
Review Article
Austin J Biosens & Bioelectron. 2023; 8(2): 1047.
Application of Nanomaterials and Biomaterials in Nanovaccinology
Naghmeh Hadidi1*; Mojgan Sheikhpour2*; Maryam Mohebbi3; Seyed Mehdi Sadat4
1Department of Clinical Research and EM Microscope, Pasteur Institute of Iran, Tehran, Iran
2Depetment of Mycobacteriology and Pulmonary Research, Pasteur Institute of Iran, Tehran, Iran
3Department of Virology, School of Medicine, Iran University of Medical Sciences, Tehran, Iran
4Department of Hepatitis and AIDS, Pasteur Institute of Iran, Tehran, Iran
*Corresponding author: Naghmeh Hadidi Department of Clinical Research and EM Microscope, Pasteur Institute of Iran, Tehran, Iran; Mojgan Sheikhpour, Depetment of Mycobacteriology and Pulmonary Research, Pasteur Institute of Iran. Tel: +98-9122054655, +98 21 64112269; +98-9122969712, +98 21 64112285 Email: hadidi@gmail.com; n_hadidi@pasteur.ac.ir; Iranmshaikhpoor@gmail.com; m_sheikhpour@pasteur.ac.ir
Received: August 30, 2023 Accepted: September 27, 2023 Published: October 04, 2023
Abstract
Over the years, the concept of vaccination has encountered great evolution. Most vaccines have been formulated in a way that mimics pathogens in order to activate immune cascades. However, vaccine development was never assumed as a simple task and it involves several studies to obtain detailed knowledge about antigen presentation and recognition by immune system. Nanovaccination has been proposed as one of the most successful break throughs and procurements in health promotion and diseases prevention. Nano vaccines can be classified into various groups based on shape, source, sizes, features and structural constriction. Therefore they are assumed to offer more opportunities and novel approaches to scientists and researches and address unmet needs in vaccine developments. Novel technologies in vaccinology mostly focus on safety-immunogenicity improvements, synergistic immunomodulation, in vivo stability, reduced toxicity and efficient delivery of stimulatory cues. Biomaterials and nano vaccines were proved as promising strategies with optimal safety and efficacy through controlling the release site and pattern for better adjustment of dosing and timing of vaccines and immunotherapies.
In this review, we have summarized future horizons and cutting-edge advances of biocompatible nano biomaterials-based platforms such as liposomes, nanoparticles, carbon based structures and membrane based vaccines. We also described the remaining challenges, limitations, and possible breakthroughs in nano vaccines’ formulation and biomaterials application in industrial scale.
Keywords: Vaccine; Carbon nanotubes; Nanotechnology; Biomaterials; Vaccinology
Introduction
Vaccine Strategies
Vaccine design generally consists of four main components: antigen, adjuvant, carrier, and delivery strategy. Antigens are foreign materials that can induce an immune response. Vaccines are categorized into four groups: live attenuated vaccine, inactivated vaccine, subunit vaccine (VLPs), and peptide vaccines based on an antigen-presenting approach. Adjuvants are stimulatory agents of vaccine formulation that exist as independent or conjugate entities and would boost the immune response to antigens. Nanoparticles are viral/non-viral Nano carriers applied to encapsulate or present antigens and/or adjuvants in live attenuated and inactivated vaccines. Adenoviral vectors, proteinaceous nanoparticles, and synthetic nanoparticles are the most common carriers for antigen delivery in vaccine formulations. Vaccines are usually administered through syringes, implants, and microneedle patches [1-10].
Contemporary vaccines would induce active immunization against complete or killed pathogens. This type of vaccine is perspective, specifically in SARS-CoV-2 vaccination. On the other hand, live attenuated (LAVs) and Inactivated Vaccines (IVs) are live a virulent viruses that normally induce immunity in single-dose administration. Nowadays, genetic code expansion has been applied to improve productivity and genetic stability of LAVs to be specifically applied in the production of SARS-CoV-2 vaccines. Inactivated Vaccines (IVs) are consisted of physically or chemically inactivated pathogens or antigen fragments. This type of vaccine is administered in multiple doses to induce sufficient immunity. IVs formulation must include adjuvants and are more stable than LAVs. However, both LAVs and IVs require a cold supply chain. The last vaccine type is called the viral vector vaccine. In this type of vaccine, genetically engineered mammalian viruses such as herpes simplex and non-replicating adenoviral vectors like Ad5-nCoV and ChAdOx1 are used [11-17].
Next Generation Vaccines
Nanotechnology and nanomaterials play important roles in the development of the next generation of vaccines and immune engineering. Nucleic acid-based (DNA and mRNA vaccines) and subunit vaccines are promising vaccine technologies. These groups are safer, more stable, and easier to scale up but have more potential in terms of risk and failure during clinical phases. Nucleic acid-based (DNA and mRNA vaccines) elicit cytotoxic T cells’ responses in addition to antibody production and T helper cells activation [18-20]. Inovio, Ethnos pharmaceuticals, and Symvivo are pharmaceutical companies running clinical trials on Covid-19 DNA vaccine candidates [21]. Meanwhile, Moderna and BioNTech-Pfizer-Fosun Pharma performed clinical trials on Covid-19 mRNA vaccine candidate. It should be noted that stability, mutagenesis, and antigen half-life are the main obstacles in the development and commercialization of nucleic acid-based vaccines. Nanotechnology has suggested some solutions for the above problems. Nanomaterials such as polymeric nanoparticles, cationic liposomes, nanoemulsions, carbon-based nanostructures, and dendrimers are supposed to facilitate nuclear translocation, antigen delivery, and trafficking to face more immune cells as well as improve formulation stability and scalability [19,22,23]. Protein nanoparticles or Virus-Like Particles (VLPs) are categorized as subunit vaccines. VLPs are stable, scalable, mono-dispersed formulations generated from antigenic subunits and biomaterials. VLPs might root from bacteriophages and mammalian, insect and plant viruses. VLPs are highly visible to immune cells and are defined as immune activators and amplifiers with non-infectious and adjuvant properties [24-30]. CanSino, AstraZeneca, Shenzhen Geno-Immune Medical Institute, Medicago-iBio’s COVID-19 vaccines, and Johnson & Jonson influenza virus vaccine Crucell are VLP vaccine candidates in the clinical development pipeline. Most of the above vaccine candidates are multivalent platforms that offer simultaneous delivery of antigen and adjuvant to lymph nodes’ Antigen-Presenting Cells (APCs) and long-acting immune stimulus. It also facilitates APCs antigen processing and antiviral antibody production by CD8+ and CD4+ T cells in MHC-I and MHC-II pathways [31-33].
Peptide-based vaccines represent the simplest platform in next generation vaccines. They are generally formulated as peptides and T cell /B cell epitopes plus suitable adjuvant or immune-informatics-derived-peptide–nanoparticle conjugates. The efficacy of Peptide-based vaccines is highly dependent on adjuvant and applied nanocarrier. For example, “albumin hitchhiking” is an emerging targeted hepatitis B virus trafficking strategy to lymph nodes’ dendritic cells and macrophages. Enhanced viral clearance and stronger humoral and cellular immune responses are pursued by antigen encapsulation and antigen surface presentation through this nanotechnology approach [34-36].
Vaccine Scalability and Manufacturing
Production cost, formulation, and scale-up of vaccine formulation are the main concerns in the development of novel, effective vaccines. The traditional manufacturing process of recombinant proteins using mammalian, bacterial, and yeast cells are still expensive and is susceptible to human contamination. Innovative manufacturing platforms are required to meet high demands during viral disease outbreaks. Plant-based expression systems are a promising production technology that was introduced during the 2014 ebola epidemic. Plant molecular farming is scalable, while fermentation-based technologies are highly sensitive to control parameters. Low production cost and safety are other advantages of molecular farming. Conventional vaccines utilize a cold supply chain, while new technologies of implants and microneedle patches exclude cold chain difficulties in product distribution and moderate to high feasibility for rapid global deployment of vaccines [37-40].
Nanomaterials Improve Vaccine Responses: Mechanisms and Mew Approaches
Nanomaterials and nanotechnology have been applied more specifically in the design and development of new vaccines against HIV (Human Immunodeficiency Virus), TB (Tuberculosis), and malaria. These three pathogens are listed by WHO (World Human Organization) among the top ten reasons for mortality in developing and low-income countries. This notification would highlight the importance of developing more efficient prophylactic strategies and more effective antigen delivery to key immune cells, including APCs, B cells, neutrophils, and macrophages [41-44]. Nano materials’ size, shape, blood circulation half-life, adjuvant properties, and complement activation potential are required during vaccine development. Antigen persistence through encapsulation or conjugation with nanostructures would enhance antigen immunogenicity. For example, Moon et al. designed ICMVs (Inter bilayer Cross-linked Multilamellar Vesicles) in which malaria antigen has been both encapsulated and conjugated to the vesicles’ surface in order to extend antigen persistence in lymph nodes [45]. Demento et al. also suggest long acting PLGA ovalbumin encapsulated PLGA nanoparticles would improve APCs' immune response and high-affinity antibody secretion from follicular helper T cells [46]. Long-acting formulation and cross-presentation of HIV, TB, and malaria antigens will potentiate cellular immune response by CD8+ if antigen fragments escape to the cytosol after lysosomal degradation of nanocarrier [47,48]. Nano materials’ physicochemical properties, such as charge, size, and flexibility, show a high impact on Lymph Nodes (LN) draining. Nanoparticles within the size range of 10-50 nm are the most suitable for LN draining. Large 50 nm nanoparticles are passively drained to LNs and are acquired by macrophages better [49-51]. Mucosal immune response and mucosal antigen delivery is an attractive field in HIV and TB vaccine design [52,53]. Mucosal mucin permeability and adhesion are also size and charge-dependent [54]. Average pore size cut-offs of 340 nm for vaginal mucus and 200 nm for respiratory mucus must be considered for appropriate antigen traverse [55]. Large (500-5000 nm) anionic nanoparticles are captured by macrophages, while small targeted (20-200 nm) nanoparticles are endocytosed by DCs (Dendritic Cells) [55-60]. C-Type lectin receptors expressed on Langerhans cells and DCs are highly suggested for targeted follicular dendritic cells that exist in LN [61-,62]. Nanomaterials might also improve adjuvant functionality, minimize their toxicity and decrease their dosing amount [63-65]. Moon et al. reported that lipid vesicles with encapsulated malaria antigen and MPLA, as an adjuvant, required adjuvant amount was reduced to 10 times less than free soluble malaria antigen-adjuvant (MPL4) and stronger induced humoral responses were achieved [66] (Figure 1). Lymph Node (LN) trafficking, persistency, controlled release pattern, APC targeting and mucosal targeting are main strategies for engineering nanomaterial vaccine delivery. Trafficking to lymph nodes is largely dependent on size, charge, hydrophobicity and flexibility. During mucosal targeting hydrophilic positively charged mucoadhesive particles create strong entanglment with mucin fiber of mucosal membrane and induce mucosal immunity. Persistency and controlled release patterns would prolongs antigen uptake through endosomal escape and cross presentation of the antigen on MHC I from reservoir systems at site of injections. Administration of anionic nanoparticles or introducing DEC-205 or B cell epitopes on nanoparticles surface would be another engineered strategy which is entitles APC targeting through Dendritic Cells (DCs) and macrophages.
