
Research Article
Austin J Biosens & Bioelectron. 2024; 9(1): 1048.
Enhanced Photocatalytic Degradation of Congo Red Dye with Iron-Cobalt Oxide Nanoparticles: A Study on Sustainable Remediation Strategies for Aquatic Contaminants
Noor Zada1*; Hamid Ullah2; Naveed Ullah2; Kashif Hussain2; Abbas Khan2; Fazal Habib2; Naseer Ul Haq2; Khalil Ullah2; Zaheer Ahmad3; Syed Muhammad Sohail4; Abdullah1; Roshni Begum1
1Department of Chemistry GDC Lal Qilla, Dir Lower KPK, Pakistan
2Department of Chemistry Government Postgraduate College Timergara, Lower Dir, Pakistan
3Department of Chemistry GDC Khanpur, Haripur, Pakistan
4Department of Chemistry GDC Badabera, Peshawar, Pakistan
*Corresponding author: Noor Zada Department of Chemistry Government Degree College Lal Qilla, Lower Dir, KPK-Pakistan. Email syednoorzada88@gmail.com
Received: February 28, 2024 Accepted: April 04, 2024 Published: April 11, 2024
Abstract
Transition metals (Iron and Cobalt), were used to synthesize bimetallic oxide nanoparticles through a wet chemical precipitation method. The Nanoparticles (NPs) were characterized using Scanning Electron Microscopy (SEM) and Energy Dispersive X-rays (EDX), which revealed that they exhibited a mostly spherical, thick, dense, and agglomerated morphology. The EDX spectra confirmed the composition of the bimetallic oxide nanoparticles, with the presence of oxygen indicating their formation in the oxide form. These bimetallic oxide nanoparticles were then utilized as photocatalysts for the degradation of Congo red dye in aqueous medium under UV irradiation. The study investigated the influence of various parameters, including irradiation time, catalyst dosage, dye concentration, and pH of the medium, on the photocatalytic degradation of the dye. The results showed that Fe-Co oxide nanoparticles exhibited high photocatalytic activity, degrading 93% of the dye within 90 minutes. Furthermore, the degradation of the dye increased with longer irradiation times and higher catalyst dosages. Additionally, increasing the pH of the medium led to an enhanced degradation rate, with 80% of the dye degraded in 45 minutes at pH 10. However, the degradation rate decreased as the concentration of the dye increased.The findings from this study not only enhance our understanding of the photocatalytic properties of Iron-Cobalt oxides but also lay the foundation for further research endeavors aimed at optimizing and extending the applicability of these NPs in diverse environmental contexts. Ultimately, the outcomes of this study carry significant implications for advancing the field of environmental science and engineering, emphasizing the continued importance of exploring innovative technologies for pollution control and sustainable resource management.
Keywords: Congo red dye; Iron-cobalt nanoparticles; Wastewater treatment; UV irradiation
Introduction
Water, a vital and irreplaceable resource for daily life, development, and industrialization, faces a critical challenge due to the impact of rapid industrialization and population growth worldwide [1]. While industrial development is hailed as the backbone of a country's progress, it simultaneously poses a significant threat by releasing life-threatening wastes into aquatic systems [2]. Industries like printing, textile, papermaking, pharmaceuticals, food processing, and cosmetics contribute to this peril by discharging effluents laden with synthetic organic compounds such as dyes and pigments into water bodies [3,4]. These substances, characterized by large-scale production, high aromaticity, chemical stability, low biodegradability, toxicity, and carcinogenic nature, emerge as major environmental pollutants. The distinctive and easily observable colors of these dyes, even at low concentrations, render water highly detrimental to the environment and human health. The permanent coloration adversely affects animals' eyes and skin, leading to carcinogenic, mutagenic, and genotoxic disorders in humans, animals, and microorganisms [5]. Moreover, the presence of these dyes in water not only mars the river's beauty but also impedes sunlight penetration, affecting the photosynthesis of aquatic green plants. Consequently, water purification becomes a focal point of research and scientific interest, employing various treatment methods like adsorption, electrolysis, membrane filtration, chemical precipitation, physisorption, chemisorption, electrokinetics, ion exchange, and coagulation, aiming to address the pressing issue of water pollution caused by the discharge of organic dyes from industries. This attention to water purification is imperative due to the scarcity of fresh drinking water, making it a global concern that demands immediate and widespread solutions [6].
