Research Article
Austin J Biotechnol Bioeng. 2014;1(3): 4.
Shelf Life Evaluation in Selected Tomato (Solanum Lycopersicum L) F7 Recombinant Inbred Lines (RILs)
Sukanta Sinha*, Ramanjini Gowda PH, Sujeet kumar and Mallikarjuna NM
Department of plant biotechnology, University of Agricultural Sciences (GKVK), India
*Corresponding author: Ramanjini Gowda PH, Department of plant biotechnology, University of Agricultural Sciences (GKVK), Bengaluru-560 065, Karnataka, India.
Received: August 25, 2014; Accepted: September 19, 2014; Published: September 22, 2014
Abstract
Tomato (Solanum lycopersicum L), one of the most important vegetable crop in the world is susceptible to rapid post harvest softening and poor shelf life leading to great post harvest losses. Among all strategies available for minimizing the postharvest losses, genetics approaches, very precisely conventional breeding coupled with modern marker technology is the promising one because of more acceptability by the consumer. We have evaluated twenty four F7 RIL'S developed from the cross L121 X Vaibhav for high shelf life. L121 is a parent having (alc gene) a ripening mutant is able to prolong shelf-life but has poor agronomic character; Vaibhav is the good agronomic cultivar released by the University of Agricultural Sciences, Bangalore and Karnataka. The evaluation of F7 RILs resulted in the identification of tomato RILs with high shelf life. Among F7 RILs, parents and checks, the maximum shelf life was observed in RIL 7-3, RIL 110-2 (110days), followed by RIL 102-1 and RIL 182-4 (100 days) with an average shelf life of the F7 RILs were 63 days. Some genetic SSR polymorphic markers were identified to be associated with the fruit shelf-life which was determined by Single Marker Analysis (SMA). SMA revealed that the markers Tom184 and Tom144 are linked to high shelf life in tomato.
Keywords: Fruit Shelf life; SSR; Single marker analysis
Introduction
Tomato (Solanum lycopersicum L), being a climacteric fruit, has a relatively short postharvest life since many processes affecting quality loss take place after harvest [1]. The main factor associated with tomato postharvest shelf-life, particularly in tropical regions where the temperature is high, is increased respiration which results in faster fruit ripening and deterioration of fruit quality [2]. In India, the aggregate post-harvest losses from farm gate to consumers in tomato ranges from 13 to 26% [3].
Several efforts have been made to increase the shelf life of tomato fruits. An early effort was done with calcium treatment to the fresh fruits. Calcium helps maintaining cell wall integrity and hence reduces the action of cell wall degrading enzymes and consequently fruit softening [4]. One of the successful efforts was by Californian Company Calgene, which had developed a tomato variety called Flavr Savr tomato using the antisense RNA technology. The introduction of this gene in the reverse form, also called antisense, resulted in low production of the poly galactonurase enzyme. Consequently, ripe tomato fruits do not lose their firmness because the cell wall of these fruits, which is made of cellulose, does not degrade as rapidly as it does in normal tomatoes. Since the Flavr Savr tomato had been taken off from the market, there is no commercial tomato variety sold in the market with high shelf life.
In the fleshy fruit model, tomato, the plant hormone, ethylene, plays a central role in ripening [5,6]. Several fruit ripening mutants have been characterized in tomato. The rin (ripening-inhibitor), nor (non-ripening) and cnr (colorless non-ripening) upstream genes were shown to act on the ethylene signaling in ripening gene. Mutations in the ethylene receptor, the Nr (Never-ripe) gene, is also shown to affect fruit ripening [7,8]. Ethylene biosynthesis requires the conversion of Aminocyclopropane- 1-Carboxylic Acid (ACC) to ethylene by the ACC oxidase (ACO). ACC oxidases are encoded by multigene families in plants and have been described to be involved in ripening, growth, and development [9,10] have used several ripening gene mutants, such as alcobaca (alc), non-ripening (nor), never ripe, and ripening inhibitor (rin) to develop lines and cultivars with delayed ripening through disruption of the ethylene signaling pathway.
All genetic markers occupy specific genomic positions within chromosomes (like genes) called 'loci' (singular 'locus') [11]. Single Marker Analysis (SMA) is the method used in earliest studies on QTL mapping [12,13]. In this method, one marker is involved at a time to find the QTL - Marker association. This single marker analysis can be implemented as a simple t test, ANOVA, linear regression and likelihood ratio test and maximum likelihood estimation [14-16].