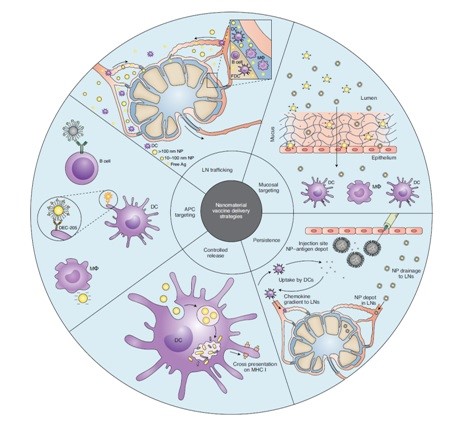
Figure 1: Strategies for engineering nanomaterial vaccine delivery [41].
Experimentals
Nanotechnology Systems for Vaccines
Low immunogenicity, in-vivo instability, toxicity, and multiple-dose administration are major problems of conventional vaccines. Nanotechnology and nanostructures provide an opportunity for enhanced cellular and humoral immune responses [67]. Higher antigen uptake by macrophages, more efficient antigen presentation and recognition, and specific and selective immunity are the main aims of nano vaccine developments [67,68]. In this review, we are going to summarize recent advances in the application of nanocarriers such as liposomes, emulsions, polymeric systems, peptide nanoparticles, carbon-based materials, and artificial VLPs in the new generation of vaccines. Table 1 summarizes nano carriers’ including liposomes, emulsions; natural and synthetic bio/polymer based systems, their composition, antigen types and route of administration. As it is listed in table 1, liposomal vaccines are mostly administrated parenteraly while emulsions, natural/synthetic polymer and carbon based systems would be applicable via parenteral, intranasal, pulmonary and oral routs. The above mentioned systems would be thoroughly explained in the next session.
Delivery System
Composition
Antigen
Route
Liposome
DDA, TDB
Ag85B-ESAT-6
Intramuscular
DDA, TDB
OVA
Intramuscular
DDA, DODA, TDB
Ag85B-ESAT-6
Intramuscular
Pegylated DDA, TDBA, TDB
Ag85B-ESAT-6
Subcutaneous
DDA, DSPC, Cholesterol, TDB
Ag85B-ESAT-6
Subcutaneous
MPL, DDA, TDB
OVA
Intraperitoneal
DDA, TDB
Trivalent influenza vaccine
Subcutaneous
DOPC, DOPG, MPB
OVA
Subcutaneous
EPC, DOGS-NTA-Ni
His-tagged heat shock protein
Intradermal
MDMPC, DMPG, Cholesterol, MPL
Polyhistidinylated OVA
Subcutaneous
Lecithin, Cholesterol
Diphtheria toxoid
Subcutaneous
Emulsion
MF59
Hemagglutinin
Intramuscular
MF59
Recombinant meningococcal B protein
Intramuscular
MF59
Recombinant meningococcal B protein
Intramuscular
W805EC
OVA
Intranasal
W805EC
OVA
Intranasal
GLA
Falciparum subunit
Subcutaneous
GLA-SE
Plasmodium vivax subunit
Subcutaneous
GLA-SE
Recombinant hemagglutinin
Intramuscular
Synthetic polymer-based system
PLGA
OVA
Subcutaneous
PLGA, Polylactic acid
Hepatitis B surface antigen
Pulmonary
Lipid-coated PLGA
OVA
Subcutaneous
Lipid-coated PLGA
Malaria antigen
Subcutaneous
Chitosan-coated polycaprolactone
H1N1 hemagglutinin
Intranasal
Polyanhydrides
Yersinia pestis antigen
Intranasal
Polylactic acid
Hepatitis B surface antigen
Subcutaneous
Deacylated cationic polyethleneimine
HIV CN54gp140 antigen
Pulmonary
PEGylated poly [2- (N, N-dimethylamino) rthylmethacrylate)
HIV gag DNA
Intranasal
Natural biopolymer-based system
N-trimethyl chitosan
OVA
Intranasal
Chitosan nanoparticles
HBsAg
Intraperitoneal
Cholesteryl-conjugated pullulan
Clostridium botulinum type-A neurotoxin subunit antigen
Intranasal
Carbon-based system
SWCNT
Tuberculin purified protein derivative
Subcutaneous
Carbon nanotube
Azoxystrobin
Intraperitoneal
Carbon magnetic nanoparticles
Hen egg lysozyme
Intravenous
Carbon nanoparticles
Bovine serum albumin
Oral
Table 1: Nanocarriers composition and application for antigens delivery in vivo [67].
Liposomes: Liposome formulation, as carrier or adjuvant, has been extensively investigated in vaccine technology, and at least eight liposomal vaccines are launched or undergoing clinical studies for human use [69]. Liposomes’ specifications, including fluidity, size, charge, lipid content, lipid types, and surface modifications, could be customized according to antigen properties to achieve optimum immunogenicity. Liposomes’ inter-bilayer space and their hydrophilic reservoir are suitable for hydrophobic, hydrophilic, and amphiphilic molecules. A combination of lipid composition and liposome size might affect the type of immune response and cytokine secretion. Small unilamellar liposomes with a size of below 500 nm and cationic lipids such as Dimethyl Dioctadecyl Ammonium (DDA) in their lipid bilayer mostly induce a stronger cellular immune response and interferon-gamma production. Surface antigen, lipid ratio, and surface antigen-lipid ratio are factors that are important in liposomal physical stability and would indirectly affect immune response intensity. There are some excipients that possess immune stimulatory properties. Trehalose Behenate (TDB), di-C14-amidine-based compounds, Monophosphoryl Lipid A (MPL), cationic lipids (e.g. DDA and DOTAP (1, 2-dioleolyl-3-trimethyl-ammonium propane)), cholesterol derivatives and imidazolium compounds are immune stimulatory candidates favorable for a stronger cellular immune response [67,68]. Inter-bilayer cross-linked multilamellar and nickel- chelating liposomes are novel liposomal vesicles designed for stable entrapment of antigen and immune stimulatory molecules within the phospholipid membrane. However, the toxicity of nickel- chelating liposomes still needs to be addressed appropriately. Subcutaneous administration of liposome-in-oil adjuvant formulations of diphtheria toxoids was another solution suggested for reducing antigen transport to LN’s draining and continuous antigen presenting to immune cells [70]. Carroll et al. also applied cationic liposome consisting of nucleic acid-based toll-like receptor agonist as an adjuvant, imidazolinium chloride and cholesterol as immunomodulator molecules, and a combination of lipoplexes -Fluzone as antigens in a new platform for influenza vaccine [71].
Emulsion and Nano Emulsion
Oil in water and water in oil Emulsions are reported to have a dual function, one as adjuvant and the second as antigen delivery system. MF59 is a well-known adjuvant emulsion which is consisted of squalene oil, span 80, tween 80, and citrate buffer.
Fluad was the first flu EMEA-approved vaccine that was formulated in MF59. MF59 was used in the development of meningococcal vaccines and was found to cause a strong humoral immune response after the administration of 3 doses in mice [72-74]. AF03 is another adjuvant emulsion which is consisted of squalene, sorbitan oleate, and cetheareth and was used in the formulation of the Humenza TM flu vaccine [75,76].
Squalene-free Nanoemulsions are another group of Nano adjuvants [77]. Makin et al. investigated adjuvant properties of water in oil emulsion of W805EC in the intranasal route. Other researchers have announced positive feedback about oil-in-water emulsification of Glucopyranosyl Lipid A (GLA) and GMZ2 in the anti-falciparum aqueous vaccine [78]. Formulation stability and biocompatibility of oil ingredients are critical in the successful commercialization of emulsions in vaccine design and development [68]. Molecular models and Transmission Electron Microscope (TEM) images of nanomaterial based vaccine against HIV and malaria have been summarized in figure 2. As it is shown, ferritin nanoparticles, cross-linked multi lamellar vesicles, self-assembling protein nanoparticles, self-assembling nanofibers and fullerene were biocompatible nanostructures and nanoadjuvants being investigated as carrier and adjuvant in vaccine formulation of HIV envelope protein, HIV trimmers and malaria epitopes [41].
