1Faculty of Pharmaceutical Science, Shri Shankaracharya Technical Campus, India
2Department of Biotechnology, Malaria Research Group, Indian Institute of Technology, India
*Corresponding author: Rohitas Deshmukh, Faculty of Pharmaceutical Science-Shri Shankaracharya Technical Campus,Bhilai, India.
*Corresponding author: Vishal Trivedi, Malaria Research Group, Department of Biotechnology, Indian Institute of Technology-Guwahati, Assam-781039, India.
Received: November 17, 2014; Accepted: December 05, 2014; Published: December 08, 2014
Cells of the body maintain and regulate a constant internal environment to maintain homeostasis. Homeostasis of the body is constantly threatened by various internal or external factors. Malaria is one of the factors where red blood cells of the body is infected by plasmodium species. Infection causes the rupture of RBC to release daughter merozoite and various pro-oxidant factors [proteinous or non-proteinous) into the blood stream. It is a complex mixture consisting of host-derived proteins, parasite secreted proteins and other metabolically derived degraded peptides. MetHb, a proteinous factor having pro-oxidant nature is potentially toxic to different cell type, organ systems and produces a considerable amount of peroxide to accelerate oxidative-mediated tissue damage. Heme, a prosthetic group of Hb/MetHb is also highly toxic in nature. Free heme exhibits toxic effects to cells and tissue to cause oxidative stress, inflammation and RBC lysis which ultimately disturbs body homeostasis. Haemozoin, a metabolic waste of malaria parasite stimulates various immune cells of the host to exhibit a change in the cytokine secretion profile and contributes to the inflammation during malaria. The malaria-infected RBC and pro-oxidant are the key players for the disturbed homeostasis condition as observed during malaria.
Keywords: Malaria; Methemoglobin; Haemozoin; Hemin; Homeostasis
RBC: Red Blood Cell; Hb: Hemoglobin; MetHb: Methemoglobin; Hz: Haemozoin; He: Hemin; PRBC: Parasitized RBC; GSH: Reduced Glutathione; PS: Phosphatidylserine; GPI: Glycosylphosphatidylinositol; ROS: Reactive Oxygen Species; IL: Interleukins; TLR: Toll like Receptors; TNF: Tumor Necrosis Factor; IFN: Interferon; NF-κB: Nuclear Factor Kappa B; MIP: Macrophage Inflammatory Proteins; MCP: Monocyte Chemo Attractant Protein; CCR: Chemokine Receptor
A healthy human person consists of more than 50 trillion cells working together to perform diverse functions. For the growth, development and survival of individual cell and whole body, it is important to maintain a constant internal environment. Thus, the various processes by which the cell or body maintain and regulates inner environment termed as homeostasis. Cell of the body perform various metabolic process and chemical reaction correctly to maintain homeostasis. However, homeostasis of the body is always threatened by many factors. One such factor is malaria where RBC is infected by Plasmodium species. Malaria is infecting nearly 10% of the world population and is responsible for 1–3 million deaths per year, mainly in children [1,2]. The parasite spreads through the bites of infected Anopheles mosquitoes, called “malaria vectors.” There are four species of plasmodium to cause malaria with the fifth most recently reported, Plasmodium knowlesi that is extremely rare [3-5]. Though malaria by Plasmodium vivax is the most common, but infection caused by Plasmodium falciparum is most lethal [1].
The clinical features include persistent fever, recurrent chills, and sweating. The symptoms are usually seen at 48h and related to the rupture of infected red blood cells. An infected individual eventually becomes weak and anemic due to loss of hemoglobin. Merozoites and malaria pigment i.e. haemozoin are known to block capillaries, causing intense headaches, renal failure, heart failure, or cerebral damage; leading to coma and death [6,7]. The observed clinical features are reflective of the disturbance in homeostasis in the host body.