In this study, twenty four Recombinant Inbred Lines (RILs) with high shelf life along with parental lines and the commercial checks like Arka Alok, Pusa Ruby and Sankranti were used. The objective of the study was to find out the best tomato lines which can stay for long time after harvesting and also the molecular evaluation of the RILs using SSR markers which are linked to the trait.
Material and Methods
The study was conducted in the field during Rabi 2013 at the Department of Plant Biotechnology, University of Agricultural Sciences, Bangalore, which is located at an altitude of 899 m above Mean Sea Level (MSL) and at 13° 00' N latitude and 77° 35' E longitude. The field experiment was conducted using Randomized block design.
Experimental material
The experimental material includes F7 lines, which is derived from crossing between L121 and Vaibhav for evaluation of shelf life. In this experiment, L121 used as female parent is a tomato line with high shelf life. Another parent Vaibhav was used as male parent with good agronomical characters.
Evaluation of F7 RILs
The best 24 F6 RILs having high shelf life were sown in the field at Department of plant Biotechnology, Bangalore during Rabi 2013 (Table 1).
RIL 5-3
RIL 7-3
RIL 15-1
RIL 34-2
RIL 45-4
RIL 53-1
RIL 54-4
RIL 60-3
RIL 60-5
RIL 61-1
RIL 61-2
RIL 96-1
RIL 102-1
RIL 104-1
RIL 110-2
RIL 124-1
RIL 135-2
RIL 145-3
RIL 169-1
RIL 171-3
RIL 182-4
RIL 189-3
RIL 200-2
RIL 201-2
Table 1: List of Tomato F7 Recombinant Inbred lines used for evaluation.
Recording of observations
The fruits harvested from each RILs, parents and checks at breaker stage was kept at room temperature (25°C) and observed for the shelf life up to 110 days. The fruit firmness was recorded at 10 days interval from the date of harvesting till 40th day. Fruit firmness was determined using a fruit penetrometer (Wagner Instruments, New Delhi, India). The fruits at breaker stage were punctured with a plunger (1 cm diameter) of penetrometer and the pressure required to penetrate the fruit pericarp was recorded and expressed in lbs/cm2.
DNA isolation
DNA was extracted from the young leaves (three to four leaves stage) using CTAB method as demonstrated by [17]. The DNA concentration was measured using Biophotometer plus (Eppendof, Geramany). The quality of the DNA was inspected using agarose gel electrophoresis (3%).
SSR marker analysis
A total of 42 SSR markers were used for the study to check the parental polymorphism, so that suitable marker can be linked to high shelf life. The PCR reactions were performed in Eppendorf Mastercycler (Germany). The 15 μl reaction volume included 10 X reaction buffer, 50 mM MgCl2, 10 mM deoxyribonucleotide triphosphate mix, 1 U of Taq DNA polymerase, and 0.25 μM each of forward and reverse SSR primers and 25 ng of genomic DNA.
The amplification reactions were carried out using the following thermal profile: 94°C for 4 min (1 cycle); 94°C for 30 sec, 47-52°C (based on the annealing temperature of the SSR primers) for 1 min, 72°C for 1 min (35 cycles); 72 °C for 7 min (1 cycle). Amplified products were screened using 5% Poly Acryl amide gel (PAGE) using 1x TBE buffer for two and half hours at 150 V and photographed. The bands were scored and analysed statistically.
Statistical analysis
The shelf life data recorded from the replicated design were analyzed using MS-Excel 2007. The single marker analysis was done using 'single factor ANOVA'.
Results and Discussion
Shelf life of the fruits
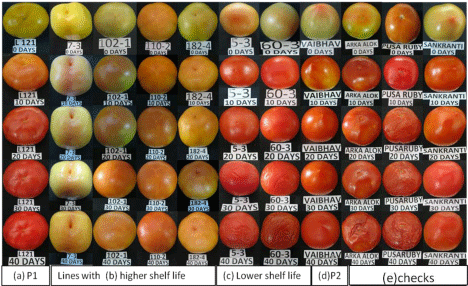
In order to identify the tomato lines with high shelf life, the days taken by fruits from breaker stage until the firmness is lost from the fruits was recorded. The fruits were harvested at breaker stage and kept at room temperature (25°C). There was a gradual change in the color, firmness and appearance of fruits. The breaker stage fruits turned to red ripe stage within 15 to 40 days depending on the tomato variety/RILs. Different stages of fruit maturity were captured in digital camera at 10 days interval up to 40 days (Figure1).