The realm of chemistry plays a pivotal role in comprehending the mechanisms and reactions involved in water pollution, offering diverse methodologies to address this issue and safeguard natural water resources [7]. Various techniques, including biological, physical, and chemical methods, are employed for treating water contaminated with toxic dyes [8,9]. Physical techniques such as adsorption, ion-exchange, coagulation, and membrane-filtration are commonly utilized for water treatment. However, these approaches have inherent limitations. Adsorption, for instance, is a sluggish process and proves inefficient in removing highly concentrated colors from polluted water [10]. Furthermore, physical techniques often suffer from incomplete dye degradation. Biological techniques, encompassing aerobic, anaerobic, and combined anaerobic-aerobic processes, leverage microorganisms for water treatment. While microorganisms play a vital role in these processes, they are less effective in degrading aromatic dyes with complex structures.
Over the past few years, the oxidation method, particularly the Advanced-Oxidation Process (AOP), has gained prominence in wastewater treatment [11-13]. This method involves the rapid formation of highly reactive free radicals capable of oxidizing and breaking down organic effluents. However, the short lifespan of oxidants poses a limitation.
An alternative, cost-effective approach involves harnessing solar light for wastewater treatment. Solar light, abundantly available on Earth at no cost, proves to be an excellent resource for degrading organic dyes present in polluted water. Nanomaterials, acting as photocatalysts, facilitate the photo-degradation of organic effluents, a process known as photocatalysis [14,15]. Heterogeneous photocatalysis, categorized as an advanced oxidation process [16], relies on metal oxide semiconductors absorbing UV or visible radiation to generate active species responsible for oxidizing organic compounds in wastewater. The efficacy of photocatalysis hinges on the adsorption of organic compounds onto the catalyst surface, a critical factor for enhancing the degradation rate. The success of this process is also contingent upon the surface area of the photocatalyst [17]. Utilizing a suitable photocatalyst and exploiting light radiation, this process initiates the generation of highly reactive hydroxyl (OH•) radicals. These radicals possess the capability to convert water pollutants into comparatively benign end products, such as CO2, H2O, and other inorganic ions [18,19], ensuring the safety of both humans and the environment. Photocatalytic degradation presents numerous advantages over traditional methods. It stands out as an efficient and straightforward instrumental technique, characterized by easily controllable operations and non-selective oxidation. Moreover, it proves cost-effective and can achieve the complete mineralization and degradation of synthetic organic dyes [20]. This process hinges on the presence of a semiconductor photocatalyst that activates upon absorbing photons. Notably, the photocatalyst can expedite the reaction without undergoing consumption [21]. Metal nanoparticles emerge as the most frequently employed photocatalysts, with their properties directly influenced by factors such as particle shape, size, geometry, and morphology [22]. Nanoparticles, characterized by their diminutive size below 100 nm, have garnered considerable attention due to their distinctive chemical and physical properties. Their potential applications span diverse fields, including medicine, solar cells, and nanodevices [23].
Congo Red dye belongs to the category of azo dyes and features two azo bonds (-N=N-) chromophores within its molecular structure. The inherent structural stability of this dye makes it notably toxic and resistant to biodegradation [24]. Widely employed in various industries, including cosmetics, paper, pharmaceuticals, chemicals, and textiles, Congo Red dye exhibits high stability in light, detergents, and water. The presence of aromatic amines in the dye's structure, responsible for carcinogenesis, poses a significant threat to both aquatic life and human beings [25].
The present research involves the synthesis of Iron-Cobalt bimetallic oxide nanoparticles (Fe-Co-NPs), as photo-catalyst, through a wet chemical precipitation method. The comprehensive investigation into the photocatalytic degradation of Congo red dye utilizing Iron-Cobalt oxides NPs holds paramount significance in addressing contemporary environmental challenges. The study not only may contribute valuable insights into the efficiency of advanced nanomaterials for pollutant remediation but also underscore the potential application of Iron-Cobalt oxides in sustainable water treatment processes. The significance lies in the urgent need to develop effective and eco-friendly strategies to mitigate the adverse impacts of industrial pollutants on aquatic ecosystems. Furthermore, the successful degradation of Congo red dye may signify a promising step towards the broader goal of developing efficient and scalable photocatalytic processes for wastewater treatment. As society continues to grapple with escalating concerns related to water pollution, this research paves the way for future advancements in the design and application of nanomaterials, offering sustainable solutions for environmental remediation and contributing to the collective efforts for a cleaner and healthier planet. The advantage of such catalysts is, if the band gap of one catalyst is high then the second one lowers its band gap and electron excitation. Moreover, the catalytic activity of the mixed bimetallic nanoparticles is much higher compared to single photo-catalyst. The morphology, structure, and elemental composition of the photo-catalyst were thoroughly examined using Scanning Electron microscopy (SEM), and energy-dispersive X-ray spectroscopy. The objectives of this study were; to develop a cost-effective and efficient photo-catalyst for the degradation of congo red dye. To mitigate water pollution caused by congo red dye and to investigate the influence of time, pH of the medium, amount of photo-catalyst, amount of recovered photo-catalyst, and dye concentration on the degradation of congo red dye.