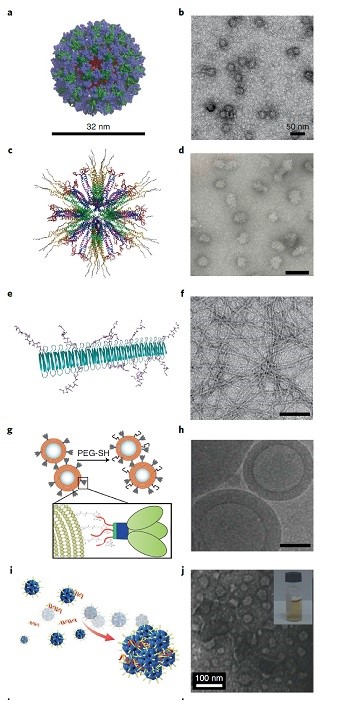
Figure 2: Molecular models and Transmission Electron Microscope (TEM) images of nanomaterial based vaccine against HIV and malaria, Ferritin nanoparticle (a,b) Self-assembling protein nanoparticles (c,d). Self-assembled nanofibres (e,f). crosslinked multilamellar vesicles (g,h), Fulleren (I,j)
Bio/Polymeric-Based Systems
PLGA (Poly-Lactic-co-Glycolic Acid) is the most extensively used biocompatible, biodegradable polymer in the synthesis of nanoparticles. Surface coating, surface charge, and particle size (>500 nm) are crucial parameters that might affect the release and presentation of antigens and/or adjuvants in oral, mucosal, and systemic delivery of vaccines [67,79]. A number of researches have been performed on encapsulated OVA (ovalbumin) in polymeric particles. Surface PEGylation, lipid adjuvants (MLP and alfa-galactosylceramide) entrapments in phospholipid PEGylated particles, and lipid content of DOPC and DOPG were shown to intensify antigen-specific IgG titre in the case of OVA and P. vivax malaria antigen (VMP001) [80,81]. Long-acting PLGA particles were found to provide a more sustained release pattern of antigens in comparison to liposomal delivery systems. This phenomenon would cause higher IgG titre in the same route of administration (e.g., sc.) [46,47]. Poly caprolactone, polyanhydrides, and chitosan are other widely used biocompatible polymers investigated in polymeric nanocarriers for delivery of H1N1 hemagglutinin, Yersinia pestis and recombinant F1-V [82-84]. Electrostatic polyplexes are another polymeric-based system that is under investigation to be applied in the development of subunit antigens (e.g., HBs Ag (Hepatitis B antigen)) or plasmid DNA vaccines [67,85].
Pulmonary administration of electrostatic complexes of cationic polymers and negatively charged plasmids were shown to be effective in the inflation of serum IgG and the production of interferon-gamma in the HIV gag DNA vaccine [86]. N-trimethyl chitosan mucoadhesive particles were administered both intranasal and intramuscular as OVA carriers. Mucosal immunity and higher antigen-specific IgA serum level were the main outcomes reported by Slutter and Sawaengsak [87,88]. Nanoparticle platform vaccine technologies and their immunization pathways are shown in Figure 3. Some Nanoparticle platform technology includes bacteriophage, ferritin nanocage, cowpea mosaic virus, liposomes, and lipid nanoparticles which varies in physical properties including size, structure and charge. VLPs are classified as non-synthetic particles with expressed or encapsulated antigens, while synthetic nanoparticle might include multi-variant vaccines, DNA vaccines, RNA vaccines, and subunit vaccines [1]. Vaccine processing and nano formulation's fate in inducing immune response has been schematically presented in Figure 3c.
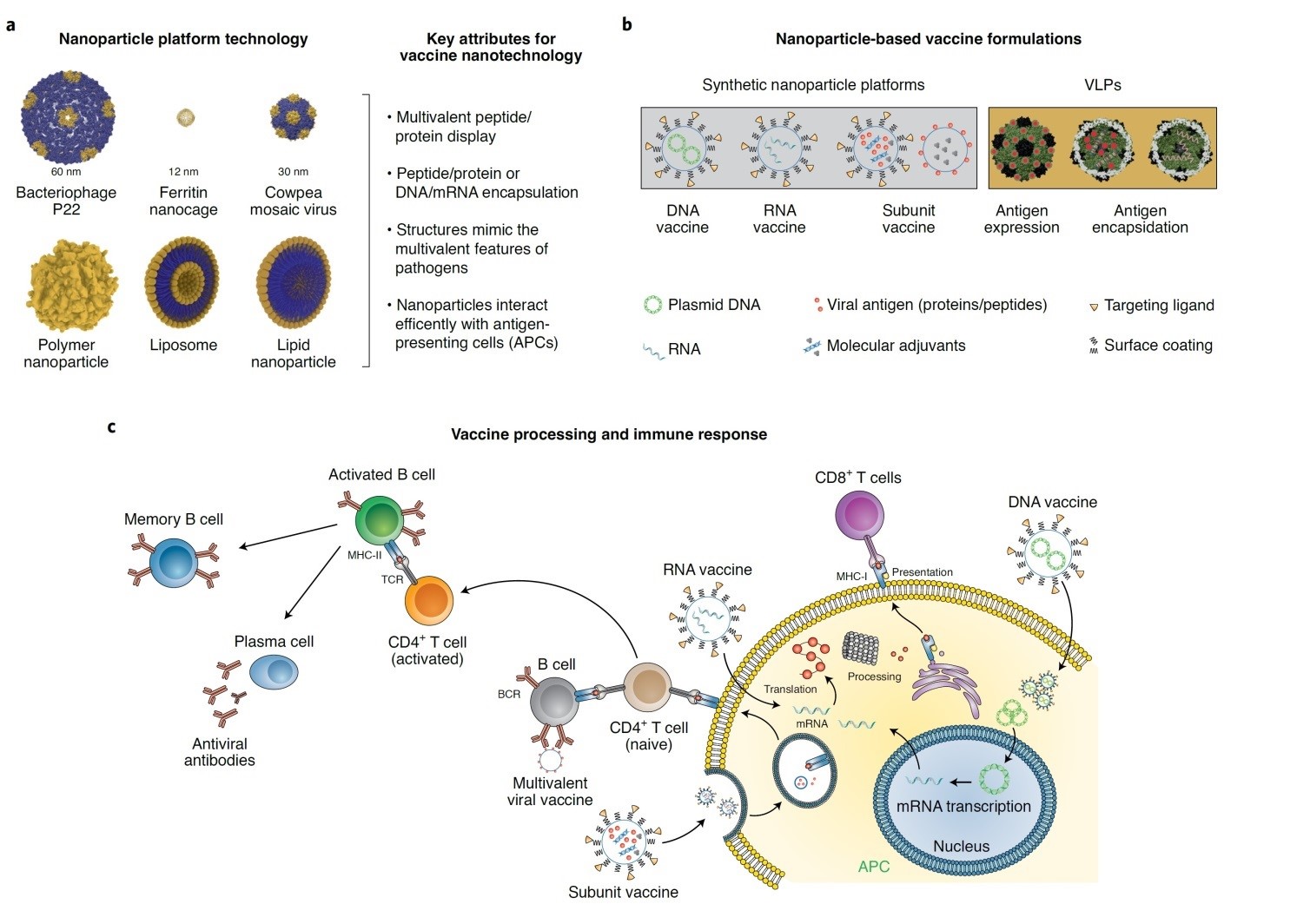
Figure 3: Nanoparticle platform vaccine technologies [1].
Self–assembled peptide nanoparticles (SAPNs): Self–assembled peptide nanoparticles were expressed and produced in Escherichia Coli as 180 repeated peptide constituents forming a scaffold with the immune stimulatory property. These platforms are suitable for a wide range of antigens but has be more specifically studied in the development of seasonal Flu and COVID-19 vaccines [68,89,90].
Carbon-based nanostructure systems: Carbon-based nanostructure systems, including Carbon Nanotubes (CNTs), carbon magnetic nanoparticles, and carbon nanoparticles, are other Nano biomaterials that are interestingly under investigation. Their dual functions of drug/ antigen carrier and adjuvant immune stimulatory potentials are being evaluated by different research teams [67,68,91-106]. The research showed that functionalized MWCNTs using a silicon reaction together with INH drug increase the level of performance and reduces the effective dose of the drug in the treatment of tuberculosis [107]. Another characteristic of functionalized CNTs is penetration into the bacterial membrane. Sheikhpour et al. found that CNTs functionalized with carboxylic acid had antimicrobial effects on Staphylococcus aureus by destroying membrane integrity and increasing drug efficiency [108]. Conventional vaccines and nano vaccines are different in activation of the immune response, dose number, cellular uptake, lymph node accumulation, antigen presentation, and migration-activation and cytokines secretion. Lower required dose, increased robust reuptake by DCs, greater accumulation in lymph nodes, increased cellular immunity, and more stimulatory cytokine secretion are shown as the most important advantages of nanotechnological vaccines [102].
CNTs are promising multidisciplinary nanostructures in biomedicine. However, toxicity of CNT and its biocompatibility are important milestones in biomedical administration [92,106]. Long-term exposure to CNTs can cause persistent inflammation, lung cancer, fibrosis, and gene damage in the lung. The presence of MWCNTs inside the body led to the production of cytokines such as TNF-α and IL-1β from immune cells, which play a role in toxicity. SWCNTs also cause acute effects such as inflammation, granuloma synthesis, collagen deposition, fibrosis, and genotoxicity. However, by using new methods such as functionalization, it is possible to produce nanotubes with greater length, greater width, and greater curvature to some extent with less toxicity [109]. Polymeric functionalization with phospholipid PEG derivatives and surfactants would remarkably improve CNTs’ biocompatibility; Moreover, functionalization would also facilitate secondary conjugation with drug molecules [94-99]. In the study, it was found that the simultaneous administration of functionalized carbon nanotubes and meropenem in nanofluid conditions caused a significant decrease in the growth of Pseudomonas aeruginosa and antibiotic resistance by increasing the stability of the drug [110]. Functionalized CNTs are not intrinsically immunogenic but are capable of activating immune system cells, including monocytes, macrophages, and DCs, after cellular reuptake. The application of SWCNTs is proposed as immune stimulator candidates and antigen carriers in vaccine studies [67,97,102]. For example, Meng et al. showed that SWCNT-conjugated tumor cell lysates resulted in better immune responses than free tumor cell lysate [97,111]. Zeinali et al. also reported that immunization with BCG vaccine as PPD-SWCNT induced a higher level of Th1 cell response in comparison to free PPD [97,112]. Hadidi et al. also confirmed the immune modulatory properties of PL-PEG-SWCNTs. Their results showed that PL-PEGSWCNTs concentration and PL-PEG-SWCNT–alum-HB vaccine concentration ratio directly affects the expression of activation and maturation markers in MDDC. These data support the idea of the co-adjuvant potential of PL-PEGSWCNT- alum compounds [97]. Different CNTs with different lengths and surface modifications were found to directly affect anti-azoxystrobin IgG antibodies in animal studies [113].