(a)Parent1 (L121), (b)Lines showing higher shelf life, (c)Lines showing lower shelf life, (d)Parent2 (Vaibhav), (e) Commercial checks.
Figure 1: Figure showing the gradual change in the fruit at 10 days interval after harvesting at breaker stage up to 40 days keeping at room temperature; the lines with high shelf life remain intact even after 40 days, whereas lines with low shelf life shriveled even at the 20th day.(a)Parent1 (L121), (b)Lines showing higher shelf life, (c)Lines showing lower shelf life, (d)Parent2 (Vaibhav), (e) Commercial checks.
The comparison of shelf life of F7 RILs with commercial checks like Arka Alok, Pusa Ruby and Sankranti shows that the RILs had higher shelf life than the checks, with an exception of the variety Sankranti.
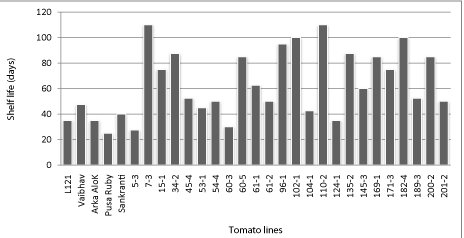
The results showed that among F7 RILs, parents and checks, the fruit shelf life was maximum in RIL 7-3, RIL 110-2 (110 days); followed by RIL 102-1 and RIL 182-4 (100 days), while least number of days were found in check Pusa Ruby (25 days), followed by RIL 5-3 (28 days). The parental line L121 and Vaibhav recorded a shelf life of 48 and 35 days respectively (Figure 2).
Figure 2: F7 tomato RILs along with parents and checks showing the shelf life (days).
Some of the RILs were superior to both the parents, whereas some were inferior to both of them in terms of fruit firmness and shelf life. According to [18] with respect to shelf-life, it was possible to identify three different groups: I- RIL 7-3, 15-1, 34-2, 45-4, 54-4, 60-5, 61-1, 96-1, 102-1, 110-2, 135-2, 145-4, 169-1, 171-3, 182-4, 189-3,200-2 and 201-2 displayed values higher than both parents; group II- RIL 53-1, 61-2, 104-1 and 124-1 displayed shelf life values between parents, and group III- RIL5-3 and 60-3, displayed values lower than both parents. The I and III groups clearly represent fixed transgressive variants as reported by [19]. Similar results were obtained by [20] using mutant gene nor. They developed a cross nor x 'Ce' in which the F1 had a shelf life of 64.1 days compared with those of nor (52.1 days) and 'Ce' (23.9 days). This difference occured because the ripening gene mutants participate in ethylene-independent signaling and impart delayed ripening in tomato [21].
Fruit firmness (lbs/cm2)
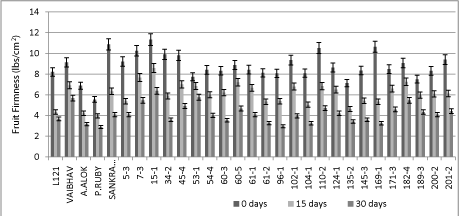
Among F7 RILs, parents and checks evaluated, the fruit firmness at 0 days was maximum in RIL5-3 (11.50 lbs/cm2), Pusa Ruby recorded minimum (5.31 lbs/cm2), while Mean of F7 RILs were 8.84 lbs/cm2, whereas at 15 days RIL15-3 (8.50 lbs/cm2) recorded maximum, Pusa Ruby recorded minimum (4.19 lbs/cm2), while Mean of F7 lines were 6.30 lbs/cm2 and at 30 days the fruit firmness recorded maximum in RIL15-3 (6.62 lbs/cm2), Pusa Ruby recorded minimum (2.62 lbs/cm2), while Mean of F7 lines were 4.52 lbs/cm2. RIL 7-3, 15-1, 110-2, 169- 1 has higher fruit firmness at the time of harvesting from both the parents but rest of the RILs had the firmness in between both parents and lower. The fruit firmness decreased significantly over time after harvesting (Figure 3).
Figure 3: F7 tomato RILs along with parents and checks showing the fruit firmness (lbs/cm2) at 15 days interval.
