Editorial
Austin J Biotechnol Bioeng. 2014;1(3): 3.
Chemokines: The Holy Messengers of Leukocyte Trafficking
Krishna Mohan Poluri*
Department of Biotechnology, Indian Institute of Technology Roorkee, India
*Corresponding author: Krishna Mohan Poluri, Department of Biotechnology, Indian Institute of Technology Roorkee (IIT-Roorkee), Roorkee-247667, Uttarakhand, India.
Received: September 02, 2014; Accepted: September 04, 2014; Published: September 06, 2014
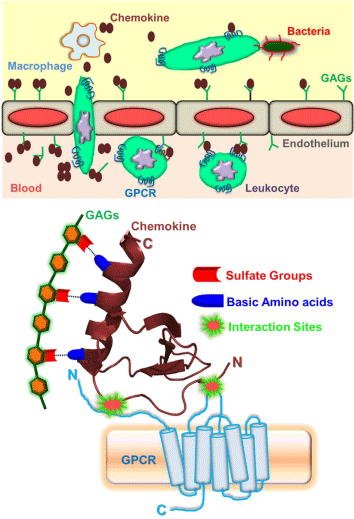
The classical panorama of an innate immune regulation during inflammation, infection, and sterile tissue injury is the rapid recruitment of circulating leukocytes throughout the organism in an effort to eliminate the infectious pathogens (such as bacteria, virus), tissue damage and other physiological insults. The salient features that are involved in the leukocyte trafficking (Figure 1) are: (a) Release of chemokines - small proteins of size 8-10 kD and are known as chemotactic cytokines, by the tissue macrophages; (b) transcytosis and decoration of chemokines on the luminal surface of endothelium forming the chemokine gradients; (c) the initial attachment and rolling of leukocytes on the inflamed endothelium; during this stage, several adhesion events are arbitrated by the different ligands and receptor pairs. For instance, L-selectin constitutively expressed by leukocytes and P, E-selectins expressed by activated endothelial cells facilitate the initial attachment and rolling of leukocytes on the endothelial surface under shear flow of blood. (d) Activation of the seven transmembrane-spanning G protein- coupled receptors (GPCRs) on the leukocytes by the chemokines bound to proteoglycans/glycosaminoglycans (GAGs) on the luminal surface of the endothelium, resulting in integrin activation and firm adhesion of rolling cells. (e) Migration of the leukocytes along the chemokine gradients, shape change, extravasations across the endothelium, and their entry into the underlying target tissue. (f) Finally, leukocytes perform the immune surveillance activities such as microbial killing and tissue repair through various mechanisms that include phagocytosis, degranulation, and extracellular traps etc [1-6]. From various stages of the recruitment process, it is obvious that chemokines emerge as "master regulators" in mediating the leukocyte trafficking in resolving infection/inflammation.
Figure 1: Schematic showing the chemokine mediated leukocyte migration (Top panel) and the cartoon representation of the structural framework of monomeric chemokine interactions with glycosaminoglycans (GAGs) and G-protein coupled receptors (GPCRs) (Bottom Panel).
The chronology of the chemokine literature presents an interesting historical perspective [7-10]. During the early years of their discovery, chemokines and their innate immunity roles attracted a little attention from the immunologists due to their maverick characteristics such as, (a) high promiscuity relationship with their receptor partners and, (b) presence of chemokine genes in two discrete chromosomal sites, thus forming two large gene clusters. In the later years, researchers discovered that, the chemokines are classified majorly into two classes. (i) Inflammatory - these are up-regulated during the inflammatory condition, play pivotal roles in immune responses, and in defining the cellular organization of the immune system thus controlling the leukocyte recruitment during inflammation/infection. (ii) Homeostatic - these are constitutively expressed in lymphoid and other organs and play major roles in the organization of the immune system. They are indeed 'maestro" of the movement and localization of lymphocyte and dendritic cell subsets in the body [8,9]. Afterwards, it has been identified that the homeostatic chemokines are clustered separately outside apart from the two major clusters that are identified as CXC and CC families. Further studies on the activation/signaling of chemokine-receptor complexes have widely established that, many of the chemokines display highly restrained ligand-receptor specificities [10]. All these findings have led to a paradigm shift in our concept of chemokine function during physiological and pathological conditions.
