Research Article
Austin J Biotechnol Bioeng. 2014;1(6): 4.
Screening the In vitro Calcium Oxalate Crystal Inhibition Potential of Abutilon indicum L.: A Common Weed Plant from the Indian Medicine System
Anita Surendra Patil*, Ankit Subhash Kale and Hariprasad Madhukar Paikrao
Department of Biotechnology, Sant Gadge Baba Amravati University, India
*Corresponding author: Anita Surendra Patil, Department of Biotechnology, Plant Secondary Metabolite Lab, Sant Gadge Baba Amravati University, Amravati (M.S), India.
Received: November 14, 2014; Accepted: November 14, 2014; Published: November 15, 2014
Abstract
Abutilon indicum L. is one of the well-known medicinal plants used in treatment of various metabolic disorders. In the present study, the aqueous extracts of fresh leaves were used for phytochemical analysis and determination of antilithiatic activity in the plant. It was found out that the plant leaves possess In vitro Calcium oxalate crystal inhibition potential i.e antilithiatic activity, which was evaluated and compared with positive control tri-sodium citrate both in various concentration (10mg to 100mg/ml). In proposed work, the antilithiatic activity was screened using slide gel method and agar gel overlay assay method, which was the novel method for qualitative and quantitative estimation of antilithiatic potential.
Keywords: Abutilon indicum L; Antilithiatic activity; Slide gel method; Agar gel overlay assay
Introduction
Kidney stone formation is one of the most common metabolic disorders of world population. Almost 12% population is suffering with these. Previous data confirms male patients are more in numbers as compare to females [7]. Prominent evidence of kidney stone has been increased over the last years, and the age of onset is decreasing. With these, the recurrence rate is high more than 50% after ten years [1,2]. This difference is higher in man as compare to women it is due to enhancing and reducing capacity of testosterone and estrogen respectively [3]. The stone formation may be at any site of the urinary system, i.e. kidney, bladder or urinary tract with variable size [4]. It has observed that in more than 60% of kidney stones are of CaOx (calcium oxalate) type, which exists in the form of COM (calcium oxalate monohydrate) and COD (calcium oxalate monohydrate). There are various factors, which enhance and prevent stone formation [5]. Our diet and lifestyle are the major biological events which are responsible for stone formation [6]. There are a number of medicinal plants used in India, which claim for treatment of kidney stones [8]. Abutilon indicum L. is one of the well-known plants possessing antilithiatic potential, as the litholytic property of Abutilon indicum L. has explained in earlier reports [9].
Abutilon indicum (Indian Abutilon, Indian mallow; is a small shrub in the Malvaceae family, native to tropic and subtropical regions of America, Africa and Australia sometimes cultivated as an ornamental [10]. A .indicum is called as "Kanghi" in Hindi, "Atibala" in Sanskrit and Marathi. It is found as a weed near Himalaya's tracts and is considered invasive on certain tropical islands [11]. A. Indicum has been with well reputed potential in "Siddha" system of Ayurveda having high potential as a remedy for jaundice, piles, ulcer and leprosy. In traditional medicine, A. indicum's various parts are used as a demulcent, aphrodisiac, laxative, diuretic, sedative, astringent, expectorant, tonic, anti-inflammatory, anthelmintic and analgesic and to treat leprosy, ulcers, headaches, gonorrhea, and bladder infection [12]. Alkaloids, flavonoids, steroids, and terpenoids have been isolated and characterized from this genus in the literature survey [13-16].
The present study explains the phytochemical evaluation of A. indicum L. by standard methods [18,19]. The calcium oxalate inhibitory potential was also studied with few novel techniques; Agar gel overlay for qualitative and quantitative characterization of antilithiatic activity and Slide gel method for antilithiatic potential using aqueous extract of A. indicum was also performed [17].
Material and Methods
Materials
All the chemicals used were of analytical grade. Double distilled water was used in all the experiments. CaCl2 (Calcium chloride), Ammonium oxalate (NH4)2C2O4 H2O), Sodium oxalate, Na2C2O4, Sodium chloride, NaCl was purchased from Qualigens, Thermo scientific. Bactoagar was purchased from Hi-media Laboratories, Mumbai, India. Alizarin red S was purchased from Sigma-Aldrich, San Diego, USA.