Carbon magnetic nanoparticles are traceable materials, potentially effective in active targeting to DCs. Graphene oxides and fullerene (C60) would potentiate antigen presentation to DC s and MHC- I APCs and T cells, respectively [67].
CNTs internalization is through direct translocation due to its needle-like structure or by endocytic-phagocytic mechanisms. Mechanism of cells reuptake is CNTs type, synthesis method, impurities, size, and surface functional groups [102]. CNTs reuptake by macrophages is mostly mediated by MACROs receptors and would generally end in activation of inflammatory pathways, cytokine secretion, and cell apoptosis /necrosis. PL-PEG-SWNTs smaller than 400 nm internalize non-phagocytic cells, including COS7 and MCF7, through passive diffusion, while larger ones would prefer the endocytosis pathway. The interaction and reuptake of CNTs by phagocytic cells are highly dependent on both natures of phagocytic cells and CNTs functionalization type. So, different cellular signaling pathways might be activated by amine or carboxyl functional groups on the CNTs surface [114-116]. Monocyte-Activating Nanotubes (MA-CNTs) are Oxidate-MWCNT-NH3+ induce maturation of dendritic cells, cytokines production of IL-6, TNF-Alfa, NFKB signaling activation, and cytokine secretion by T helpers. So, Oxidate-MWCNT-NH3+ would be prospecting immune therapeutics in cancer management [117,118]. Carboxylate Pl-PEG-CNTs activate DC maturation and activation as well as IL6, IL10, and TNF and NFKB production. On the other hand, pure CNTs would activate oxidative stress and caspase-1 pathways, IL-1 production, and cause cytotoxicity [102]. It has been stated that ammonium-functionalized CNTs and ox-CNTs would modulate the immune system without induction of cell apoptosis [119,120]. Allen et al. first describe CNTs’ enzymatic biodegradation and elimination by Horseradish Peroxidase (HRP) [102,121]. PEGylated CNTs would experience auto degradation by Myeloperoxidase (MOP), Eosinophil Peroxidase (EPO), and hypochlorous acid in side neutrophils [122]. Macrophage NADPH oxidase-dependent ROS, lignin peroxidase, xanthine oxidases, and manganese peroxidases are responsible for CNTs biodegradation [123,124]. Biodegraded CNTs would finally eliminate by exocytosis through the trans-Golgi complex [102].
CNTs immune modulation capacities include immune stimulation and immune suppression. Immuno stimulation would happen through cellular (macrophage-monocyte) response, DCs, lymphocyte and complement system activation, plus IL-6, IL-12, and Il-2 production. Immunosuppression would occur through the Cyclooxygenase (Cox) pathway, prostaglandin, and Il-10 secretion [102].
It could be concluded that functionalized CNTs might be a potential candidate in vaccine developments. CNTs’ physiochemical and structural properties are of great concern in biocompatibility, biodegradability, immunomodulatory, and design of antigen cargos. Further studies on how CNTs physicochemical modification would alter its interaction with immune cells have become necessary in finding the best options for cancer and infectious diseases, including HIV and Covid-19 [102].
Physicochemical properties of nanomaterial: It should be noted that physicochemical properties play important roles in the design and development of nano formulations with improved antigen delivery and presentation that target vaccine molecules to specific sites and induce desired immune responses [125-127]. From this point of view, shape, size, surface charge, surface volume ratio, porosity, hydrophobicity, hydrophilicity, and crystallinity are key factors that affect nanoparticles’ pharmacokinetic and pharmacodynamics parameters as well as antigen release and degradation kinetics [127].
The sizes of nanomaterial determine the mechanism of cellular uptake and specificity [127]. It has been reported that large PLGA nanoparticles (1, 7, and 17μm) showed a reduced internalization rate in comparison to smaller ones (300 nm). Smaller nanomaterial (20–200 nm) were readily endocytosed by the resident DCs, whereas larger sizes (500– 2,000 nm) were effectively taken up by the migratory DCs, and particles less than 200 nm size were drained into the lymph nodes. On the other hand, particles up to the 20 nm range were suitably transported to the APCs [127].
Particle shape also affects the cellular interaction, intracellular trafficking, and rate of antigen release inside the host cells, phagocytosis rate and the activation of signaling pathways, and improvement of antigen processing and presentation to T-cells. For example, Spherical gold nanoparticles were actively internalized by bone marrow-derived dendritic cells and were able to induce a stronger immune response in comparison to cube,rod, or worm-shaped particles. Particle shape would also affect localization. It is said that nanorods were practically delivered to the cell nucleus; however, nanosheets have remained in the cytoplasm after endocytosis [127,128].
Surface charge is also effective in antigen internalization. This electrostatic interaction was exemplified by the observation of significantly improved internalization of cationic polystyrene nanoparticles by the APCs in comparison to neutrally charged particles. Surface functionalization and modification with TLR-7, TLR-8, TLR9 agonists, CD47 molecules, TLR2, and TLR4 agonists, and galactose were also reported to activate the complement pathway, increased cytokine production and the expression of immune regulatory genes [127,129].
Hydrophobicity and hydrophobicity also matter in nano vaccines. Hydrophobic polymeric nanostructures are strong inducers of pro-inflammatory cytokines and co-stimulatory molecules than hydrophilic structures. Hydrophobic polymeric nanostructures also facilitate opsonization by increasing the immunoglobulin adsorption on the cell surfaces [127,128].
Nano Vaccines
Nano vaccines are suitable tools for targeting organs or tissue where disease or infection originated from, while conventional vaccines would affect the whole body. Nanoparticles are applicable to improve the solubility of hydrophobic compounds for parenteral administration. They maintain the integrity of antigens against degradation, stabilize peptides, proteins, or nucleic acids and reduce required doses. In addition, mucosal immunity, antigens protection against enzymatic‐acidic degradation, and a depot reservoir system with controlled release patterns are listed as other advantages of nano vaccines [130].
Membrane based cancer nano vaccines: Traditional membrane-based cancer nano vaccines are classified as a single-cell type (e.g. erythrocytes, lymphocytes, etc.); however emerging membrane-based cancer nano vaccines are categorized as exoxomes, hybrid cells and microcapsules with outstanding antitumor capacities (Figure 4). The membrane fraction of hybrid cells group might be separated from cancerous cells, dendritic cells and erythrocytes. Hybrid membranes were reported to demonstrate enhanced antigen delivery efficiency and precise targeting via lymph node guiding. There are currently 12 ongoing clinical trials conducting hybrid membrane strategy in vaccine development. Nie et al. developed an adjuvant and antigen co-delivery nano vaccine based on Escherichia coli and tumour cell membranes with a potent antitumor activity in vivo [131,132].
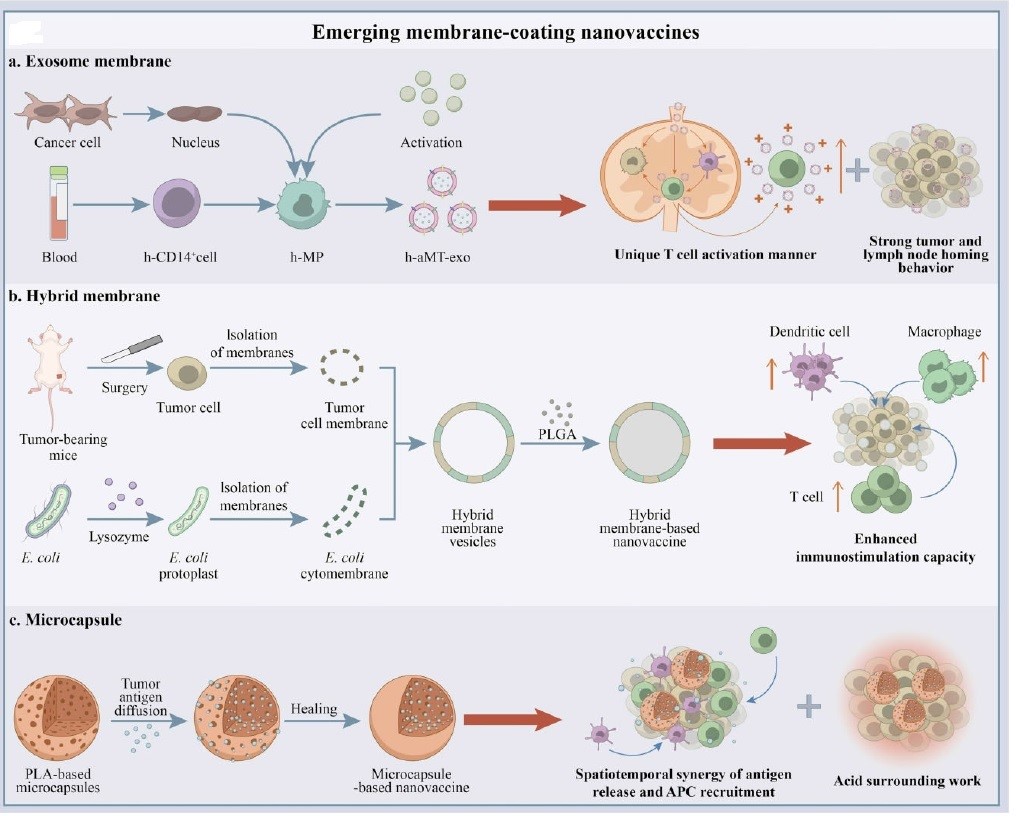
Figure 4: Emerging membrane-based nano vaccines: a) exosome membrane, b) hybride membrane, c) microcapsule [132].
Exosomes are one of the most unique nannocarriers from membrane-enclosed Extracellular Vehicle group (EV). Exsomes’ size normally varies between 30 to 150 nm. Desirable size, biocompatibility, in vivo stability, and target-specifi c delivery makes them potential candidate as adjuvant and antigen carriers. Exosomes are also considered as agents for local and systemic cell-to-cell communication through transfering functional substances to recipient cells. The studies’ results reveal that exosomes can be exploited as biomarkers and immunotherapeutic agents for nano vaccines development [131,132]. DC-derived Exosomes (DEXs) form a new class of vaccines for cancer immunotherapy which elicit strong immune responses and tumor suppression in animal cancer models. DEXs efficacy have been investigated in patients with advanced melanoma and Hepatocellular Carcinoma (HCC) [133]. Promising outcomes reveal that DEXs can serve as novel cancer nano vaccines due to their in herited antitumor properties. Schirrmacher and Barz reported that Tumor-Derived Exosomes (TDEs) displayed antigens similar to their corresponding tumor cells in Cytotoxic Lymphocytes (CTLs [134].