Materials and Methods
Chemicals and Instrumentation
In terms of the chemicals and instrumentation utilized in this study, we sourced Manganese Chloride (MnCl4.2H2O), silver nitrate (AgNO3), sodium hydroxide (NaOH), nitric acid (HNO3), hydrochloric Acid (HCl), and sulfuric acid (H2SO4) from Frontier Chemical Company. Additionally, the Congo red dye crucial for the experiments was acquired from Danyal Trading Company located in Mingora, Pakistan.
For the morphological analysis of bimetallic oxide nanoparticles (NPs), we employed a JEOL JSM-5910 Scanning Electron Microscope (SEM). To delve into the elemental composition, both A-MWCNT-supported and unsupported bimetallic oxide NPs underwent scrutiny through energy-dispersive X-ray spectroscopy (EDX) utilizing a Model INCA 200/Oxford Instruments, based in Oxford, UK.
The investigation extended to the photodegradation study of CR in an aqueous medium. This particular aspect of the research involved the use of a UV–visible spectrophotometer. To delve into the structural aspects of the prepared catalyst, X-Ray Diffraction (XRD) patterns were meticulously collected. This process was facilitated by a PHILIPS X’ Pert PRO X-ray diffractometer employing Cu K-α radiation. The XRD pattern collection spanned from 10° to 80°, with an incremental step size set at 0.01°. These comprehensive methodologies were integral to the analytical processes undertaken in the study, providing a robust foundation for the subsequent findings and interpretations.
Preparation of Fe-Co oxides NPs
To synthesize Iron-Cobalt bimetallic oxide nanoparticles (Fe-Co oxides NPs), a round bottom flask containing 100 mL each of 0.1M CoCl2 and 0.1M FeCl3·3H2O solutions underwent a controlled addition of 0.2M NaOH drop by drop with constant stirring until reaching a basic pH of 10. The resulting mixture was then heated at 60°C for 2 hours under continuous stirring, leading to the formation of Fe-Co oxides nanoparticles in the form of a precipitate. After cooling, the NPs were filtered, washed with distilled water to remove any residual chemicals or impurities, and subsequently dried either under sunlight or using an oven before storage.
Photodegradation of Congo red dye by using Fe-Co oxides NPs
For the photo-degradation of CR dye, the prepared Fe-Co oxides NPs served as a photocatalyst under UV-light irradiation (254 nm, 15 W). An 80 ppm solution of CR dye was created, and 0.002g of Fe-Co oxides NPs were added to 20 mL of the dye solution in 50 mL beakers. After allowing for a 15-minute adsorption-desorption equilibrium in the dark, the mixture underwent UV-light exposure with constant stirring for varying time periods. Following UV-irradiation, the NPs catalyst was separated from the dye solution through filtration. The UV-Visible spectrophotometer was employed to monitor the photo-degradation of CR dye. Additionally, the influence of catalyst dosage and pH on the photo-degradation process was investigated. The catalyst dosage study involved varying amounts of catalyst while keeping other parameters constant, while the pH study explored the effects of different pH levels (4, 7, and 10) on the dye's photo-degradation. Acidic and basic solutions were prepared by adding HNO3 (0.1M) and NaOH (0.1M) dropwise to distilled water, respectively, before the dye solutions were prepared in these media.
UV-Vis Analysis
The photo-degradation study of the CR dye was carried out by using UV-Vis spectrophotometer. The % degradation of dyes was calculated by using the following formulas.
Degradation rate (%) =
Degradation rate (%) =
where Co stands for initial dye concentrations, C for the concentration of dye after sun-light irradiation, while Ao shows initial absorbance, and A is the dye absorbance after sun-light irradiation.
Results and Discussion
Characterization
In Fig. S1, Scanning Electron Microscope (SEM) micrographs of Fe-Co oxides nanoparticles are displayed at magnifications of 2500, 5000, and 10000, respectively. The Fe-Co oxide nanoparticles predominantly exhibit a spherical shape in the images. Throughout the micrographs, a consistent and homogeneous distribution of the nanoparticles is evident. Some particles appear in aggregated, agglomerated, and clustered formations, indicating certain degrees of nanoparticle clustering. Notably, the sizes of the nanoparticles remain nearly uniform, with no significant variation observed. This information provides valuable insights into the morphology, distribution, and size consistency of the Fe-Co oxide nanoparticles, essential for understanding their characteristics and potential applications. In Figure 1, the Energy-Dispersive X-ray (EDX) profiles of the Fe-Co oxide nanoparticles are also depicted. The long peaks observed in the figure correspond to the elemental composition of Iron (Fe), indicating the presence of iron in the nanoparticles. Conversely, short peaks represent the elemental composition of Cobalt (Co), confirming the existence of cobalt in the nanoparticles. The presence of oxygen peaks in the EDX profiles further validates that the Fe and Co nanoparticles are in their oxide form. This information is crucial for characterizing the chemical composition and oxidation state of the nanoparticles, providing insights into their properties and potential applications.