Collection of plant
The plant material Abutilon indicum was collected from Melghat forest region, Amravati. The Herbarium specimen was prepared, authenticated by its morphological characters and submitted to Department of Biotechnology, Sant Gadge Baba Amravati University with accession number SGBAU-DBT-06.
Extract preparation
Leaves of A. indicum were thoroughly washed in tap water and allowed to dry completely in shade. Further the 15 gm material was crushed in mortar and pestle in 100ml distilled water. The extract was collected in 50 ml centrifuge tubes and centrifuged at 4000 rpm at 4°C temperature. The supernatant was collected and filtered by muslin cloth. Next it was allowed to evaporate and dried completely in sunlight. Further stored in deep freeze and used for analysis.
Qualitative phytochemical screening
The different qualitative chemical tests were performed for establishing profile of prepared plant extract for its chemical composition. Qualitative Phytochemical analyses were done using the standard procedures. All the tests performed on extracts to detect various phyto constituents present with the results were shown in Table 1.
Sr. No.
NAME OF TEST
TYPE OF TEST
INFERENCE
ALKALOIDS
WAGNER'S TEST
+
MAYER'S TEST
+
HAGER'S TEST
+
DRAGENDORFF'S TEST
+
CARBOHYDRATES
MOLISCH'S TEST
+
BENEDICT'S TEST
-
FEHLING'S TEST
-
PHENOLS & TANNINS
FERRIC CHLRIDE TEST
+
GELATIN TEST
-
LEAD ACETATE TEST
+
GLYCOSIDES
BORNTRAGERS TEST
-
PROTEINS & AMINO ACIDS
BIURET TEST
-
NINHYDRIN TEST
-
SAPONINS
FOAM TEST
+
FATS & OILS
SPOT TEST
-
GUM & MUCILAGE
+
STEROIDS
+
ANTHRAQUINONES
BORNTRAGERS TEST
-
Table 1: Phytochemical screening of aqueous extracts of A. indicum L. showing antilithiatic activity.
Screening the antilithiatic activity of Abutilon indicum extract
Slide gel assay method: Slide gel method for screening antilithiatic metabolites was performed as per Schneider et al. (1983). One percent (1%) of bactoagar prepared in double distilled water, was heated to liquefy the agar and then smeared on the clean glass slides, the agar was allowed to solidify. On each slide four wells were prepared (spacing between wells was 0.5 cm distance longitudinally x 1.25 cm distance perpendicularly). The slides prepared were divided into negative control (D/W only) sample (aqueous plant extract of A. indicum and positive controls (tri-sodium citrate).
A. indicum extract were prepared in sterile distill water at 100mg/ml stock solution along with tri-sodium citrate and their dilutions were prepared in 10.0 mg/ml, 25mg/ml, 40mg/ml, 55mg/ ml, 70mg/ml, 85mg/ml and 100 mg/ml for comparative evaluation of antilithiatic activity in variable assay methods.
The formation of CaOX crystals was carried out by introducing 20 μl of 0.2 M calcium chloride and 20 μl of 0. 2M ammonium oxalate in the opposite wells and extract of A. indicum was added in two longitudinal wells for each concentrations. All the experimental slides were kept in the moist chamber for two hours.
Statistically significant differences within the area of the crystals formation between blank, control and A. indicum extract were studied. The effects of the A. indicum extract and positive control on the in vitro growth of calcium oxalate crystals compared with the blank by using the inhibition index (I). Absence of inhibition is indicated by I equals 0; whereas complete inhibition is shown by I equals 1.
Inhibitory indexes were calculated by:
I = 1- (As/Ac)x100
Where, As = area of calcium oxalate crystals in presence of sample tested and
Ac = area of calcium oxalate crystals formed for the corresponding blank.
After the incubation, the activity was seen visually, and Inhibitory indexes were recorded. The results for inhibitory potential of selected plants by slide gel method were recorded.