Malaria caused by plasmodium species completes life cycle in host RBC and liver [8]. Infection in humans begins starts when infected mosquito takes a blood meal which results in the release of sporozoites from the salivary glands of the mosquito into the bloodstream. Sporozoite then enters the liver for replication resulting in the release of thousands of merozoite into the blood stream. In the blood, each merozoite invades an RBC and engulfs a portion of RBC and appears as thin ring, so called as the “Ring” stage. The ring stage parasite grows to form Trophozoite whereas at the Schizont stage; the parasite produces 20–30 daughter merozoites to start another cycle [6]. Rupture of RBCs release various pro-oxidants such as such as Hemoglobin (Hb), Methemoglobin (MetHb), Hemin (He) and Haemozoin (Hz) into the circulation [4, 6].
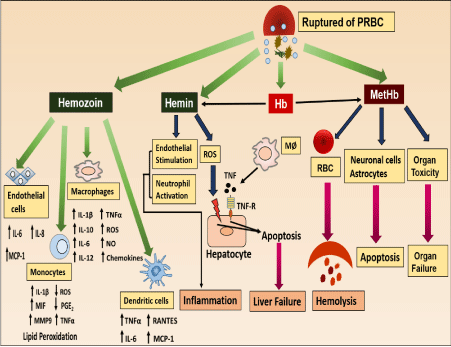
The pro-oxidant molecules released from the malaria-infected RBC can affect the different cells, tissue and organs to disturb body homeostasis. Effects of these pro-oxidants in disturbing homeostasis are summarized in Figure 1. The following section discussion will be on the pro-oxidants and the effect on various cells, tissue or organ system and the role in the disturbance of homeostasis.
Methemoglobin: During malaria, lysis of malaria-infected RBCs result in the release of hemoglobin (Hb) into the circulation [9]. Free Hb is highly unstable and is readily form methemoglobin (MetHb) with Fe3+ or oxidation by Reactive Oxygen Species (ROS) into Ferryl hemoglobin [Fe [IV] = O) [10, 11]. However, ferryl Hb is highly unstable and gets converted into the MetHb through electron transfer. In addition to direct oxidation of Hb, other extrinsic factors such as toxic drug molecules, metabolic by-products of pathogenic organisms, and pro-inflammatory cytokines caused redox imbalance and oxidized intracellular Hb to form MetHb.
MetHb and homeostasis: In normal physiology, MetHb level remains below 2% but during severe malaria condition the concentration reaches up-to toxic levels (>4%) and it effects various cells and organ. Thus, the increased level of methemoglobin is responsible for the disturbed homeostasis.
MetHb and RBC physiology: MetHb is pro-oxidant in nature and exhibits toxicity towards RBCs to disturb the homeostasis. During malaria, lysis of individual Parasitized RBC (PRBC) causes lysis of more than ten uninfected RBCs, leading to the development of severe hemolytic anemia [4,12]. MetHb dose-dependently makes RBC susceptible to osmotic stress and causes hemolysis. Pseudo-peroxidase activity of MetHb causes production of a large amount of ROS in microenvironment. It disturbs the redox balance of RBC to develop oxidative stress as indicated by increase in lipid peroxidation, protein carbonyl and decreased GSH level. As a result of oxidative stress, cytoskeleton changes occur which results in loss of membrane fragility and hemolysis. MetHb initiates a type of chain reaction where initially released MetHb causes destruction of more RBC and amplify the lysis reaction to cause anemia as observed during severe malaria [13]. Oxidative stress within RBC causes Phosphatidylserine [PS) externalization to generate sticky patches to initiate aggregation. Higher degree of RBC aggregation (5-10) is accountable for vascular blockage and associated with the development of pathophysiology of malaria.