According to our current knowledge, humans express ~50 chemokines and many of them share the fundamental property of oligomerization. They are divided into four subclasses depending on the position of N-terminal cysteine residues (CC, CXC, CX3C, and C), where "X" can be any amino acid. The first chemokine structure was solved 25 years back for CXCL8/IL8 [11]. Since then, structures were determined for several of the members from each subclass. To an extent of surprise and frustration of the structural biologists, it had been observed that, irrespective of the subclass, the "canonical tertiary structure" of the chemokine essentially remained the same. The high structural homology and stability of the monomeric chemokine structures can be partially attributed to the presence of one to three disulfide bonds that are mostly fixed in sequence and structure. A typical monomeric chemokine structure consists of a long disordered N-terminal domain followed by a 310-helix, anti parallel three stranded β-sheet and a C-terminal a-helix [11,12]. The monomeric scaffold of a chemokine is sufficient enough to interact with its cellular partner's glycosaminoglycans and G-protein coupled receptors in order to regulate the leukocyte trafficking as described above. The disordered N-terminal region of the chemokine engages its residues in interacting with the extracellular loops (including the N-domain) of the GPCR; whereas, the glycosaminoglycans can interactions are primarily electrostatic in nature and are mediated by the positively charged amino acids (such as His/Lys/Arg) of the C-terminal helix of the chemokine with the sulfate groups of the GAGs (Figure 1) [12-15]. However, the mechanism and molecular level details of chemokine mediated leukocyte migration are more complex due to its ability to undergo reversible oligomerization, and yet to be unraveled in great detail.
The structural diversity of the chemokine molecules originates from its oligomerization behavior. Chemokines can reversibly exist as monomers, dimers, tetramers and/or higher order oligomers. For example, the dimeric structural architecture attained by the CXC and CC chemo kinesis completely different. CXC chemokines dimerize by involving the residues in the first β-strand, thereby forming a six stranded β-sheet structure topped by two a-helices. The CC chemokines dimerize through the nucleated residues around the N-terminus, and forms a β-sheet in the dimer. In contrast to those two forms of dimers, the XC chemokines form a non-canonical dimer that has no similarity to that of any of anorthodox chemokine tertiary structure [16,17]. Along with the dimeric states, tetrameric structures also have been reported for few of the chemokines. Indeed, three different forms of tetrameric structure had been reported for CXCL10 [18]. Furthermore, chemokines also have an extraordinary ability to undergo hetero-oligomerization to assemble into hetero dimers/ tetramers within or outside their subclass members.
The propensity of chemokine oligomerization has been reported to vary significantly by orders of magnitude (nM to mM), and depends on the environmental and cellular milieu. Moreover, the glycosaminoglycans are known to induce oligomerization in chemokines [16]. Indeed, such sort of GAG induced oligomerization can efficiently modulate the chemotactic gradients on the inflamed endothelium. This dynamic oligomerization process of chemokines on the endothelium manifests to differential binding to the glycosaminoglycans and binding/activation of the receptors respectively and can also influence the kinetics of binding and their longevity on endothelium for sustained leukocyte homing. In addition, as the leukocyte activation triggers diverse signal transduction cascades; which cascade is triggered may not only be depends on the chemokine and the receptor engaged but also on the oligomerization state of the chemokines, receptors and/or nature of chemotactic/haptotactic gradients. Such type of selective activation of distinct pathways indicates that the receptors couple not only to their G proteins but also to the chemokine oligomeric state for targeted leukocyte migration.
Although, the leukocyte trafficking is tightly regulated in cooperation with various cellular partners, sometimes under various pathological conditions and organ transplantations (allograft/ xenografts), these infiltrating leukocytes become invasive as they are not only the effector cells combating invading pathogens but are also involved in tissue degradation. As such, an excessive or reduced influx or activation of infiltrating leukocytes into the infected/ damaged tissue may have profound effects on its quality of the tissue healing response [19]. Such an altered influx of leukocyte migration is also very common during some of the viral infections as these viruses adopt various mechanisms of "molecular mimicry" to hijack the human immune system. Some viruses can even produce their own chemokine and receptor homolog's and, proteins that bind human chemokines (chemokine binding proteins) to counter attack [20].
A complete abrogation of a chemokine activity or blockade of a chemokine receptor is most likely to be accompanied by severe side effects on immune functioning. Alternatively, attenuating a particular chemokine-receptor axis during disease might suffice the purpose, while leaving normal physiological functions intact. Owing to the diversity in the regulation of the chemokine system, several strategies have been developed to regulate/block, restore and control/ manipulate the leukocyte migration. They include small-molecule receptor antagonists, GAG analogs, peptide inhibitors, modified chemokine ligands and the disruption of chemokine pairs [21-25].
Considering the fact that "healing as a matter of opportunity rather than a matter of time", given the dependence of chemokine activity on GAG binding and the importance of homo and hetero oligomer formation of chemokines and their receptors, many new entry points and additional novel strategies have to be considered for potential therapeutic approaches. Modification of GAG binding and chemokine oligomerization properties of chemokines might be one of the valid methodologies. Small molecule, low molecular weight GAG mimetic and engineered "super chemokines" should be other plausible solution. Further, the recently discovered heterothallic interactions of chemokines can be considered by designing peptides, antibodies or small molecules. Pursuing all these potential therapeutic avenues along with a comprehensive knowledge of the structural and energetic basis of chemokine interactions with receptors and GAGs will aid human community to combat against several inflammatory/ infectious disorders.