Agar gel overlay assay: Agar gel overlay method is one of the most significant methods for qualitative and quantitative potential of compounds and any plant extracts [20]. In this method, a thick 20x30 cm clean glass plate was used. On the glass plate 0.8% Bactoagar prepared in 0.2 M Calcium chloride (CaCl2) solution was spread and allowed it to solidify for 30 minutes. On the agar plate, five wells were prepared with spacing of 5 cm between each well by borer for each sample dilution, including positive control (tri-sodium citrate). Tri-sodium citrate was added in the range of 100 mg/ml to 500 mg/ ml. Aqueous extract of Abutilon indicum was prepared in 100 mg/ ml stock and further diluted in different concentrations ranging from 20 mg/ 20 μl, 1.6mg/20μl, 1.2 mg/20μl, 0.8 mg/20μl and 0.4 mg/20μl. After loading of test samples, the entire assembly was incubated in moist condition in a closed chamber for 24 hours for diffusion of sample loaded in wells. Ready experimental plates were deepened in 0.2 M ammonium oxalate solution for 10 minutes. The results become visible in the form clear zone i.e crystal inhibited zone against the white background. In order to visualize evident results the plates were further stained with 0.2% Alizarin red (S) at pH 6.5 for 5 minutes. The plates were removed carefully and dipped in distilled water for 15 seconds for destaining unwanted color. The zones were measured for both positive control and sample and were statistically analyzed.
Result and Discussion
Phytochemical analysis
A. indicum has been subjected for aqueous extraction and preliminary phytochemical evaluation. The concentrated extract of A. indicum was brownish, gummy and sticky; it was stored in cold conditions for further study. The phytochemical analysis shown the existence of the number of phyto-constituents including Alkaloid, Carbohydrate, Phenol, Tannin, Saponins, Steroids, Gum and Mucilage whereas Glycosides, Anthraquinones, Proteins and Amino acid were found to be absent. The results are shown in Table 1. Earlier reports also support the presence of phytochemicals especially for alkaloids and Phenols in A. indicum [21].
Slide gel assay
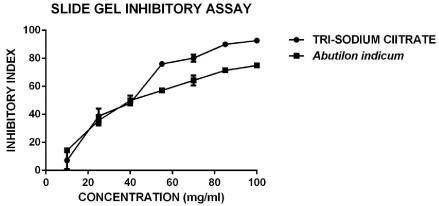
The slide gel assay for calcium oxalate crystal Inhibition was performed for aqueous extract of A. indicum along with positive control The visible streak of CaOx crystal was Tri-sodium citrate, the inhibition of streak formation was measured by measuring the area of crystal streak which was deduced in the form of Inhibitory index using formula given in methods; results were statistically analyzed and shown in Table 2 and plotted in Graph I. The IC50 values were calculated using corresponding linear regression equations and goodness of fit values were presented. The inhibitory index plot represents the comparative CaOx inhibition activity in aqueous extract of A. indicum and Tri-sodium citrate (Positive control) in the range of 10 mg/ml to 100 mg/ml. Results suggest, In case with A. indicum activity was observed with 50% inhibitory concentration i.e. IC50 at 51.01 mg/ml, while in comparison, Tri-sodium citrate exhibited more significant IC50 value at 42.26 mg/ml. Similarly the slide gel method is reported to be suitable for the screening of antilithiatic activity in Plantaga major L. and Orthosiphon stamineus respectively [22,23].
Graph I: Effect of increasing concentrations of positive control (Tri-sodium citrate) and Abutilon indicum L. extracts on Calcium oxalate Inhibitory index.
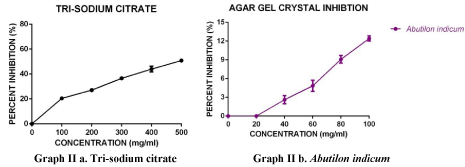
Graph II: Effect of increasing concentrations of Tri-sodium citrate and Abutilon indicum L. extract on calcium oxalate Percent area inhibition. Error bars represent ± SEM (n=3).