Molecular characterization
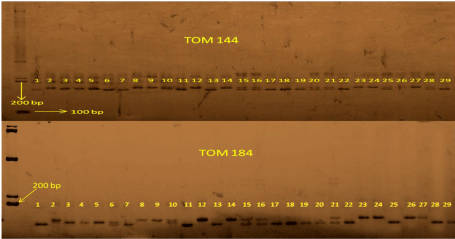
Molecular characterization was done by SSR markers. SSR markers which are codominant in nature have specific positions on chromosome regions. The association of a single marker to a number of traits is important because it reveals the nature of the particular marker. In the present study 42 SSR markers were used out of which only 5 markers were found to be polymorphic. SMA is the method used in earlier studies on QTL mapping where one marker is involved at a time to find the QTL - Marker association. The SMA revealed that for fruit keeping quality only two SSR markers TOM 184, TOM 144 were associated (Table 2). A single QTL was identified for shelf life on linkage group six. This QTL, fr_ke_qlty1 was flanked by LEgata 2 and LEta16 [22]. Physiological and genetic studies have resulted in the identification and characterization of several ripening mutants such as never ripe (Nr), non-ripening (nor), and ripening inhibitor (rin), genes of which are located on chromosomes 9, 10, and 5, respectively. While fruits of Nr mutant ripen slowly, fruits of nor and rin fail to ripen and do not exhibit any climacteric rise [23]. Several QTLs have been identified in L. esculentum which are linked to high shelf life which are distributed on chromosome 2 and chromosome 4 [24]. In the present study the SSR markers TOM 184 and TOM 144 found to be linked with the high shelf life trait but these two markers are present on chromosome number 4 and 11 respectively (Figure 4).
1.L121 2.Vaibhav 3.Arka Alok 4.Pusa Ruby 5.Sankranti 6.RIL 5-3 7.RIL 7-3 8.RIL 15-1 9.RIL 34-2
10.RIL 45-4 11.RIL 53-1 12.RIL 54-4 13.RIL 60-3 14.RIL 60-5 15.RIL 61-1 16.RIL 61-2 17. RIL 96-1
18.RIL 102-1 19.RIL 104-1 20.RIL 110-2 21.RIL 124-1 22.RIL 135-2 23.RIL 145-3 24.RIL 169-1
25.RIL 171-3 26.RIL 182-4 27.RIL 189-3 28.RIL 200-2 29.RIL 201-2.
Figure 4: Poly Acryl amide Gel Electrophoresis (PAGE) image of SSR marker TOM184 and TOM1441.L121 2.Vaibhav 3.Arka Alok 4.Pusa Ruby 5.Sankranti 6.RIL 5-3 7.RIL 7-3 8.RIL 15-1 9.RIL 34-2
10.RIL 45-4 11.RIL 53-1 12.RIL 54-4 13.RIL 60-3 14.RIL 60-5 15.RIL 61-1 16.RIL 61-2 17. RIL 96-1
18.RIL 102-1 19.RIL 104-1 20.RIL 110-2 21.RIL 124-1 22.RIL 135-2 23.RIL 145-3 24.RIL 169-1
25.RIL 171-3 26.RIL 182-4 27.RIL 189-3 28.RIL 200-2 29.RIL 201-2.
Sl. No.
Trait
Primer
P value
F (crit.)
1
Fruit shelf life
TOM144
0.0018*
4.01
TOM184
0.0028*
2.28
Table 2: Single factor ANOVA for fruit shelf life.
Conclusion
Among F7 RILs, parents and checks the maximum shelf life was observed in RIL 7-3, RIL 110-2(110days), followed by RIL 102-1 and RIL 182-4 (100 days). Molecular study involving single marker analysis confirms that TOM 184 and TOM 144 are linked to high shelf life trait. These RILs with high shelf life can be used for crop improvement programme in combination with Marker Assisted Selection (MAS).
Acknowledgement
The authors are highly thankful to DBT-HRD, and Department of Biotechnology, New Delhi (DBT Project No-BI/PR 3405/ PBD/16/943) for providing funds to carry out the research project.
References
- Zapata PJ, Guillén F, Martínez-Romero D, Castillo S, Valero D, Serrano M. Use of alginate or zein as edible coatings to delay postharvest ripening process and to maintain tomato (Solanum lycopersicon Mill) quality. J Sci Food Agric. 2008; 88: 1287-1293.
- Bailén G, Guillén F, Castillo S, Serrano M, Valero D, Martínez-Romero D. Use of activated carbon inside modified atmosphere packages to maintain tomato fruit quality during cold storage. J Agric Food Chem. 2006; 54: 2229-2235.