MetHb and cellular toxicity: Plasmodium infection is associated with oxidative stress in RBC, responsible for membrane lipids/proteins oxidation, leading to band-3 clustering as well as PS externalization and opsonization of RBC membranes. The modifications on the surface of the infected RBCs are sufficient for the recognition of the cells by macrophages and consequent phagocytosis. Moreover, free Hb is also taken up by macrophages from circulation as a detoxification mechanism. In an in-vitro system, extracellular Hb effects the normal functioning of macrophages and induces death [14]. MetHb has a potential to produce a large amount of peroxide which accelerate oxidative-mediated cellular and tissue damage [15,16]. Pro-oxidant nature of MetHb leads to changes in physiology and biochemical behavior of vascular endothelium and smooth muscles. MetHb activates endothelial cells to up-regulate the production and release of IL-8, IL-6 and adhesion molecule E-selectin. This result in inflammation and vascular occlusion in brain and explains the severe pathophysiological outcomes during cerebral malaria [17].
MetHb and organ toxicity: Released MetHb gets accumulated at various tissues sites and exhibits toxicity towards different organ systems. Hemolysis of RBC leads to increased accumulation of MetHb in the cardiac tissue and increased iron load to cause cardiac toxicity. It was also believed that in certain animal species MetHb scavenge the Nitric Oxide (NO) to cause myocardial tissue necrosis [18]. MetHb released after intra-cerebral hemorrhage plays an important role in neurotoxicity and edema development. In an in-vitro culture MetHb shows cytotoxicity towards brain and spinal cell to cause death in an oxidative stress-dependent mechanism to develop coma and death as evidenced in the cases of cerebral malaria [19,20]. Moreover, exposure of MetHb to brain cells cause up-regulation of heme oxygenase-I in microglia cells, implying that MetHb was taken up by microglia cells. At high concentration the uptake results in death of microglia cells to disturb the homeostasis [21]. MetHb exposure to the pulmonary system causes changes in the vascular compliance, elasticity, distensibility, and stiffness of pulmonary cells. Generation of ROS, inflammation and autacoid dis-regulation are the overall pathology of MetHb in lungs that lead to disturbed homeostasis [22].
Hemin is an essential molecule in living system which plays vital role in multiple biological processes like respiration, oxygen transport and signal transduction. It is present as prosthetic group in hemo-proteins that are important for cellular function and metabolism. Structurally hemin consists of an iron surrounded by four porphyrin ring to form iron-protoporphyrin complex. During patho-physiological state, hemin is being released from the protein and present in free form. In case of malaria, free hemin is released from the rupture of plasmodium infected RBC or from MetHb in presence of reactive oxygen species (ROS). As a result the concentration of hemin reaches more than 20μM which is cytotoxic concentration. Such high concentration is responsible for the observed malaria patho-physiology [23,24].
Hemin and homeostasis: Hemin exhibits toxic effects to cells and tissues to disturb body homeostasis. The pro-oxidant nature of hemin is known to cause toxicity towards various cells, tissue and organ system.
Hemin and RBC physiology: The hydrophobic nature of hemin facilitates insertion into the cell membrane and increases cell’s susceptibility to oxidant-mediated killing [25]. It is reported that hemin after getting incorporated into the intact RBC results in mechanical disruption of the RBC membrane, oxidation of sulfhydryl groups, lipid peroxidation, potassium loss and swelling [26]. In addition, it also disturbs cation gradient and induces hemolysis by a colloid–osmotic mechanism. There are 2 phases for the hemolytic process induced by hemin. In phase I, loss of potassium ion leads to the depletion of glutathione and ATP whereas in phase II, massive hemoglobin loss takes place [27]. Hemin mediated cross-linking of cytoskeletal proteins [such as spectrin and protein 4.1) induces conformational structural abnormality in RBC [28]. Exposure of hemin to RBC causes externalization of Phosphatidylserine (PS), an early signal of apoptosis (eryptosis). PS expressing RBCs are engulfed by macrophages and are rapidly eliminated from circulation to cause anemia [29]. It is also reported that eryptotic cells may adhere to the vascular wall and may compromise the microcirculation [30]. Thus, hemin mediated eryptosis further increases the pro-oxidants burden in the circulation to severely affect host homeostasis.