CONC
(mg/ml)
Inhibitory Index
IC 50
Linearity Equation
R2 (GOODNESS OF FIT)
Tri-sodium citrate (±S.E.M.)
A.indicum
(±S.E.M.)
Tri sodium citrate
A.indicum
Tri sodium citrate
A.indicum
Tri sodium citrate
A.
indicum
10
7.20±6.15
14.28±00
42.26
51.01
Y = 0.9307*X + 10.66
Y = 0.6377*X + 17.47
0.9117
0.9239
25
38.80±5.4
35.71±3.57
40
48.10±1.01
49.99±3.57
55
76.00±1.23
57.14±00
70
80.20±2.47
64.27±3.58
85
90.00±00
71.42±00
100
92.67±1.22
74.99±1.79
Table 2: Calcium oxalate Inhibitory Index of A.indicum L. aqueous extract by slide gel assay method.
Agar gel overlay assay
The aqueous extracts of A. indicum and Tri-sodium citrate (positive control) were analyzed for calcium oxalate crystal inhibition on agar gel by well diffusion technique. The results were recorded in the form of inhibitory area and percent area inhibition, as summarized in Table3 and graphs IIa, IIb. The percent inhibition values were calculated for aqueous extract of A. indicum and tri-sodium citrate, which confirm that the Tri-sodium citrate showed more CaOx crystal repressive activity followed by A. indicum, as shown in Figure 1&2.
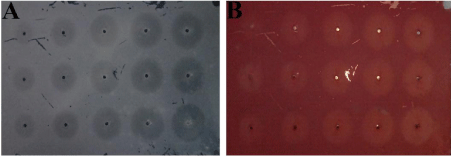
Figure 1: Effect of positive control Tri-sodium citrate on CaOx crystal formation in agar gel inhibition assay (A-Before Staining with Alizarin red S, B-After staining with Alizarin red S).
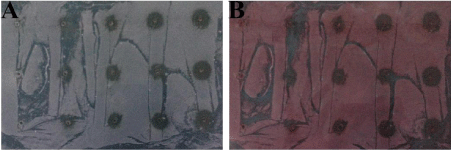
Figure 2: Effect of Abutilon indicum L. aqueous extract on CaOx crystal formation in agar gel inhibition assay (A-Before Staining with Alizarin red S, B-After staining with Alizarin red S).
CONC. (mg/ml)
Tri- sodium citrate
Abutilon indicum L.
Percent inhibition (± S.E )
IC 50 (mg/ml)
CONC. (mg/ml)
Percent inhibition (± S.E)
IC 50 (mg/ml)
100
20.48±1.25
466.90
20
0.00±0
395.64
200
27.12±1.19
40
0.65±0.16
300
36.59±1.27
60
1.21±0.22
400
43.98±2.27
80
2.29±0.13
500
50.81±1.17
100
3.03±0.10
Table 3: Percent Inhibitory values for A. indicum L. using agar overlay method.
Conclusion
The earlier studies proposed methods for measurement of antilithiatic activity of medicinal plants, but the present studies provide the simple, rapid and robust method of qualitative and quantitative screening for antilithiaitc potential of medicinal plant extracts agar gel by well diffusion technique. A. indicum was never explored for its antilithiaitc activity, hence proposed study is extensively useful in herbal remediation in kidney stone disorders.
Acknowledgement
The authors would like to thank Rajiv Gandhi Science and Technology Commission, Mumbai, India for research grant (RGSTC/File- 2012/DPP-94/CR-12) under the major research project sanctioned to Prof. Anita Patil and also to Department of Biotechnology, Sant Gadge Baba Amravati University, Amravati (M.S) India for providing the necessary research facilities.
References
- Moe OW. Kidney stones: pathophysiology and medical management. Lancet. 2006; 367: 333-344.
- Bartoletti R, Cai T, Mondaini N, Melone F, Travaglini F, Carini M, Rizzo M. Epidemiology and risk factors in urolithiasis. Urol Int. 2007; 79: 3-7.
