Research Article
Austin J Biotechnol Bioeng. 2014;1(2): 5.
Adsorption of Hexavalent Chromium onto Activated Carbon
Mohanty S, Bal B and Das AP*
Bioengineering Laboratory, Centre of Biotechnology, Siksha O Anusandhan University, India
*Corresponding author: Das AP, Bioengineering Laboratory, Centre of Biotechnology, Siksha O Anusandhan University, Khandagiri Square, Bhubaneswar, India.
Received: July 18, 2014; Accepted: August 11, 2014; Published: August 12, 2014
Abstract
Background: Hexavalent chromium is a well established carcinogenic and mutagenic contaminant, frequently cause to come into the surroundings through various related study of origins and development of human activities. Among a variety of approaches, adsorption of toxicant hexavalent chromium by activated carbon is measured to be a feasible option to remediate Cr (VI) contagion, from ground and water beds, effusing from mopes of chromite quarry and mine waste.
Methods: In this present lesson, removal of Cr (VI) via adsorption on activated carbon was investigated. The studies were conducted by varying different parametric quantity such as initial pH, adsorbent dosage, contact time and temperature. Experimental studies found that under optimized condition, the activated carbon is able to remove 99% of Cr (VI) with initial concentration of 10 mg/L in 6 hr, at pH- 6 and temperature-30°C.
Results: The result suggests that the rate of adsorption is highly influenced by the process parameters. The percentage of adsorption increases with increase in adsorbent dose (1.5 gm). In the initial stages the rate of Cr (VI) adsorption was high, then gradually decreases and remained constant. At pH 6 effectiveness of adsorption of activated carbon was found to be highest. With increase in temperature the adsorption increased i.e. at 30°C almost 95% Cr (VI) was adsorbed. The optimal parameter for chromium uptake was dependent on chromium concentration, absorbent dose-1.5 gm, pH -6, temperature-30°C, contact time-6 hr. The maximum efficiencies of chromium removal were found 99% with all above optimized parameters using activated carbon.
Conclusion: The current investigation reports the removal of Cr (VI) from an aquatic system through adsorption on to activated carbon equipped from coconut shells with a maximum removal efficiency of 99% within 6 hours. Thus activated carbon prepared from coconut shell can perform as a noble adsorbent for the adsorption of chromium from industrial effluents.
Keywords: Adsorption; Activated carbon; Hexavalent chromium; Bioremediation
Introduction
Hexavalent chromium is highly toxic for the environment ensuing from haphazard mining, industrial effluents, tanning wastes and steel alloys etc [1]. Although chromium (Cr) is measured as a necessary nutrient but it is one of the toxic pollutants in chromite mines and several industries which has become a major area of concern. The centralized maximum concentration level (MCL) for Chromium water consumption is 100 ug/L and the National Institute for Occupational Health and Safety (NIOSH) urge of an coverage border for Cr (VI) of 1 ug/m3 and an overexposure frontier for Cr (0), Cr (II) and Cr (III) of 500 ug/m3 for a 10 hr workday, 40 hr week. From all anthropogenetic origin approximately 35% of Cr is released as hexavalent chromium Cr (VI) into the environment [2]. The greatest anthropogenic sources emissions are chromite mining, Cr plating, chemical industrialized of Cr and cooling system by evaporation [3]. Chromium have an existence in numerous oxidation states ranging from Cr (-II) to Cr (VI) [4].