- Kalidas K, Akila K. Micro level investigation of marketing and post harvest losses of tomato in Coimbatore district of Tamilnadu. J. Stored Prod. Postharvest Res. 2014; 5: 1-7.
- Poovaiah BW. Role of calcium in prolonging storage life of fruits and vegetables. Food Technol. 1986; 40: 86-89.
- Giovannoni J. Molecular Biology Of Fruit Maturation And Ripening. Annu Rev Plant Physiol Plant Mol Biol. 2001; 52: 725-749.
- Seymour G, Poole M, Manning K, King GJ. Genetics and epigenetics of fruit development and ripening. Curr Opin Plant Biol. 2008; 11: 58-63.
- Giovannoni JJ. Fruit ripening mutants yield insights into ripening control. Curr Opin Plant Biol. 2007; 10: 283-289.
- Vrebalov J, Ruezinsky D, Padmanabhan V, White R, Medrano D, Drake R, Schuch W. A MADS-box gene necessary for fruit ripening at the tomato ripening-inhibitor (rin) locus. Science. 2002; 296: 343-346.
- Alexander L, Grierson D. Ethylene biosynthesis and action in tomato: a model for climacteric fruit ripening. J Exp Bot. 2002; 53: 2039-2055.
- Kopeliovitch E, Mizrahi Y, Rabinowitch HD, Kedar N. Effect of the fruit-ripening mutant genes rin and nor on the flavor of tomato fruit. J Amer Soc Hort Sci. 1979; 107: 361-364.
- Collard BCY, Jahufer MZZ, Brouwer JB, Pang ECK. An introduction to markers, quantitative trait loci (QTL) mapping and marker-assisted selection for crop improvement: The basic concepts. Euphytica. 2005; 142: 169-196.
- Edwards MD, Stuber CW, Wendel JF. Molecular-marker-facilitated investigations of quantitative-trait loci in maize. I. Numbers, genomic distribution and types of gene action. Genetics. 1987; 116: 113-125.
- Weller JI, Soller M, Brody T. Linkage analysis of quantitative trait in an intraspecific cross of tomato (Lycopersicon esculentum x Lycopersicon pimpinellifolium) by means of genetic markers. Genetics. 1988; 118: 329-339.
- Haley CS, Knott SA. A simple regression method for mapping quantitative trait loci in line crosses using flanking markers. Heredity (Edinb). 1992; 69: 315-324.
- Nienhuis J, Helentjaris T, Slocum M, Ruggero B, Schaefer A. Restriction fragment length polymorphism analysis of loci associated with insect resistance in tomato. Crop Sci. 1987; 27: 797-803.
- Wang Z, Weber JL, Zhong G, Tanksley SD. Survey of plant short tandem DNA repeats. Theor Appl Genet. 1994; 88: 1-6.
- Saghai-Maroof MA, Soliman KM, Jorgensen RA, Allard RW. Ribosomal DNA spacer-length polymorphisms in barley: mendelian inheritance, chromosomal location, and population dynamics. Proc Natl Acad Sci. USA. 1984; 81: 8014-8018.
- Rodriguez GR, Pratta GR, Zorzoli R, Picardi L. A, Recombinant lines obtained from an interspecific cross among Lycopersicon species selected by fruit weight and fruit shelf life. J Am Soc Hortic Sci. 2006; 131: 651-656.
- deVicente MC, Tanksley SD. QTL analysis of transgressive segregation in an interspecific tomato cross. Genetics. 1993; 134: 585-596.
- Rodriguez GR, Pratta GR, Libertti DR, Zorzoli R. Inheritance of shelf life and other quality traits of tomato fruit estimated from F1's, F2's and backcross generations derived from standard cultivar, nor homozygote and wild cherry tomato. Euphytica. 2010; 176: 137-147.
- Barry CS, Giovannoni JJ. Ripening in the tomato Green-ripe mutant is inhibited by ectopic expression of a protein that disrupts ethylene signaling. Proc Natl Acad Sci. USA. 2006; 103: 7923-7928.
- Yogendra KN, Ramanjini Gowda PH. Phenotypic and molecular characterization of a tomato (Solanum lycopersicum L.) F2 population segregation for improving shelf life. Genet Mol Res. 2013; 12: 506-518.
- Tigchelaar EC, Mcglasson W, Buescher R. Genetic regulation of tomato fruit ripening. Hort Science. 1978; 13:508-513.
- Foolad MR. Genome mapping and molecular breeding of tomato. Int J Plant Genomics. 2007; 2007: 64358.