Figure 1: Photographic images of dilution and catalytic behaviour of CR dye after degradation at different time intervals (a);
UV-visible graphs and percent degradation of CR dye at different time intervals (b and c respectively).
Photocatalytic degradation study of Congo red dye
The photocatalytic activity of the Fe-Co oxide nanoparticles, synthesized in this study, was assessed by catalytically degrading Congo red dye in an aqueous medium under UV irradiation. The progression of the degradation process was visually captured through photographs of the CR dye solution at various time intervals (15, 30, 45, 60, 75, and 90 minutes), as depicted in Figures 1a. Upon examination of the images, a discernible trend emerges. The color changes observed in the pictures provide visual evidence of the photodegradation of Congo red dye over time. Specifically, the gradual increase in irradiation time corresponds to a visible enhancement in the degradation of the dye. At earlier time points, the characteristic color of Congo red is more pronounced, indicating a higher concentration of the dye in the solution. However, as the irradiation time extends, there is a noticeable fading and attenuation of the Congo red color, signifying the progressive degradation of the dye molecules facilitated by the Fe-Co oxide nanoparticles under UV irradiation. These visual observations align with the quantitative analysis of the photocatalytic degradation process, confirming that the synthesized Fe-Co oxide nanoparticles exhibit a time-dependent enhancement in their ability to degrade Congo red dye, thus emphasizing the efficacy of the photocatalytic system over extended irradiation periods.
The photo-degradation of Congo Red (CR) was assessed by monitoring the relative intensity of the UV-Vis spectral peak associated with CR. The peak of maximum absorbance for CR occurs at 497 nm. In Figure 2c, the percentage of degradation of Congo red dye is illustrated over time. The graph indicates a clear trend of increasing degradation with the passage of time. Specifically, the percent degradation of the dye is recorded as follows: 33% at 15 minutes, 50% at 30 minutes, 66% at 45 minutes, 83% at 60 minutes, 86% at 75 minutes, and 93% at 90 minutes. These results suggest that the photo-degradation of CR is a time-dependent process, with a higher duration leading to a more substantial degradation of the dye.
Effect of dye concentration on photocatalytic degradation
The impact of dye concentration on the photocatalytic degradation, while maintaining a constant amount of catalyst for a duration of 20 minutes, was examined. The findings revealed that the degradation rate decreases with an increase in dye concentration. As depicted in Figure 2, the photo-degradation of CR dye diminishes as the dye concentration rises. This trend can be explained by the Beer–Lambert law, which posits that higher dye concentrations result in a reduction of the path length traversed by entering photons. Consequently, there is a lower adsorption of photons on the catalyst surface, leading to a diminished occurrence of photo-degradation. Figure 2c illustrates the percentage of degradation of CR dye, showcasing a decline as the dye concentration increases. Specifically, the percent degradation in a 20-minute time frame for dye solutions of 70, 80, and 90 ppm is recorded as 50%, 33%, and 11%, respectively. This outcome underscores the inverse relationship between dye concentration and the efficiency of photocatalytic degradation, providing valuable insights into optimizing conditions for effective dye removal.
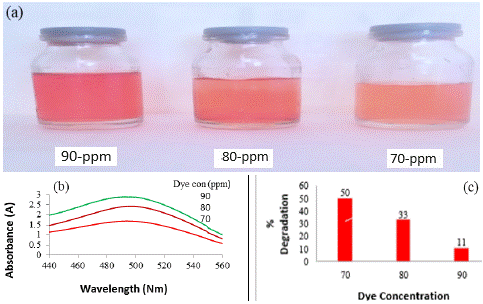
Figure 2: Photographic images of dilution at different dye concentration (a); UV-Visible graphs and percent degradation of CR dye of different concentrations (b and c respectively).
Effect of pH on the photo degradation
The influence of pH on the photo degradation of CR emerges as a crucial factor, considering the diverse pH levels at which industrial effluents, particularly from sectors like dyes, paints, and textiles, are typically discharged. The variation in pH levels in these effluents necessitates a comprehensive exploration of how pH impacts the photo-degradation process.