Wei et al. designed a chimeric-membrane nano vaccine based on exosomes from macrophage-tumor hybrid cells. Their customized nano vaccines targeted lymph nodes and T-cells in a unique ‘direct exosome interaction’ manner with long-lasting induction of tumor regression in various cancer models, especially when combined with anti-PD1 therapy [132,134]. Exosomes’ capability as adjuvant was also investigated in combination with genetically improved murine melanoma B16 cells. They successfully induced immunostimulatory signals in mice 7 days after the last immunization. These results reveals the potential benefits of exosomes as adjuvant and carriers for future cancer vaccine development [135]. Exosome-based vaccine candidates for cancer, hepatitis B, AIDS, and other infectious diseases are under investigation by researchers all over the world [131].
As for microcapsule-based nano vaccines, Wang et al. prepared a self-healing microcapsule system that can generate a desirable tumor microenvironment in situ, wherein antigen release, APC cells recruitment and acid microenvironment are deigned to work in a synergetic manner to promote anti-tumor activity [132]. Currently, Wei et al. have also designed two microcapsule-based nano vaccines with potent antitumor activity in various hematological cancer and solid tumor models in vivo [132].
New era in vaccinology and Biomaterials’ Role: Weak immunogenicity and short-term stability are most common limitations associated with subunit antigens. Biomaterials offer many advantages including biocompatibility, adjustable immunogenicity, low immunological reaction and desirable stability in comparison to conventional vaccine delivery systems. In the recent decade, biomaterial-based platforms such as synthetic and natural polymers, lipids, crystalline scaffolds, microneedles, and other particles have rapidly come out in order to improve vaccine essentials including efficacy, safety and stability simultaneously but only a few of suggested systems provide sustained or controlled release properties. For example, synthetic biodegradable polyesters are suitable for antigen encapsulation (e.g., single / double emulsion solvent evaporation and spray drying). Polyesters are compatible with various administration routes (e.g., dermal, intranasal and subcutaneous) and offer a flexible formulations and platforms for enriched immune response. However, stability and production of a local acidic environment following hydrolysis is their main bottle neck in proteins formulation.
Melt extrusion or co-extrusion with other materials has been investigated to design implantable vaccines for HPV (human papillomavirus) and seems promising. Figure 5 illustrates some of the commonly used synthetic polymers in vaccine formulations [136-139]. Biomaterials application have been suggested as one of the standard protective solutions to overcome these problems and augment immunization. Biomaterials are good in stabilizing host antigens and achieving sustained release pattern. However, there is still a significant challenge in vaccine formulation to achieve optimal therapeutic efficacy. Figure 6 illustrates some commonly used polysaccharides in vaccine formulations that might overcome some of formulation challenges in vaccine developments. Dextran, alginate, chitosan, hyaluronic acid and starch been described as applicable polysaccharides in controlled vaccine delivery.
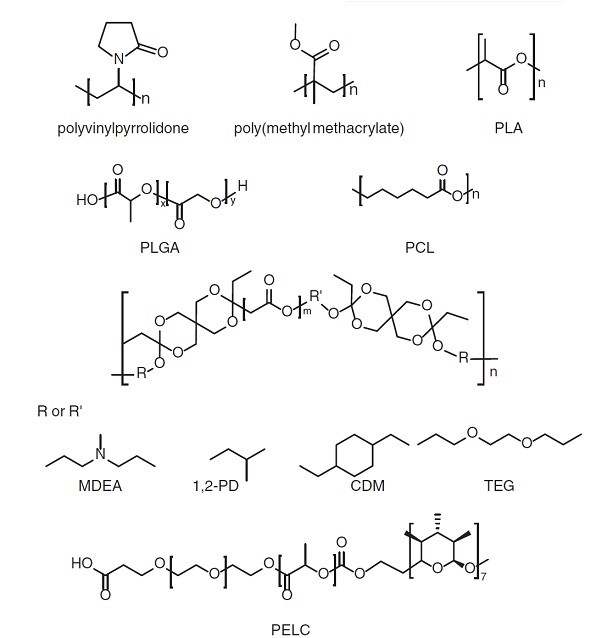
Figure 5: Commonly used synthetic polymers in vaccine formulations [136].
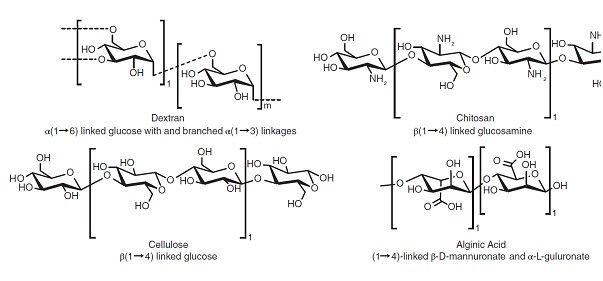
Figure 6: Common polysaccharides in vaccine formulations [136].
Distinguished properties of natural polysaccharides that have attracted attention are their desirable water solubility, ease of preparation, simple chemical modification and flexibility of administration in oral and intranasal routs [136].
Chitosan has been highly recommended in vaccine formulation of hydrogels and Metal–Organic Frameworks (MOFs) against infectious diseases owing to its high safety and ease of clearance. This phenomenon might be explained by chitosan’s strong electrostatic interaction owing to its positively charged nitrogen that synergistically enhances APC uptake and immune activation.
Figure 7 briefly illustrates several antigen/biomaterial interaction approaches. The interaction might be classified into five categories; surface adsorption, mixing, encapsulation, conjugation. Surface adsorption is completely based on electrostatic or hydrophilic/hydrophobic interactions that lead to the weak antigen attachment and burst release in vivo. However, encapsulation and conjugation through chemical bonding and cross linkage of antigen with selected biomaterials would lead to improved immunogenicity. This assumed to be happened because of gradual degradation of biomaterials intra/extracellular environment. Currently, simultaneous adsorption and encapsulation interactions are the most commonly applied interactions for improving vaccines sustained release pattern. Pulsatile release seems to be a better alternative in comparison to a single injection followed by several booster shots. This methodology is suggested to avoid immune cell exhaustion and reduced antibody-antigen affinity that normally occur in booster single shots. It might be concluded that biomaterials application in antigen delivery and vaccine development seems effective in improving vaccine stability and performance but there are still questions that need to be addressed by researches [136].
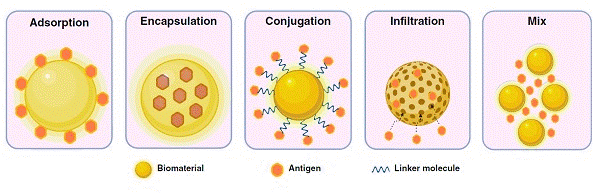
Figure 7: Schematic antigen/biomaterial interaction approaches [136].
Conclusions
Low rate of patient response and off-target adverse events of nanomaterial’s application in vaccine development indicate that many challenges exist and should be addressed to achieve more successful platforms [128]. The key principle is how to trigger appropriate antigen-specific immune responses by stimulating immune cells and inducing innate/adaptive immune responses. The flexible design of nanostructures endows nano vaccines with improved specific immune responses. These vaccine types mainly benefit from their unique drug/antigen delivery, adjuvant properties and customized immunomodulation properties in nano scale [127]. Currently, most vaccines are administered by a parenteral route, which is invasive and has poor patient compliance. However, nanomaterial application in vaccine development provided various options for vaccine administration, including topical, intranasal, inhalation, and oral administration for both therapeutic cancer vaccines and preventive vaccines for infectious diseases [128].
In general, toxicity, scale‐up process in sterile conditions, and difficulties in presenting naive antigens are critical limitations in vaccine platforms. However, with the advent of new techniques such as scaled‐up methodology for spray drying, some obstacles of scale‐up are eliminated, but there is still a long path to omit this millstone [128,129]. Nanotechnology platforms tend to intensify immunogenicity by effective targeted antigen delivery through their immune-modulatory properties; improved formulation stability, controlled release pattern, less immune toxicity and immunosuppression, surface modification, co-delivery of antigens and adjuvants, customized differentiate cellular and humoral immune responses and scalability. Additionally, nanoparticles could be tailored for single dose, non-invasive administration of antigens through immune engineering methods and co-encapsulation with stimulatory molecules.
Although nanotechnology-based vaccines are currently in different stages of clinical trials, these considerations would potentiate ongoing strategies in nanomaterial application, nano vaccines, and anti-infective treatments. More effective vaccines are to be developed by compromising nano vaccines and immune cell interactions. It has been well established that physicochemical properties of nanomaterial, including type, size, shape, surface charge, and hydrophobicity level, are the main factors affecting interactions, antigenicity level, adjuvant properties, and host immune response. Thus it might me concluded that, emerging nano vaccines and nanobiomaterials are beneficial tools for next generation vaccines with optimum efficacy for different route of administrations and targeted immune cells as well as improved safety and more flexible dosing regimen.
Author Statements
Acknowledgements
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Conflict of Interest
We should acknowledge our team in Pasteur Institute of Iran that shows high attribution in writing this manuscript.
References
- Shin MD, Shukla S, Chung YH, Beiss V, Chan SK, et al. COVID-19 vaccine development and a potential nanomaterial path forward. Nat Nanotechnol. 2020; 15: 646-55.
- Wrapp D, Wang N, Corbett KS, Goldsmith JA, Hsieh CL, Abiona O, et al. Cryo-EM structure of the 2019-nCoV spike in the prefusion conformation. Science. 2020; 367: 1260-3.
- Andersen KG, Rambaut A, Lipkin WI, Holmes EC, Garry RF. The proximal origin of SARS-CoV-2. Nat Med. 2020; 26: 450-2.