The major leading oxidation states of chromium are Cr (VI) and Cr (III) which are obtained from the environment. Cr (VI) is extremely portable and has a capability of being dissolved quickly whereas Cr (III) is comparatively inert, chemically steadier and less bio-available due to its insignificant porosity to bio-membranes [5]. Cr (VI) causes genetic mutations and cancer in the level brings out ecological and wellness harm because of its elevated hypertoxicity [6], mutagenicity [7] and carcinogenicity [8]. Presently waste matter is treated with chemical removal, ferrous sulfate, followed by precipitation with alkaline or ion exchange removal; nevertheless, the process of adsorption is mostly based on precipitation and supplemented treatment to remediate those effluents is to be sorted. The quest for alternative novel and modern skill has close attention of awareness on bioremediation of toxic heavy elements. Detoxification mechanism used by the engineers' could be an alternative unconventional method for the removal of Cr (VI) pollution [9]. Chromium is essential to human activity: a dose of 0.1-0.3 ppm per day is necessary for usual growth which generally comes through different foods accessories & fruit drinks. Cr (III) plays a crucial responsibility in animal and plant metabolic activity, whereas hexavalent chromium is straightly toxic to animals, humans and plants. Thus management of the heavy metal to remediate the waste material before emitting into the surroundings becomes inevitable.
Contamination and elevated concentration of hexavalent chromium effluence has contributed a major health hazard affecting more than 2 lakh mine workers and population residing in the Sukinda chromite mine of Odisha, India. Several studies suggest all forms of Cr (VI) are regarded as carcinogenic to workers however the most significant routes of professional exposure to contaminant are breathing and dermal contact [10-12]. Different methods have been suggested for the removals of metal contaminant such as bioremediation, solvent extraction, electrolysis, reverse osmosis, ion exchange adsorption & electrochemical precipitation [13,9]. Adsorption is the good and promising method & a possible unconventional way for the removal of the effluent among all those techniques. Different types of absorbents for the removal pollutant have been reported earlier by means of adsorbents like granular activated carbon, activated shedge, soya cake, husk of rice based activated carbon and fly ash, etc [14]. In the present study, the adsorption capacity of Cr (VI) onto activated carbon was studied. The effect of adsorbent dose, contact time, temperature, pH, and concentration of adsorbate on percentage of adsorption has also been investigated. The overall schematic presentation of the Cr (VI) adsorption process is explained in (Figure 1).
Figure 1: Schematic diagram of experimental procedure.
Adsorption studies
The study of stabilized adsorption onto different parameters was analyzed by using various concentration of Cr (VI) with (10-40 mg) and different activated carbon dosages (0.5-2.5 gm). The pH standards were attuned with NaOH solutions and diluted HCl. Adsorption experiments were carried out by varying adsorbent dose, pH, contact time, temperature required to reach the optimized parameters to remove Cr (VI). The adsorption potential of activated carbon toward Cr (VI) was analyzed by means of their aqueous solutions. The stock solution (1000 mg/L) was diluted as required to obtain standard solutions of concentration ranging from 10-40 mg/L. The effect of different parameters was investigated on Cr (VI) percentage of adsorption with doses of activated carbon.
Material and Methodology
A sequence of flasks containing dichromate solutions of different concentrations from 10- 40 mg/L was prepared from the stock solution of 1000 mg/L. Experiments were carried out with 50ml as whole volume of the reaction mixture with varying concentration of activated carbon. The removal of hexavalent chromium was analyzed by interacting with 1, 5-diphenyl carbazide (DPC) and the absorption was observed using spectrophotometer at 540 nm. In the present practical, all chemicals used were of AR grade purchased from Himedia. The details of experiments were described as followed.
Preparation of Cr (VI) solution
The stock solution of Cr (VI) with concentration (1000 mg/L) was prepared in distilled water with potassium dichromate (K2Cr2O7). All working concentrations varying from 10-40 mg/L were obtained by diluting the stock solution with double distilled water.
Estimation of Cr (VI) concentration
To each test tube 100 ul collected sample was added, to that 400 ul of water, 200 ul of 6NH2SO4, 100 ul 1.5 DPC was added and the volume was make up to 1 ml. Then it was incubated for 10 minutes at room temperature. The absorbance of the obtained solution was measured at 540 nm.