As depicted in Figure 3, the photo-degradation rate of CR dye exhibits a discernible dependence on the pH of the medium. This correlation underscores the pivotal role of pH in influencing both the overall photo-degradation efficiency and the generation of hydroxyl radicals.
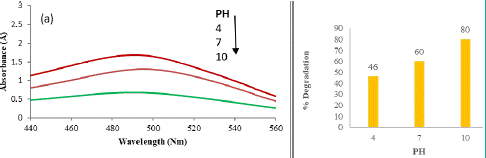
Figure 3: UV Visible graphs and percent degradation study of Congo red dye at pH 4, 7 and 10 for 20 minutes.
The graph illustrates a positive relationship between pH and the rate of photocatalytic degradation, with a notable increase in degradation as the pH of the medium rises. At higher pH levels, there is a corresponding escalation in the formation of active hydroxyl radicals (•OH), contributing to an enhanced degradation rate. The clear trend observed in the graph indicates a proportional rise in the percentage of degradation with an increase in pH. Specifically, the percentage degradations after 45 minutes at pH 4, 7, and 10 are recorded at 46%, 60%, and 80%, respectively.
The observed pH-dependent enhancement in the photo-degradation process can be attributed to the impact of pH on the generation of hydroxyl radicals. The higher availability of hydroxyl radicals at elevated pH levels facilitates a more efficient degradation of the CR dye. This understanding is crucial for optimizing the photo-degradation process based on the specific pH conditions of industrial effluents, contributing to the development of tailored and effective wastewater treatment strategies.
Effect of the catalyst dosage on the photo degradation
The impact of catalyst dosage on the photo-degradation rate of CR dye was investigated across varying catalyst quantities, ranging from 0.002 g to a maximum of 0.008 g per 20 mL dye solution, while maintaining a constant duration of 20 minutes. The visual representation in Fig. 4 illustrates the progression of Congo red degradation in aqueous media with different catalyst amounts. Notably, there is a discernible enhancement in the photo-degradation rate as the catalyst's quantity increases.
To delve deeper into the quantitative aspect, Figure 4-b,c provides insight into the UV and percent degradation of CR dye over a 15-minute interval, utilizing different amounts of the photo catalyst. The corresponding percent degradation values stand at 33%, 46%, 64%, and 75% for catalyst quantities of 0.002 g, 0.004 g, 0.006 g, and 0.008 g, respectively. This data reinforces the positive correlation between catalyst dosage and the efficiency of Congo red dye degradation, demonstrating the potential for improved treatment outcomes with higher catalyst concentrations.
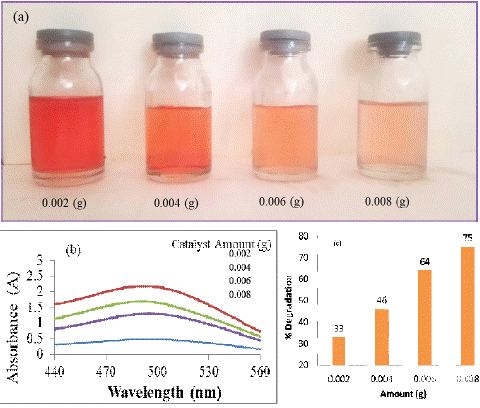
Figure 4: CR dye in aqueous media by using different amounts of the photo catalyst.UV –Visible and Percent degradation graph of CR dye degraded by different amount of catalyst.
Effect of the Recover catalyst on photo degradation
The exploration of the recovered catalyst's photodegradation performance, conducted under consistent experimental conditions, aimed to assess its effectiveness following the recovery process. The recovery involved multiple washes with deionized water to ensure the removal of any adsorbed dye on the catalyst. Despite demonstrating a considerable capacity to degrade CR dye, the recovered catalyst displayed a notable reduction in activity compared to the original catalyst.
The observed decline in the photocatalytic efficacy of the recovered catalyst can be attributed to the potential obstruction of active sites on the photocatalyst's surface. This hindrance arises from the accumulation of photosensitive hydroxides during the recovery process. These hydroxides may deposit on the catalyst's surface, leading to a compromised ability to initiate photodegradation reactions efficiently. Figure 5 provides a visual representation of the percentage degradation of Congo red dye by both the original and recovered photocatalyst. The data illustrates that the original catalyst achieved an impressive 93% dye degradation within the 90-minute experimental duration. In contrast, the recovered catalyst demonstrated a reduced performance, achieving an 82% degradation within the same timeframe. Several factors contribute to the diminished activity of the recovered catalyst. The washing steps, while essential for removing adsorbed dye, may inadvertently alter the catalyst's surface properties or compromise its structural integrity. Additionally, the presence of residual impurities or changes in the catalyst's composition during the recovery process could influence its photocatalytic performance.