- Devi VK, Baskar R, Varalakshmi P. Biochemical effects in normal and stone forming rats treated with the ripe kernel juice of plantain (musa paradisiaca). Anc Sci Life. 1993; 12: 451-461.
- Havagiray R, Chitme, Shashi Alok, Jain SK, Monika Sabharwal. Herbal treatment for urinary stones. IJPSR, 2010.
- Pareta SK, Patra KC, Harvansh R. Invitro crystallization inhibition by Achyranthes indica linn. Hydroacohalicextract: An approach to antilithiasis. Int J of Pha and biosci, 432.
- Gilhotra UKR, Christina AJM. Effect of Rotunda aquatic Lour. On ethylene-glycol induced urolithiasis in rats. Int. J. Drug Dev. And Res. 2011; 3: 273-280.
- Soundararajan P, Mahesh R, Ramesh T, Begum VH. Effect of Aerva lanata on calcium oxalate urolithiasis in rats. Indian J Exp Biol. 2006; 44: 981-986.
- Khan NJ, Shinge JS, Naikwade NS. Antilithiatic effect of Helianthus annus Linn. Leaf extract in ethylene glycol and ammonium chloride induced nephrolithiasis. Int J of pharm sci. 2010; 2: 181.
- Prachi, Chauhan N, Kumar D, Kasana MS. Medicinal plant of Muzaffarnagar districtused in treatment of Urinary tract and Kidney stones. Indian Journal of Traditional Knowledge. 2009; 8: 191-195.
- "Abutilon indicum", Pacific Island Ecosystems at Risk. Retrieved. 2008; 6: 18.
- Matławska I, Sikorska M. Flavonoid compounds in the flowers of Abutilon indicum (L.) Sweet (Malvaceae). Acta Pol Pharm. 2002; 59: 227-229.
- Rajakaruna N, Cory SH,Towers GHN. "Antimicrobial Activity of Plants Collected from Serpentine Outcrops in Sri Lanka". Pharmaceutical Biology. 2002; 40: 235-244.
- Gaind KN, Chopra KS. Phytochemical investigation of Abutilon indicum. Planta Med. 1976; 30: 174-188.
- Subramanian SS, Nair AGR. Flavonoids of four malvaceous plants. Phytochem. 1972; 1: 1518.
- Sharma PV, Ahmad ZA. Two sesquiterpene lactones from Abutilon indicum. Phytochem. 1989; 28: 3525.
- Dhanalaksmi S, Lakshmanan KK, Subramanian MS. Chemical constituents from Abutilon indicum. J Res Edn Indian Med.1990; 9: 21.
- Schneider HJ, Bothor W, Berg W, Börner RH, Jakob M. A gel model for measuring crystallization inhibitor activities. Urol Int. 1983; 38: 33-38.
- Kokate CK, Practical Pharmacognosy, Vallabh Prakashan, 116-120, 123-124.1994.
- Kokate CK, Purohit AP and Gokhale SB, Pharmacognosy, 3rd edn. Nirali Prakashan, Pune;1995.
- Patil AS, Paikrao HM, Kale AS, Patil SR. Method for in vitro qualitative and quantitative detection of calcium oxalate inhibitory activity of plant extracts. Indian patent. 2014
- Raja RR. Recent pharmacognostical, phytochemical and antifungal analysis of Abutilon indicum (Tamil- Manjal Thuthi) in disease of Ringworm infection - Research report. Int. J. Pharmacy and Pharmaceutical Sci. 2012; 4: 97-100.
- Sharifa AA, Jamaludin J, Kion LS, Chia LA, Osman K. Anti-Urolithiatic Terpenoid Compound from Plantago major Linn. (Ekor Anjing). Sains Malaysiana. 2012; 411: 33-39.
- Dharmaraj S., Hossain MA, Zhari S, Harn GL, Ismail Z. The use of principal component analysis and self-organizing map to monitor inhibition of calcium oxalate crystal growth by Orthosiphon stamineus extract. Chemometrics and Intelligent Laboratory Systems. 2006; 81: 21-28.