- Benvenuto D, Giovanetti M, Ciccozzi A, Spoto S, Angeletti S, Ciccozzi M. The 2019‐new coronavirus epidemic: evidence for virus evolution. J Med Virol. 2020; 92: 455-9.
- Yuan M, Wu NC, Zhu X, Lee CD, So RTY, Lv H, et al. A highly conserved cryptic epitope in the receptor binding domains of SARS-CoV-2 and SARS-CoV. Science. 2020; 368: 630-3.
- Yan R, Zhang Y, Li Y, Xia L, Guo Y, Zhou Q. Structural basis for the recognition of SARS-CoV-2 by full-length human ACE2. Science. 2020; 367: 1444-8.
- Lucchese G. Epitopes for a 2019-nCoV vaccine. Cell Mol Immunol. 2020; 17: 539-40.
- Grifoni A, Sidney J, Zhang Y, Scheuermann RH, Peters B, Sette A. A sequence homology and bioinformatic approach can predict candidate targets for immune responses to SARS-CoV-2. Cell Host Microbe. 2020; 27: 671-680.e2.
- Baruah V, Bose S. Immunoinformatics‐aided identification of T cell and B cell epitopes in the surface glycoprotein of 2019‐ nCoV. J Med Virol. 2020; 92: 495-500.
- Ahmed SF, Quadeer AA, McKay MR. Preliminary identification of potential vaccine targets for the COVID-19 coronavirus (SARS-CoV-2) based on SARS-CoV immunological studies. Viruses. 2020; 12: 254.
- Bull JJ. Evolutionary reversion of live viral vaccines: can genetic engineering subdue it? Virus Evol. 2015; 1: vev005.
- Si L, Xu H, Zhou X, Zhang Z, Tian Z, Wang Y, et al. Generation of influenza A viruses as live but replication-incompetent virus vaccines. Science. 2016; 354: 1170-3.
- Thi Nhu Thao T, Labroussaa F, Ebert N, V’kovski P, Stalder H, Portmann J, et al. Rapid reconstruction of SARS-CoV-2 using a synthetic genomics platform. Nature. 2020; 582: 561-5.
- Xie X, Muruato A, Lokugamage KG, Narayanan K, Zhang X, Zou J, et al. An infectious cDNA clone of SARS-CoV-2. Cell Host Microbe. 2020; 27: 841-848.e3.
- Ciabattini A, et al. Vaccination in the elderly: the challenge of immune changes with aging. In: Seminars in immunology. Elsevier. 2018; 40: 83-94.
- Dicks MD, Spencer AJ, Edwards NJ, Wadell G, Bojang K, Gilbert SC, et al. A novel chimpanzee adenovirus vector with low human seroprevalence: improved systems for vector derivation and comparative immunogenicity. PLOS ONE. 2012; 7: e40385.
- Fausther-Bovendo H, Kobinger GP. Pre-existing immunity against Ad vectors: humoral, cellular, and innate response, what’s important? Hum Vaccin Immunother. 2014; 10: 2875-84.
- Alberer M, Gnad-Vogt U, Hong HS, Mehr KT, Backert L, Finak G, et al. Safety and immunogenicity of a mRNA rabies vaccine in healthy adults: an open-label, non-randomised, prospective, first-in-human phase 1 clinical trial. Lancet. 2017; 390: 1511-20.
- Pardi N, Hogan MJ, Porter FW, Weissman D. mRNA vaccines—a new era in vaccinology. Nat Rev Drug Discov. 2018; 17: 261-79.
- Smith TRF, Patel A, Ramos S, Elwood D, Zhu X, Yan J, et al. Immunogenicity of a DNA vaccine candidate for COVID-19. Nat Commun. 2020; 11: 2601.
- Iavarone C, O’hagan DT, Yu D, Delahaye NF, Ulmer JB. Mechanism of action of mRNA-based vaccines. Expert Rev Vaccines. 2017; 16: 871-81.
- Zeng C, Hou X, Yan J, Zhang C, Li W, Zhao W, et al. Leveraging mRNAs sequences to express SARS-CoV-2 antigens in vivo. bioRxiv. 2020.
- Lim M, Badruddoza AZM, Firdous J, Azad M, Mannan A, Al-Hilal TA, et al. Engineered nanodelivery systems to improve DNA vaccine technologies. Pharmaceutics. 2020; 12: 30.
- Kanekiyo M, Bu W, Joyce MG, Meng G, Whittle JR, Baxa U, et al. Rational design of an Epstein-Barr virus vaccine targeting the receptor-binding site. Cell. 2015; 162: 1090-100.
- Kanekiyo M, Joyce MG, Gillespie RA, Gallagher JR, Andrews SF, Yassine HM, et al. Mosaic nanoparticle display of diverse influenza virus hemagglutinins elicits broad B cell responses. Nat Immunol. 2019; 20: 362-72.
- Sharma J, Shepardson K, Johns LL, Wellham J, Avera J, Schwarz B, et al. A self-adjuvanted, modular, antigenic VLP for rapid response to influenza virus variability. ACS Appl Mater Interfaces. 2020; 12: 18211-24.
- Brune KD, Howarth M. New routes and opportunities for modular construction of particulate vaccines: stick, click, and glue. Front Immunol. 2018; 9: 1432.
- Ross K, Senapati S, Alley J, Darling R, Goodman J, Jefferson M, et al. Single dose combination nanovaccine provides protection against influenza A virus in young and aged mice. Biomater Sci. 2019; 7: 809-21.
- Patterson DP, Rynda-Apple A, Harmsen AL, Harmsen AG, Douglas T. Biomimetic antigenic nanoparticles elicit controlled protective immune response to influenza. ACS Nano. 2013; 7: 3036-44.
- Bachmann MF, Jennings GT. Vaccine delivery: a matter of size, geometry, kinetics and molecular patterns. Nat Rev Immunol. 2010; 10: 787-96.
- Herzog C, Hartmann K, Künzi V, Kürsteiner O, Mischler R, Lazar H, et al. Eleven years of Inflexal® V—a virosomal adjuvanted influenza vaccine. Vaccine. 2009; 27: 4381-7.
- Wang S, Qin L, Yamankurt G, Skakuj K, Huang Z, Chen PC, et al. Rational vaccinology with spherical nucleic acids. Proc Natl Acad Sci USA. 2019; 116: 10473-81.
- Smith DM, Simon JK, Baker JR Jr. Applications of nanotechnology for immunology. Nat Rev Immunol. 2013; 13: 592-605.
- Mohsen MO, et al. Major findings and recent advances in virus–like particle (VLP)-based vaccines. In: Seminars in immunology. Elsevier. 2017; 34: 123-132.
- Bezu L, Kepp O, Cerrato G, Pol J, Fucikova J, Spisek R, et al. Trial watch: peptide-based vaccines in anticancer therapy. Oncoimmunology. 2018; 7: e1511506.
- Liu H, Moynihan KD, Zheng Y, Szeto GL, Li AV, Huang B, et al. Structure-based programming of lymph-node targeting in molecular vaccines. Nature. 2014; 507: 519-22.
- Clemente M, Corigliano MG. Overview of plant-made vaccine antigens against malaria. J Biomed Biotechnol. 2012; 2012: 206918.
- Nandi S, et al. Techno-economic analysis of a transient plant-based platform for monoclonal antibody production. In: MAbs. Taylor & Francis. 2016; 8: 1456-1466.
- Qiu X, Wong G, Audet J, Bello A, Fernando L, Alimonti JB, et al. Reversion of advanced Ebola virus disease in nonhuman primates with ZMapp. Nature. 2014; 514: 47-53.
- PREVAIL II Writing Group, Multi-National PREVAIL II Study Team, Davey RT, Dodd L, Proschan MA, Neaton J et al. A randomized, controlled trial of ZMapp for Ebola virus infection. N Engl J Med. 2016; 375: 1448-56.
- Fries CN, Curvino EJ, Chen JL, Permar SR, Fouda GG, Collier JH. Advances in nanomaterial vaccine strategies to address infectious diseases impacting global health. Nat Nanotechnol. 2021; 16: 1-14.
- World health organization. The top 10 causes of death https://www. who. int/news-room/fact-sheets/detail/the-top-10-causes-of-death. Last consultation: November 2019.
- Chen I, Cooney R, Feachem RGA, Lal A, Mpanju-Shumbusho W. The Lancet Commission on malaria eradication. Lancet. 2018; 391: 1556-8.
- HIV, U.G., AIDS statistics. Fact sheet. Moon. 2018.
- Moon JJ, Suh H, Li AV, Ockenhouse CF, Yadava A, Irvine DJ. Enhancing humoral responses to a malaria antigen with nanoparticle vaccines that expand Tfh cells and promote germinal center induction. Proc Natl Acad Sci USA. 2012; 109: 1080-5.
- Demento SL, Cui W, Criscione JM, Stern E, Tulipan J, Kaech SM, et al. Role of sustained antigen release from nanoparticle vaccines in shaping the T cell memory phenotype. Biomaterials. 2012; 33: 4957-64.
- Swartz MA, Hirosue S, Hubbell JA. Engineering approaches to immunotherapy. Sci Transl Med. 2012; 4: 148rv9.
- Hirosue S, Kourtis IC, van der Vlies AJ, Hubbell JA, Swartz MA. Antigen delivery to dendritic cells by poly (propylene sulfide) nanoparticles with disulfide conjugated peptides: cross-presentation and T cell activation. Vaccine. 2010; 28: 7897-906.
- Reddy ST, van der Vlies AJ, Simeoni E, Angeli V, Randolph GJ, O’Neil CP, et al. Exploiting lymphatic transport and complement activation in nanoparticle vaccines. Nat Biotechnol. 2007; 25: 1159-64.
- Kaminskas LM, Porter CJ. Targeting the lymphatics using dendritic polymers (dendrimers). Adv Drug Deliv Rev. 2011; 63: 890-900.
- Kourtis IC, Hirosue S, de Titta A, Kontos S, Stegmann T, Hubbell JA, et al. Peripherally administered nanoparticles target monocytic myeloid cells, secondary lymphoid organs and tumors in mice. PLOS ONE. 2013; 8: e61646.
- Lai SK, Wang YY, Wirtz D, Hanes J. Micro- and macrorheology of mucus. Adv Drug Deliv Rev. 2009; 61: 86-100.