Acknowledgement
I thank Ms. Khushboo Gulati and Mr. Krishnakant Gangele for technical comments. This work is supported by the Grants IITR-BIO-FIG-100637, DBT-IYBA and SERB/LS-1059/2013 from Government of India to KMP.
References
- Blanchet X, Langer M, Weber C, Koenen RR, von Hundelshausen P. Touch of chemokines. Front Immunol. 2012; 3: 175.
- Colditz IG, Schneider MA, Pruenster M, Rot A. Chemokines at large: in-vivo mechanisms of their transport, presentation and clearance. Thromb Haemost. 2007; 97: 688-693.
- Muller WA. Leukocyte-endothelial-cell interactions in leukocyte transmigration and the inflammatory response. Trends Immunol. 2003; 24: 327-334.
- Lowe JB. Glycan-dependent leukocyte adhesion and recruitment in inflammation. Curr Opin Cell Biol. 2003; 15: 531-538.
- Kolaczkowska E, Kubes P. Neutrophil recruitment and function in health and inflammation. Nat Rev Immunol. 2013; 13: 159-175.
- Wang L, Fuster M, Sriramarao P, Esko JD. Endothelial heparan sulfate deficiency impairs L-selectin- and chemokine-mediated neutrophil trafficking during inflammatory responses. Nat Immunol. 2005; 6: 902-910.
- Baggiolini M. Chemokines and leukocyte traffic. Nature. 1998; 392: 565-568.
- Sallusto F, Baggiolini M. Chemokines and leukocyte traffic. Nat Immunol. 2008; 9: 949-952.
- Zlotnik A, Yoshie O. The chemokine superfamily revisited. Immunity. 2012; 36: 705-716.
- Rossi D, Zlotnik A. The biology of chemokines and their receptors. Annu Rev Immunol. 2000; 18: 217-242.
- Clore GM, Appella E, Yamada M, Matsushima K, Gronenborn AM. Three-dimensional structure of interleukin 8 in solution. Biochemistry. 1990; 29: 1689-1696.
- Fernandez EJ, Lolis E. Structure, function, and inhibition of chemokines. Annu Rev Pharmacol Toxicol. 2002; 42: 469-499.
- Lau EK, Allen S, Hsu AR, Handel TM. Chemokine-receptor interactions: GPCRs, glycosaminoglycans and viral chemokine binding proteins. Adv Protein Chem. 2004; 68: 351-391.
- Poluri KM, Joseph PR, Sawant KV, Rajarathnam K. Molecular basis of glycosaminoglycan heparin binding to the chemokine CXCL1 dimer. J Biol Chem. 2013; 288: 25143-25153.
- Lortat-Jacob H. The molecular basis and functional implications of chemokine interactions with heparan sulphate. Curr Opin Struct Biol. 2009; 19: 543-548.
- Salanga CL, Handel TM. Chemokine oligomerization and interactions with receptors and glycosaminoglycans: the role of structural dynamics in function. Exp Cell Res. 2011; 317: 590-601.
- Wang X, Sharp JS, Handel TM, Prestegard JH. Chemokine oligomerization in cell signaling and migration. Prog Mol Biol Transl Sci. 2013; 117: 531-578.
- Swaminathan GJ, Holloway DE, Colvin RA, Campanella GK, Papageorgiou AC, Luster AD, et al. Crystal structures of oligomeric forms of the IP-10/CXCL10 chemokine. Structure. 2003; 11: 521-532.
- Eming SA, Krieg T, Davidson JM. Inflammation in wound repair: molecular and cellular mechanisms. J Invest Dermatol. 2007; 127: 514-525.
- Lalani AS, McFadden G. Evasion and exploitation of chemokines by viruses. Cytokine Growth Factor Rev. 1999; 10: 219-233.
- Koenen RR, Weber C. Therapeutic targeting of chemokine interactions in atherosclerosis. Nat Rev Drug Discov. 2010; 9: 141-153.
- Das AM, Brodmerkel CM. Chemokines and chemokine receptors: Large and small therapeutic strategies for inflammatory diseases. Drug discovery today: Therapeutic Strategies. 2006; 3: 381-388.
- Garin A, Proudfoot AE. Chemokines as targets for therapy. Exp Cell Res. 2011; 317: 602-612.
- Wells TN, Power CA, Shaw JP, Proudfoot AE. Chemokine blockers--therapeutics in the making? Trends Pharmacol Sci. 2006; 27: 41-47.
- Severin IC, Soares A, Hantson J, Teixeira M, Sachs D, Valognes D, et al. Glycosaminoglycan analogs as a novel anti-inflammatory strategy. Front Immunol. 2012; 3: 293.