Preparation of activated carbon
Dry coconut shell samples were sieved after mechanical grinding to obtain particle sizes less than 4mm. Chemical activation process was performed using 10% HCl and carbonized at optimum temperature (50°C) for a definite time period using a Tubular furnace. The dried samples were crushed to -70 meshes. Pyrolysis of the impregnated sample was carried out in an inert environment. The sample were washed and dried at 105°C and kept in sealed bags for further experimentation.
Effect of adsorbent dose
Adsorption of Cr (VI) was studied using different dose of activated carbon and different concentration of Cr (VI). Effect of different adsorbent dose with concentration from 0.5-2 mg/L on the removal rate of hexavalent chromium was investigated. Different concentrations of hexavalent chromium were serially taken in flasks with 10-40 mg/L. All the adsorption procedures were measured at normal room temperature, using speed of (150 rpm) for adsorption.
Effect of contact time
The adsorbents were added with different doses to the solutions. The procedure of adsorption was taken place in a flask with a shaking velocity (at 150 rpm) at particular temperatures (37°C), so that constant contact time between adsorbent and the metal ion was maintained. At regular interval of 6hr the suspension was filtered through filter paper and the filtrate was analyzed to evaluate the concentration of Cr (VI) metal in the solution. Metal analysis was carried out by using a UV-visible spectrophotometer.
Effect of pH
To determine the effect of pH, experiments were conducted in different activated carbon dosage from 0.5-2 gm, initial concentration of Cr (VI) from 10-40 mg/L and solution pH from 2 to 10. Shaking velocity was maintained 150rpm to control the adsorption mechanism constantly. The kinetics study of the process was done at pH 2, 4, 6, 8, 10 with adsorbent dosage and Cr (VI) concentration. The samples were taken and analyzed at every 1hr.
Result and Discussion
Effect of adsorbent dose
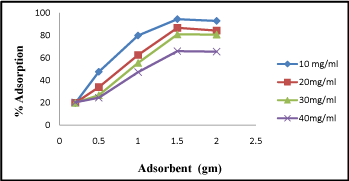
It is evident from the experiment that the competence of activated carbon to remove Cr (VI) was found to be highest at 1.5gm of dose (Figure 2). The adsorption percentage was plotted against adsorbent dose. At lower adsorbent dose (0.2 gm/L) the percentage of adsorption was found 20%, but when the adsorbent dose increases (1.5-2 gm/L) the percentage of adsorption increases to 94%. From above investigation we conclude that with increase in adsorbent dose the percentage adsorption increased.
Figure 2: Adsorption of Cr (VI) by different adsorbent concentrations (0.5-2.5gm).
However other researchers have reported equivalent trends in Cr (VI) sorption [9]. The decrease in Cr (VI) at higher adsorbent dose may be due to struggle of ion for the sites available.
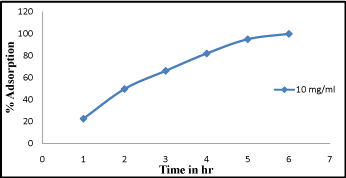
Effect of contact time
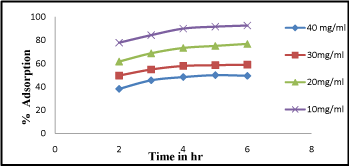
From the recorded observations graph was plotted against adsorption percentage and adsorption time i.e. shown in (Figure 3). It is cleared from the figure that the equilibrium time is dependent on the adsorbate concentration. At 2-3hr percentage of adsorption was found slightly increased. At higher adsorbate concentration (40 mg/L) percentage of adsorption was found to be high at 93% and gradually becomes constant. The slope of the plot in the initial stage was found at 1 but with increasing time it becomes constant. From above observation in the early stages it is exposed that the rate of uptake was fast but progressively decreased and become constant when equilibrium reached, similar reports has also been reported [14].
Figure 3: Effect of contact time Cr (VI) adsorption at different time interval from 1-6 hr.