- Lai SK, Wang YY, Hanes J. Mucus-penetrating nanoparticles for drug and gene delivery to mucosal tissues. Adv Drug Deliv Rev. 2009; 61: 158-71.
- Lai SK, Wang YY, Hida K, Cone R, Hanes J. Nanoparticles reveal that human cervicovaginal mucus is riddled with pores larger than viruses. Proc Natl Acad Sci USA. 2010; 107: 598-603.
- Schuster BS, Suk JS, Woodworth GF, Hanes J. Nanoparticle diffusion in respiratory mucus from humans without lung disease. Biomaterials. 2013; 34: 3439-46.
- Champion JA, Mitragotri S. Role of target geometry in phagocytosis. Proc Natl Acad Sci USA. 2006; 103: 4930-4.
- Yu SS, Lau CM, Thomas SN, Jerome WG, Maron DJ, et al. Size- and charge-dependent non-specific uptake of pegylated nanoparticles by macrophages. Int J Nanomedicine. 2012; 7: 799.
- Fröhlich E. The role of surface charge in cellular uptake and cytotoxicity of medical nanoparticles. Int J Nanomedicine. 2012; 7: 5577-91.
- Xiang SD, Scholzen A, Minigo G, David C, Apostolopoulos V, Mottram PL, et al. Pathogen recognition and development of particulate vaccines: does size matter? Methods. 2006; 40: 1-9.
- Leleux J, Atalis A, Roy K. Engineering immunity: modulating dendritic cell subsets and lymph node response to direct immune-polarization and vaccine efficacy. J Control Release. 2015; 219: 610-21.
- Figdor CG, Van Kooyk Y, Adema GJ. C-type lectin receptors on dendritic cells and Langerhans cells. Nat Rev Immunol. 2002; 2: 77-84.
- Tokatlian T, Read BJ, Jones CA, Kulp DW, Menis S, Chang JYH, et al. Innate immune recognition of glycans targets HIV nanoparticle immunogens to germinal centers. Science. 2019; 363: 649-54.
- Smirnov D, Schmidt JJ, Capecchi JT, Wightman PD. Vaccine adjuvant activity of 3M-052: an imidazoquinoline designed for local activity without systemic cytokine induction. Vaccine. 2011; 29: 5434-42.
- Ilyinskii PO, Roy CJ, O’Neil CP, Browning EA, Pittet LA, Altreuter DH, et al. Adjuvant-carrying synthetic vaccine particles augment the immune response to encapsulated antigen and exhibit strong local immune activation without inducing systemic cytokine release. Vaccine. 2014; 32: 2882-95.
- Hanson MC, Crespo MP, Abraham W, Moynihan KD, Szeto GL, Chen SH, et al. Nanoparticulate STING agonists are potent lymph node–targeted vaccine adjuvants. J Clin Invest. 2015; 125: 2532-46.
- Friede M, Muller S, Briand JP, Van Regenmortel MH, Schuber F. Induction of immune response against a short synthetic peptide antigen coupled to small neutral liposomes containing monophosphoryl lipid A. Mol Immunol. 1993; 30: 539-47.
- Kim M-G, Park JY, Shon Y, Kim G, Shim G, Oh Y. Nanotechnology and vaccine development. Asian J Pharm Sci. 2014; 9: 227-35.
- Keikha R, Daliri K, Jebali A. The use of nanobiotechnology in immunology and vaccination. Vaccines. 2021; 9: 74.
- Watson DS, Endsley AN, Huang L. Design considerations for liposomal vaccines: influence of formulation parameters on antibody and cell-mediated immune responses to liposome associated antigens. Vaccine. 2012; 30: 2256-72.
- de Veer M, Neeland M, Burke M, Pleasance J, Nathanielsz J, Elhay M, et al. Cell recruitment and antigen trafficking in afferent lymph after injection of antigen and poly (I: C) containing liposomes, in aqueous or oil-based formulations. Vaccine. 2013; 31: 1012-8.
- Lay M, Callejo B, Chang S, Hong DK, Lewis DB, Carroll TD, et al. Cationic lipid/DNA complexes (JVRS-100) combined with influenza vaccine (Fluzone®) increases antibody response, cellular immunity, and antigenically drifted protection. Vaccine. 2009; 27: 3811-20.
- O’Hagan DT. MF59 is a safe and potent vaccine adjuvant that enhances protection against influenza virus infection. Expert Rev Vaccines. 2007; 6: 699-710.
- Vesikari T, Knuf M, Wutzler P, Karvonen A, Kieninger-Baum D, Schmitt HJ, et al. Oil-in-water emulsion adjuvant with influenza vaccine in young children. N Engl J Med. 2011; 365: 1406-16.
- Calabro S, Tritto E, Pezzotti A, Taccone M, Muzzi A, Bertholet S, et al. The adjuvant effect of MF59 is due to the oil-in-water emulsion formulation, none of the individual components induce a comparable adjuvant effect. Vaccine. 2013; 31: 3363-9.
- Klucker MF, Dalençon F, Probeck P, Haensler J. AF03, an alternative squalene emulsion‐based vaccine adjuvant prepared by a phase inversion temperature method. J Pharm Sci. 2012; 101: 4490-500.
- Cotte JF, Sonnery S, Martial F, Dubayle J, Dalençon F, Haensler J, et al. Characterization of surfactants in an oil-in-water emulsion-based vaccine adjuvant using MS and HPLC–MS: structural analysis and quantification. Int J Pharm. 2012; 436: 233-9.
- Makidon PE, Nigavekar SS, Bielinska AU, Mank N, Shetty AM, Suman J, et al. Characterization of stability and nasal delivery systems for immunization with nanoemulsion-based vaccines. J Aerosol Med Pulm Drug Deliv. 2010; 23: 77-89.
- Lousada-Dietrich S, Jogdand PS, Jepsen S, Pinto VV, Ditlev SB, Christiansen M, et al. A synthetic TLR4 agonist formulated in an emulsion enhances humoral and Type 1 cellular immune responses against GMZ2–A GLURP–MSP3 fusion protein malaria vaccine candidate. Vaccine. 2011; 29: 3284-92.
- Thomas C, Rawat A, Hope-Weeks L, Ahsan F. Aerosolized PLA and PLGA nanoparticles enhance humoral, mucosal and cytokine responses to hepatitis B vaccine. Mol Pharm. 2011; 8: 405-15.
- Bershteyn A, Hanson MC, Crespo MP, Moon JJ, Li AV, Suh H, et al. Robust IgG responses to nanograms of antigen using a biomimetic lipid-coated particle vaccine. J Control Release. 2012; 157: 354-65.
- Moon JJ, Suh H, Polhemus ME, Ockenhouse CF, Yadava A, Irvine DJ. Antigen-displaying lipid-enveloped PLGA nanoparticles as delivery agents for a Plasmodium vivax malaria vaccine. PLOS ONE. 2012; 7: e31472.
- Gupta PN, Vyas SP. Investigation of lectinized liposomes as M-cell targeted carrier-adjuvant for mucosal immunization. Colloids Surf B Biointerfaces. 2011; 82: 118-25.
- Ulery BD, Kumar D, Ramer-Tait AE, Metzger DW, Wannemuehler MJ, Narasimhan B. Design of a protective single-dose intranasal nanoparticle-based vaccine platform for respiratory infectious diseases. PLOS ONE. 2011; 6: e17642.
- Torres MP, Wilson-Welder JH, Lopac SK, Phanse Y, Carrillo-Conde B, Ramer-Tait AE, et al. Polyanhydride microparticles enhance dendritic cell antigen presentation and activation. Acta Biomater. 2011; 7: 2857-64.
- Saini V, Jain V, Sudheesh MS, Dixit S, Gaur RL, Sahoo MK, et al. Humoral and cell-mediated immune-responses after administration of a single-shot recombinant hepatitis B surface antigen vaccine formulated with cationic poly (l-lactide) microspheres. J Drug Target. 2010; 18: 212-22.
- Mann JF, McKay PF, Arokiasamy S, Patel RK, Klein K, Shattock RJ. Pulmonary delivery of DNA vaccine constructs using deacylated PEI elicits immune responses and protects against viral challenge infection. J Control Release. 2013; 170: 452-9.
- Slütter B, Bal S, Keijzer C, Mallants R, Hagenaars N, Que I, et al. Nasal vaccination with N-trimethyl chitosan and PLGA based nanoparticles: nanoparticle characteristics determine quality and strength of the antibody response in mice against the encapsulated antigen. Vaccine. 2010; 28: 6282-91.
- Sawaengsak C, Mori Y, Yamanishi K, Mitrevej A, Sinchaipanid N. Chitosan nanoparticle encapsulated hemagglutinin-split influenza virus mucosal vaccine. AAPS PharmSciTech. 2014; 15: 317-25.
- Raman S, Machaidze G, Lustig A, Aebi U, Burkhard P. Structure-based design of peptides that self-assemble into regular polyhedral nanoparticles. Nanomedicine. 2006; 2: 95-102.
- Doll TA, Neef T, Duong N, Lanar DE, Ringler P, Müller SA, et al. Optimizing the design of protein nanoparticles as carriers for vaccine applications. Nanomedicine. 2015; 11: 1705-13.
- Yang S, Wang Z, Ping Y, Miao Y, Xiao Y, Qu L, et al. PEG/PEI-functionalized single-walled carbon nanotubes as delivery carriers for doxorubicin: synthesis, characterization, and in vitro evaluation. Beilstein J Nanotechnol. 2020; 11: 1728-41.
- Debnath SK, Srivastava R. Drug delivery with carbon-based nanomaterials as versatile nanocarriers: progress and prospects. Front Nanotechnol. 2021; 3: 644564.
- Maiti D, Tong X, Mou X, Yang K. Carbon-based nanomaterials for biomedical applications: a recent study. Front Pharmacol. 2018; 9: 1401.
- Hadidi N, Kobarfard F, Nafissi-Varcheh N, Aboofazeli R. Optimization of single-walled carbon nanotube solubility by noncovalent pegylation using experimental design methods. Int J Nanomedicine. 2011; 6: 737-46.