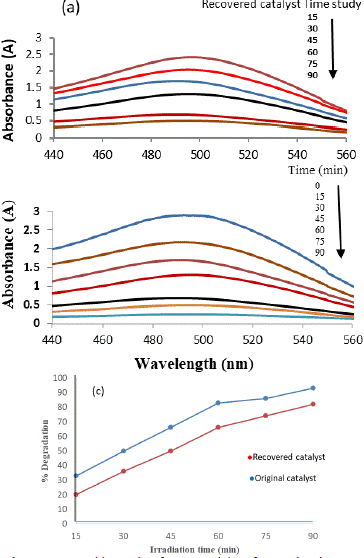
Figure 5: UV- visible graphs of Congo red dye of original and recovered photo catalyst.
In conclusion, the investigation underscores the impact of the recovery process on the photocatalytic activity of the catalyst. While recovery is crucial for sustainability and cost-effectiveness, careful consideration of the potential alterations to the catalyst's properties is essential. The observed reduction in photodegradation efficiency highlights the need for further optimization of recovery methods to maintain or enhance the catalyst's performance in subsequent applications.
Mechanism of Photocatalytic degradation and analysis of Bandgap Energy
Figure 6 display the proposed mechanism for photocatalytic degradation of the targeted CR dye. The photocatalytic degradation of dyes occurs through the following mechanism: Under UV radiation, a catalyst is activated, causing electrons to move from the valence band to the conduction band, resulting in the generation of an electron-hole pair.
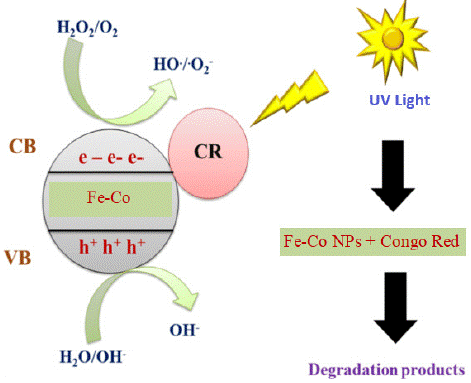
Figure 6: Schematic illustration for the mechanism of photocatalytic degradation of CR dye.
Catalysts (NPs)+ h →NPs (e-+ h+) (3)
Here, e − and h + represent the electron in the conduction band and the positive hole in the valence band, respectively. Both of these species migrate to the catalyst surface, where they interact with other species present on the surface. The positive hole can either directly oxidize the dye molecule due to its high oxidative potential or react with water molecules to produce hydroxyl radicals. The electrons present in the conduction band react with oxygen molecules, reducing them to superoxide radicals, which are highly reactive and contribute to the degradation of dyes. The possible reactions are summarized in the following equations [26, 27].
NPs + hv → NPs (h+ + e−) (4)
h+ + Dye → Oxidation of the dye molecules (5)
h+ + OH−/H2O → ∙OH (6)
e− + O2 → ∙O2− (7)
∙OH + ∙O2− + dye → degradable/less toxic species (8)
The band gap is a crucial factor in the context of photocatalysis. When a material is exposed to light, especially in the visible light spectrum, electrons in the valence band can be excited to the conduction band, creating electron-hole pairs. This process is essential for various applications, including photocatalysis.
In the case of metal oxides like cobalt oxide (Co3O4), having a band gap of 2.7 eV [28], this implies that it primarily absorbs light in the ultraviolet (UV) region, limiting its efficiency in utilizing visible light for photocatalysis. However, when iron oxide is added, resulting in a decrease in the band gap to 1.3 eV (as depicted in Figure 7, the material becomes more responsive to visible light.
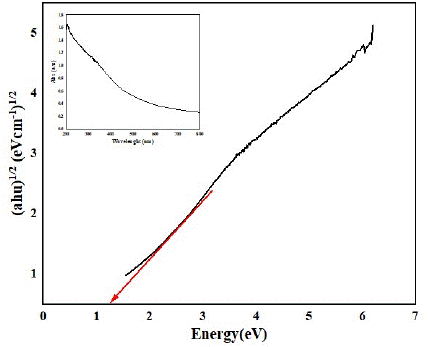
Figure 7: Bandgap plot of the prepared Fe-Co oxide nanoparticles from the UV–vis DRS results.