Effect of pH
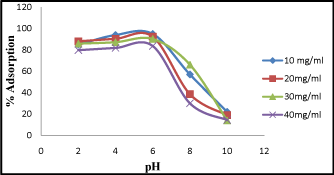
The adsorption experiment was carried out showing the effect of pH ranging from 2-10. The observed result suggest that at lower pH, percentage of adsorption is higher. But at maximum pH range from 5-6, chromium removal was found to be 94%. The adsorption decreases with increasing in pH up to 9, (Figure 4). The decrease of standard redox potential for Cr (VI) at lower pH may also contribute to the overall Cr (VI) removal as earlier suggested by [15]. Their exterior part might be positively charged or negatively charged. The pH dependence of Cr (VI) adsorption can chiefly be interrelated to the kind and ionic state of these functional groups and also on the adsorbate in the solution [16,17].
Figure 4: Effect of pH on Cr (VI) adsorption at different pH ranging from 2-10.
To clarify the experimental actions of Cr (VI) adsorption with varying pH, it is essential to study various mechanisms such as chemical interaction, electrostatic attraction or repulsion, and ion exchange, which are accountable for absorption on adsorbent surface as described by [18].
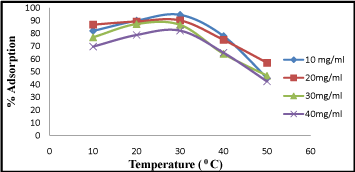
Effect of temperature
Effect of temperature on process of chromium adsorption seems a significant parameter. In the temperature range studied (20-40°C), the extent of Cr (VI) (10 mg/L-40 mg/L) adsorption increased with increase in temperature (Figure5). About 95% Cr (VI) adsorption was observed at 300C but it gradually decreases with again increase in temperature at 40°C. Similar reports [11,19] also suggest a similar optimal temperature for Cr (VI) adsorption with activated carbon. The decrease in adsorption percentage with rise in temperature may be due to desorption caused by the existing thermal energy.
Figure 5: Effect of temperature on Cr (VI) adsorption at different ranges 10-40°C.
Chromium (VI) removal under optimized parameters
From the observed report, it is revealed that with activated carbon dose-1.5 mg, at pH-6, temperature-30°C, and Cr (VI) concentration-10 mg/L are the optimized parameters where Cr (VI) is found to be best removed [20]. It was revealed that at optimized parameters the maximum adsorption rate was found highest, with a rate of 99 % within6 h (Figure6).
Figure 6: Cr (VI) removal under optimized parameters of carbon dose 1.5 gm, temperature-30°C, pH-6 within 6 hr.
Conclusion
The present work evaluates the use of activated carbon for the elimination of Cr (VI) from aqueous solutions. The removal of Cr (VI) was found to be dependent on the different parameters such as absorbent dose, contact time, pH, and temperature.
Hexavalent chromium was rapidly adsorbed when higher concentrations of adsorbent dose were used. Thus we conclude that the percentage of adsorption increases to 94% with the increase in adsorbent dose (1.5gm). Percentage of adsorption was found to be maximum within 6 hr with lower absorbent dose (10 mg/L) and a slight increase is seen within 4-6hr. Effective adsorption was occurred in the pH of 6. The above result indicates that the adsorption capacity depends upon temperature of activation of the activated carbon. With increase in temperature the amount of contaminant adsorption capability increased i.e. at 30°C almost 95% Cr (VI) was adsorbed. The optimum parameters value for chromium uptake was dependent on chromium concentration, with pH -6, temperature-30°C, and contact time-6 hr. The maximum efficiencies of chromium removal were found 99% with all above optimized parameters using activated carbon.
It was concluded that activated carbon produced from coconut shell has an efficient adsorption capacity and is a good adsorbent for the adsorption of chromium from industrial and mining effluents. Activated carbon prepared from coconut shells which are treated as a waste can offer a low-cost and readily available, activated carbon source. Thus this study affords a cost effective means for removing metal ions from contaminated effluents.
