- Starodub NF, Taran MV, Shpirka NF, Shavanova KE. Fiber optic SOS-type biosensor for the control of the genotoxicity of some environmental objects. World J of Eng Res and Technol. 2016; 2: 123-130.
- Starodub NF, Samodumova IM, Starodub VN. Usage of organosilanes for integration of enzymes and immunocomponents with electrochemical and optical transducers. Sensors and Actuators. 1995; 24: 173-176.
- Starodub NF, Rebriev AV. Liquid photopolymerizable compositions as immobilized matrix of biosensors. Bioelectrochem. 2007; 71: 29-32.
- Starodub NF. Photopolymerizable Materials in Biosensorics. In: Environmental Monitoring, Ema Ekundayo (ed). 2011; 299-326.
- Premkumar RJ, Rosen R, Belkin Sh, Lev O. Sol–gel luminescence biosensors: Encapsulation of recombinant E. coli reporters in thick silicate films. Anal Chim Acta. 2002; 462: 11-23.
- Bettaieb F, Ponsonnet L, Lejeune P, Ouada HB, Martelet C, Bakhrouf A, et al. Immobilization of E. coli bacteria in three-dimensional matrices for ISFET biosensor design. Bioelectrochem. 2007; 71: 118-125.
- Colpo P, Ruiz A, Ceriotti L, Rossi F. Surface Functionalization for Protein and Cell Patterning. Adv Biochem Engin/Biotechnol. 2010; 117: 109–130.
- Ikariyama Y, Nishiguchi S, Koyama T, Kobatake E, Aizawa M, Tsuda M, et al. Fiber-optic-based biomonitoring of benzene derivatives by recombinant E. coli bearing luciferase gene-fused TOL-plasmid immobilized on the fiber-optic end. Anal Chem. 1997; 69: 2600-2605.
- Lyngberg OK, Stemke DJ, Schottel JL, Flickinger MC. A single-use luciferasebased mercury biosensor using Escherichia coli HB101 immobilized in a latex copolymer film. J Ind Microbiol Biotechnol. 1999; 23: 668-676.
- Starodub NF, Torbicz W, Pijanowska D, Starodub VM, Kanjuka MI, Dawgul M, et al. Optimisation methods of enzyme integration with transducers for analysis of irreversible inhibitors. Sensors and Actuators. 1999; 58: 420-426.
- Starodub NF, Rebrijev AV. Photopolymers as immobilization mtrix in biosensorics. Ukr Biochim Zhurn. 2001; 73: 5-17.
- Lead T, Lee JH, Belkin S, Gu MB. Microbial whole-cell arrays. Microbial Biotech. 2008: 2; 137–148.