A UV–Vis spectrophotometer was utilized to examine both the absorption region and optical activity of the Fe-Co oxides catalyst under investigation. The determination of optical bandgaps employed the Tauc equation (Equation 3), expressed as
(ahν)1/2 = A(hν − Eg) (9)
By graphing (αhν)1/2 against hν and extrapolating a linear fit to the X-axis, valuable insights into the bandgaps (Eg) were obtained. The Tauc plot application validated that the Fe-Co NPs possessed a bandgap of 1.3 eV, visually illustrated in Figure 8. This bandgap value signifies the potential of the prepared photocatalyst for efficient utilization of visible light in various photocatalytic processes [29]. The reduction in band gap allows the metal oxide nanoparticles to absorb a broader range of light, including visible light, which constitutes a significant portion of sunlight. This enhanced light absorption promotes the generation of more electron-hole pairs, improving the photocatalytic activity of the material.
In summary, the decrease in band gap values, as observed in the case of adding iron oxide to cobalt oxide, facilitates better utilization of visible light, leading to increased electron-hole pair formation and ultimately improving the photocatalytic performance of the metal oxide nanoparticles.
Conclusion
The research findings indicate that Fe-Co oxide nanoparticles exhibit a notable level of photocatalytic activity, particularly in the process of degrading Cong red dye through photo degradation. Impressively, within a span of 90 minutes, a substantial 93% of the dye was successfully degraded. Notably, the observed trend reveals that as the duration of exposure increases, there is a corresponding augmentation in the photo degradation of the dye. Furthermore, the study highlights the influence of the catalyst's quantity on the photo degradation process. It was evident that an escalation in the amount of catalyst positively correlated with the degradation efficiency. For instance, utilizing 0.002g of the photo catalyst resulted in the degradation of 66% of the dye within a 45-minute timeframe. The impact of the medium's pH on the degradation process was also investigated. The findings illustrated a direct relationship between pH levels and dye degradation. Specifically, at a pH of 10, an impressive 80% of the dye underwent degradation within a relatively short span of 45 minutes, indicating the significance of the medium's alkalinity in enhancing the degradation efficiency. However, an intriguing observation emerged concerning the concentration of the dye. Surprisingly, the research demonstrated that as the concentration of the dye increased, the rate of photo degradation exhibited a counter-intuitive decrease. This suggests a nuanced relationship between dye concentration and the efficacy of the photo degradation process.
References
- Shivaraju HP, Midhun G, Kumar KMA, Pallavi S, Pallavi N, Behzad S. Degradation of selected industrial dyes using Mg-doped TiO2 polyscales under natural sun light as an alternative driving energy. Applied Water Science. 2017; 7: 3937.
- Shah A, Shahzad S, Munir A, Nadagouda MN, Khan GS, Shams F, et al. Micelles as soil and water decontamination agents. Chemical Reviews. 2016; 116: 6042.
- Thilagavathi M, Arivoli S, Vijayakumaran V. Adsorption of malachite green from waste water using Prosopis juliflora bark carbon. Kuwait Journal of Science. 2015; 42: 120-33.
- Avasarala BK, Tirukkovalluri SR, Bojja S. Magnesium doped titania for photocatalytic degradation of dyes in visible light. Journal of Environmental and Analytical Toxicology. 2016; 6: 1.
- Velusamy P, Lakshmi G. Enhanced photocatalytic performance of (ZnO/CeO2)-b-CD system for the effective decolorization of rhodamine B under UV light irradiation. Applied Water Science. 2017; 7: 4025.
- Zada N, Khan I, Shah T, Gul T, Khan N, Saeed K. Ag–Co oxides nanoparticles supported on carbon nanotubes as an effective catalyst for the photodegradation of Congo red dye in aqueous medium. Inorganic and Nano-Metal Chemistry. 2020; 50: 333–340.
- Lu WQ, Xie SH, Zhou WS, Zhang SH, Liu AL. Water pollution and health impact in China: a mini review. Open Environ Sci J. 2008; 2: 1-5.
- Bafana A, Devi SS, Chakrabarti T. Azo dyes: past, present and the future. Environ Rev. 2011; 19: 350-371.
- Ahmad A, Mohd-Setapar SH, Chuong CS, Khatoon A, Wani WA, Kumar R, et al. Recent advances in new generation dye removal technologies: novel search for approaches to reprocess wastewater. RSC Adv. 2015; 5: 30801-30818.
- Gupta V. Application of low-cost adsorbents for dye removal–a review J. Environ. Manage. 2009; 90: 2313-2342.
- Oturan MA, Aaron JJ. Advanced oxidation processes in water/wastewater treatment: principles and applications. A review. Crit Rev Environ Sci Technol. 2014; 44: 2577-2641.
- Divyapriya G, Nambi IM, Senthilnathan J. Nanocatalysts in Fenton based advanced oxidation process for water and wastewater treatment. J Bionanosci. 2016; 10: 356-368.