- Hadidi N, Kobarfard F, Nafissi-Varcheh N, Aboofazeli R. Pegylated single-walled carbon nanotubes as nanocarriers for cyclosporin A delivery. AAPS PharmSciTech. 2013; 14: 593-600.
- Hadidi N, Hosseini Shirazi SF, Kobarfard F, Nafissi-Varchehd N, Aboofazeli R. Evaluation of the effect of pegylated single-walled carbon nanotubes on viability and proliferation of Jurkat cells. Iran J Pharm Res. 2012; 11: 27-37.
- Hadidi N, Sharifnia Z, Eteghadi A, Shokrgozar MA, Mosaffa N. Pegylated single-walled carbon nanotubes as co-adjuvants enhance expression of maturation markers in monocyte-derived dendritic cells. Nanomedicine (Lond). 2021; 16: 171-88.
- Hadidi N, Shahbahrami Moghadam N, Pazuki G, Parvin P, Shahi F. In vitro evaluation of DSPE-PEG (5000) amine SWCNT toxicity and efficacy as a novel nanovector candidate in photothermal therapy by response surface methodology (RSM). Cells. 2021; 10: 2874.
- Hadidi N, Ramezani L, Shokrghozar MA, Amanzadeh A, Saffari M. The effect of surface modification of single-wall carbon nanotubes on cytotoxicity reduction in the liver cell model (HEPG2). KAUMS J (Feyz). 2015; 19: 302-8.
- Paliwal S, Pandey K, Pawar S, Joshi H, Bisht N. A review on carbon nanotubes: as a Nano carrier drug delivery system. Indian J Pharm Sci. 2020; 82: 766-72.
- Zare H, Ahmadi S, Ghasemi A, Ghanbari M, Rabiee N, Bagherzadeh M, et al. Carbon nanotubes: smart drug/gene delivery carriers. Int J Nanomedicine. 2021; 16: 1681-706.
- de Carvalho Lima EN, Diaz RS, Justo JF, Castilho Piqueira JR. Advances and perspectives in the use of carbon nanotubes in vaccine development. Int J Nanomedicine. 2021; 16: 5411-35.
- Joshi P, Riley P, Gupta S, Narayan RJ, Narayan J. Advances in laser-assisted conversion of polymeric and graphitic carbon into nanodiamond films. Nanotechnology. 2021; 32: 432001.
- Jampilek J, Kralova K. Advances in drug delivery nanosystems using graphene-based materials and carbon nanotubes. Materials (Basel). 2021; 14: 1059.
- Yang ST, Luo J, Zhou Q, Wang H. Pharmacokinetics, metabolism and toxicity of carbon nanotubes for biomedical purposes. Theranostics. 2012; 2: 271-82.
- Bardhan NM, Jansen P, Belcher AM. Graphene, carbon nanotube and plasmonic nanosensors for detection of viral pathogens: opportunities for rapid testing in pandemics like COVID-19. Front Nanotechnol. 2021; 3: 64.
- Zomorodbakhsh S, Abbasian Y, Naghinejad M, Sheikhpour M. The effects study of isoniazid conjugated multi-wall carbon nanotubes nanofluid on Mycobacterium tuberculosis. Int J Nanomedicine. 2020; 15: 5901-9.
- Jannati H, Sheikhpour M, Siadat SD, Safarian P. Antimicrobial activity and drug delivery ability of functionalized Multi-Walled Carbon nanotubes Nanofluid on staphylococcus aureus. Nanomed Res J. 2021; 6: 248-56.
- Sheikhpour M, Naghinejad M, Kasaeian A, Lohrasbi A, Shahraeini SS, Zomorodbakhsh S. The applications of carbon nanotubes in the diagnosis and treatment of lung cancer: a critical review. Int J Nanomedicine. 2020; 15: 7063-78.
- Amiri V, Sheikhpour M, Shooraj F, Parzadeh M, Masoumi M. Antibacterial effects study of nanofluid containing carbon nanotubes and evaluation of its efficacy on reducing antibiotic resistance of Pseudomonas aeruginosa Journal of Islamic Azad Univesity-Tehran Medical Branch. MEDICAL SCIENCES. 2021; 31: 276-83.
- Meng J, Meng J, Duan J, Kong H, Li L, Wang C, et al. Carbon nanotubes conjugated to tumor lysate protein enhance the efficacy of an antitumor immunotherapy. Small. 2008; 4: 1364-70.
- Zeinali M, Jammalan M, Ardestani SK, Mosaveri N. Immunological and cytotoxicological characterization of tuberculin purified protein derivative (PPD) conjugated to single-walled carbon nanotubes. Immunol Lett. 2009; 126: 48-53.
- Parra J, Abad-Somovilla A, Mercader JV, Taton TA, Abad-Fuentes A. Carbon nanotube-protein carriers enhance size-dependent self-adjuvant antibody response to haptens. J Control Release. 2013; 170: 242-51.
- Kim CG, Kye YC, Yun CH. The role of nanovaccine in cross-presentation of antigen-presenting cells for the activation of CD8+ T cell responses. Pharmaceutics. 2019; 11: 612.
- Lin J, et al. Understanding the synergistic effect of physicochemical properties of nanoparticles and their cellular entry pathways. Commun Biol. 2020; 3: 1-10.
- Mohammadi MR, Nojoomi A, Mozafari M, Dubnika A, Inayathullah M, Rajadas J. Nanomaterials engineering for drug delivery: a hybridization approach. J Mater Chem B. 2017; 5: 3995-4018.
- Pescatori M, Bedognetti D, Venturelli E, Ménard-Moyon C, Bernardini C, Muresu E, et al. Functionalized carbon nanotubes as immunomodulator systems. Biomaterials. 2013; 34: 4395-403.
- Orecchioni M, Bedognetti D, Sgarrella F, Marincola FM, Bianco A, Delogu LG. Impact of carbon nanotubes and graphene on immune cells. J Transl Med. 2014; 12: 138.
- Meunier E, Coste A, Olagnier D, Authier H, Lefèvre L, Dardenne C, et al. Double-walled carbon nanotubes trigger IL-1β release in human monocytes through Nlrp3 inflammasome activation. Nanomedicine. 2012; 8: 987-95.
- Delogu LG, Venturelli E, Manetti R, Pinna GA, Carru C, Madeddu R, et al. Ex vivo impact of functionalized carbon nanotubes on human immune cells. Nanomedicine (Lond). 2012; 7: 231-43.
- Allen BL, Kichambare PD, Gou P, Vlasova II, Kapralov AA, Konduru N, et al. Biodegradation of single-walled carbon nanotubes through enzymatic catalysis. Nano Lett. 2008; 8: 3899-903.
- Vickers NJ. Animal communication: when I’m calling you, will you answer too? Curr Biol. 2017; 27: R713-5.
- Vlasova II, Kapralov AA, Michael ZP, Burkert SC, Shurin MR, Star A, et al. Enzymatic oxidative biodegradation of nanoparticles: mechanisms, significance and applications. Toxicol Appl Pharmacol. 2016; 299: 58-69.
- Kotchey GP, Gaugler JA, Kapralov AA, Kagan VE, Star A. Effect of antioxidants on enzyme-catalysed biodegradation of carbon nanotubes. J Mater Chem B. 2013; 1: 302-9.
- Prashant CK, Kumar M, Dinda AK. Nanoparticle based tailoring of adjuvant function: the role in vaccine development. J Biomed Nanotechnol. 2014; 10: 2317-31.
- Yan X, Zhou M, Yu S, Jin Z, Zhao K. An overview of biodegradable nanomaterials and applications in vaccines. Vaccine. 2020; 38: 1096-104.
- Pati R, Shevtsov M, Sonawane A. Nanoparticle vaccines against infectious diseases. Front Immunol. 2018; 9: 2224.
- Das A, Ali N. Nanovaccine: an emerging strategy. Expert Rev Vaccines. 2021; 20: 1273-90.
- Gheibi Hayat SM, Darroudi M. Nanovaccine: A novel approach in immunization. J Cell Physiol. 2019; 234: 12530-6.
- Feng C, Li Y, Ferdows BE, Patel DN, Ouyang J, Tang Z, et al. Emerging vaccine nanotechnology: from defense against infection to sniping cancer. Acta Pharm Sin B. 2022; 12: 2206-23.
- Santos P, Almeida F. Exosome-based vaccines: history, current state, and clinical trials. Front Immunol. 2021; 12: 711565.
- Zhao G, Jiang Y, Ma P, Wang S, Nie G, et al. Membrane-based cancer nanovaccines: the time is now. QJM An Int J Med. 2023; 116: 621-624.
- Diao L, Liu M. Rethinking antigen source: cancer vaccines based on whole tumor cell/tissue lysate or whole tumor cell. Adv Sci (Weinh). 2023; 10: e2300121.
- Wang S, Li F, Ye T, Wang J, Lyu C, Qing S, et al. Macrophage-tumor chimeric exosomes accumulate in lymph node and tumor to activate the immune response and the tumor microenvironment. Sci Transl Med. 2021; 13: eabb6981.
- Morishita M, Takahashi Y, Matsumoto A, Nishikawa M, Takakura Y. Exosome-based tumor antigens–adjuvant co-delivery utilizing genetically engineered tumor cell-derived exosomes with immunostimulatory CpG DNA. Biomaterials. 2016; 111: 55-65.
- Luzuriaga MA, Shahrivarkevishahi A, Herbert FC, Wijesundara YH, Gassensmith JJ. Biomaterials and nanomaterials for sustained release vaccine delivery. Wiley Interdiscip Rev Nanomed Nanobiotechnology. 2021; 13: e1735.
- Yang J, Li Y, Jin S, Xu J, Wang PC, Liang XJ, et al. Engineered biomaterials for development of nucleic acid vaccines. Biomater Res. 2015; 19: 5.
- Uppu DSSM, Turvey ME, Sharif ARM, Bidet K, He Y, Ho V, et al. Temporal release of a three-component protein subunit vaccine from polymer multilayers. J Control Release. 2020; 317: 130-41.
- Repka MA, Shah S, Lu J, Maddineni S, Morott J, Patwardhan K, et al. Melt extrusion: process to product. Expert Opin Drug Deliv. 2012; 9: 105-25.