References
- U.S. Environmental Protection Agency (USEPA). Code of Federal Regulations. 40 CFR. 1999; 141: 32.
- Wiley J, Sons. Calder LM. Chromium contamination of groundwater, in chromium in the natural and human environments. Wiley Series in Advances in Environmental Science and Technology. 1988; 202: 15-229.
- Agency for Toxic Substances and Disease Registry (ATSDR). Toxicological Profile for Chromium. U.S. Department of Health and Human Services, Public Health Service. 2000.
- Cardenas-Robles A, Martinez E, Rendon-Alcantar I, Frontana C, Gonzalez-Gutierrez L. Development of an activated carbon-packed microbial bioelectrochemical system for azo dye degradation. Bioresour Technol. 2013; 127: 37-43.
- Othman ZA, Ali R, Naushad Mu. Hexavalent chromium removal from aqueous medium by activated carbon prepared from peanut shell: adsorption kinetics, equilibrium and thermodynamic studies. Chemical Engineering Journal. 2012; 184: 238- 247.
- Esmaeili A, Ghasemi S, Rustaiyan A. Removal of hexavalent chromium using activated carbons derived from marine algae gracilaria and sargassum sp. Journal of Marine Science and Technology. 2010; 18: 587-592.
- Nishioka H. Mutagenic activities of metal compounds in bacteria. Mutat Res. 1975; 31: 185-189.
- Venitt S, Levy LS. Mutagenicity of chromates in bacteria and its relevance to chromate carcinogenesis. Nature. 1974; 250: 493-495.
- Donmez GC, Aksu Z, Ozturk A, Kutsal T. A comparative study on heavy metal biosorption characteristics of some algage. Process Biochem. 1999; 34: 885-892.
- Sharma DC, Forster CE. Hexavalent chromium using low-cost adsorbent. Bioresource Technology. 1994; 47: 257-264.
- Bernard E, Jimoh A, Odigure JO. Heavy Metals Removal from Industrial Wastewater by Activated Carbon Prepared from Coconut Shell. Research Journal of Chemical Sciences. 2013; 3: 8-9.
- Das AP, Mishra S. Biodegradation of the metallic carcinogen hexavalent chromium Cr(VI) by an indigenously isolated bacterial strain. J Carcinog. 2010; 9: 6.
- Das AP, Singh S. Occupational health assessment of chromite toxicity among Indian miners. Indian J Occup Environ Med. 2011; 15: 6-13.
- Esmaeili A, Ghasemi S, Rustaiyan A. Removal of hexavalent chromium using activated carbons derived from marine algae gracilaria and sargassumsp. Journal of Marine Science and Technology. 2010; 18: 587-592.
- Agarwal GS, Bhuptawat HK, Chaudhari S. Biosorption of aqueous chromium(VI) by Tamarindus indica seeds. Bioresour Technol. 2006; 97: 949-956.
- Gholipour M, Hashemipour H, Mollashahi M. Hexavalent chromium removal from aqueous solution via adsorption on granular activated carbon: adsorption, desorption, modeling and simulation studies. Journal of Engineering and Applied Sciences. 2011; 6: 10.
- IIyas M, Malik MA, Andrabi SMZ, Khan Z. Kinetics and mechanism of paracetamol oxidation by chromium(VI) in absence and presence of manganese(II) and sodium dodecylsulphate. Res Lett Phys Chem. 2007; 1-5.
- Sharma DC, Chatterjee C. Chromium accumulation and its effects on wheat (Triticumaestivum L. cv. HD 2204) metabolism. Plant Science. 1995; 111: 145-151.
- Wang T, Xu H. Reduction of Cr(VI) by hydrazine in solution saturated with KHCO3. J Hazard Mater. 2005; 123: 176-180.
- Sharma DC, Forster CE. A preliminary examination into the adsorption of hexavalent chromium using low-cost adsorbents. Bioresource Technology. 1994; 47: 257-264.