- Kanakaraju D, Glass BD, Oelgemöller M. Advanced oxidation process-mediated removal of pharmaceuticals from water: a review. J Environ Manage. 2018; 219: 189-207.
- Pimentel D, Berger B, Filiberto D, Newton M, Wolfe B, Karabinakis E, et al. Water resources: agricultural and environmental issues. Bioscience. 2004; 54: 909-918.
- Kumar A, Kumar A, Krishnan V. Perovskite oxide-based materials for energy and environment-oriented photocatalysis. ACS Catal. 2020; 10: 10253-10315
- Kumar A, Raizada P, Singh P, Saini RV, Saini AK, Hosseini-Bandegharaei A. Perspective and status of polymeric graphitic carbon nitride based Z-scheme photocatalytic systems for sustainable photocatalytic water purification. Chem Eng J. 2020; 391: 123496.
- Singh P, Sudhaik A, Raizada P, Shandilya P, Sharma R, Hosseini-Bandegharaei A. Photocatalytic performance and quick recovery of BiOI/Fe3O4@graphene oxide ternary photocatalyst for photodegradation of 2,4-dintirophenol under visible light Mater. Today Chem. 2019; 12: 85-95
- Kamat P. Manipulation of charge transfer across semiconductor interface a criterion that cannot be ignored in photo catalyst design. J Phys Chem Lett. 2012; 3: 663–672.
- Hammad A, Haitham M, El-Bery HM, EL-Shazly AH, Elkady M. Effect of WO3 Morphological Structure on its Photoelectrochemical Properties. Int J Electrochem Sci. 2018; 13: 362–372.
- Velusamy P, Lakshmi G. Enhanced Photocatalytic Performance of (ZnO/CeO2 )-b-CD System for the Effective Decolorization of Rhodamine B under UV Light Irradiation. Appl Water Sci. 2017; 7: 4025–4036.
- Saeed K, Khan I, Gul T, Sadiq M. Efficient Photodegradation of Methyl Violet Dye Using TiO2/Pt and TiO2/Pd Photocatalysts. Appl Water Sci. 2017; 7: 3841–3848.
- Nasrollahzadeh M, Sajadi SM, Maham M, Kohsari I. Biosynthesis, Characterization and Catalytic Activity of the Pd/Bentonite Nanocomposite for Base- and Ligand-Free Oxidative Hydroxylation of Phenylboronic Acid and Reduction of Chromium (VI) and Nitro Compounds. Microporous Microporous Mater. 2018; 271: 128–137.
- Zada N, Khan I, Shah T, Gul T, Khan N, Saeed K. Ag–Co oxides nanoparticles supported on carbon nanotubes as an effective catalyst for the photodegradation of Congo red dye in aqueous medium. Inorg. Nano-Metal Chem. 2020; 50: 333–340.
- Asses N, Ayed L, Hkiri LN, Hamdi M. Congo Red Decolorization and Detoxification by Aspergillus niger” Removal Mechanisim and Dye Degradation pathway. Hin BioM Re Intel. 2018; 6.
- Bhat SA, Zafar F, Mondal AH, Kareem A, Miraza AU, Khan S, et al. Photocatalytic degradation of carcinogenic Congo red dye in aqueous solution, antioxidant activity and bacterial effect of Nio nanoparticles. J Iran Chem Soci. 2019; 17: 215-227.
- Indira K, Shanmugam S, Hari A, Vasantharaj S, Sathiyavimal S, Brindhadevi K, et al. Photocatalytic degradation of congo red dye using nickel–titanium dioxide nanoflakes synthesized by Mukia madrasapatna leaf extract. Environmental Research. 2021; 202: 111647.
- Jabbar ZH, Graimed BH, Issa MA, Ammar SH, Ebrahim SE, Khadim HJ, et al. Photocatalytic degradation of Congo red dye using magnetic silica-coated Ag2WO4/Ag2S as Type I heterojunction photocatalyst: Stability and mechanisms studies. Materials Science in Semiconductor Processing. 2023; 153: 107151.
- Hitkari G, Sandhya S, Gajanan P, Manoj K Shrivash, Deepak Kumar. Synthesis of Chromium Doped Cobalt Oxide (Cr:Co3O4) Nanoparticles by Co-Precipitation Method and Enhanced Photocatalytic Properties in the Visible Region. Journal of Material Science & Engineering. 2018; 07: 3–9.
- Sharfalddin A, Alzahrani E, Alamoudi M. Micro, Sono, Photocatalytic Degradation of Eosin B Using Ferric Oxide Doped with Cobalt. Am Chem Sci J. 2016; 13: 1–13.