Hemin and macrophages: During malaria, macrophages perform robust erythrophagocytosis. Hemin accumulation within the macrophages induces ROS and may cause lipid peroxidation, DNA damage and/or protein aggregation, leading to cell death via apoptotic pathway [14]. It is reported that hemin causes early macrophage death characterized by the loss of plasma membrane integrity and morphologic features resembling necrosis. Hemin induced macrophage necrosis follows TLR4/Myd88 dependent expression of TNF-α and TLR4 independent ROS generation [31].
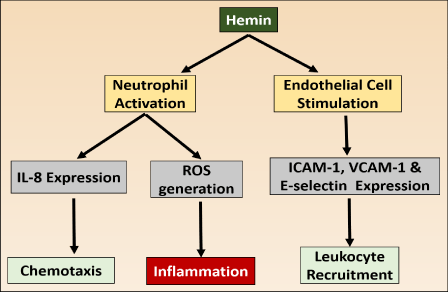
Hemin and inflammation: Inflammation is a defense system of body which is essential for protection against pathogens and for cleaning up of damaged cells after injury. Under normal physiology there is resolution of inflammation after elimination of pathogens to maintain homeostasis. But during malaria hemin causes inflammatory reactions for prolong period to disturb homeostasis. It causes generation of intracellular ROS to stimulates the expression of intracellular adhesion molecules (ICAM-1), vascular cell adhesion molecule1 (VCAM-1) and endothelial leukocyte adhesion molecules (E-selectin) from endothelial cells. The endothelial adhesion molecule recruits leukocytes to initiate various inflammatory responses (Figure 1.2). Moreover, hemin promotes increase in vascular permeability and the infiltration of leukocytes (32,33). The activated immune cells release proteases and ROS result in severe tissue damage. Hemin mediated inflammation is involved in the pathology of atherosclerosis, renal failure, complications after artificial blood transfusion, peritoneal endometriosis, and heart transplant failure. The inflammation caused by hemin also damages the liver. It sensitizes the hepatocytes to undergo apoptosis in response to TNF-α mediated pro-inflammatory signals [34].
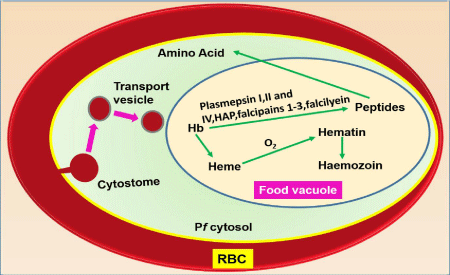
Malaria parasite during its intraerythrocytic stage of its life cycle digest about 80% hemoglobin of RBC into globin and hemin. Globin part is used as major source of amino acids for their growth and development whereas free hemin is converted into insoluble, inert, crystalline and less toxic Haemozoin (Hz) or malaria pigment [35- 37]. Degradation process of hemoglobin to form Hz is summarized in Figure 1.3. Hz is heme polymer containing hematin dimers; composed of hematin molecules linked through bonding between iron of first hematin to the carboxylate of the adjacent hematin [38,39]. Powder diffraction study confirms that haemozoin from the malaria parasite is structurally identical to synthetic β-hematin [39].
Haemozoin and homeostasis: Haemozoin synthesis by the malaria parasite as a protective mechanism against the pro-oxidant hemin is regarded as an inert metabolic waste of the malaria parasite. But now it is well established that Hz is also responsible for malaria pathology. Macrophages, monocytes, neutrophils, endothelial cells and dendritic cells phagocytize Hz result in dysregulation of immune functions of these cells [6]. Hz-fed macrophages are viable but functionally impaired. They are unable to digest Hz or repeat phagocytosis to generate the oxidative burst upon appropriate stimulation or kill ingested bacteria, fungi, or tumor cells [40]. Hz-laden macrophages stimulated with interferon is defective in induction of MHC class-II and resulting immunodepression during malaria [41]. The internalized Hz remains within the phagocytic cells for prolong period but the mechanistic details are not clear. It is suggested that Hz inhibits phagolysosome formation or inhibition of lysosomal acidification and it is accompanied with inhibition of Protein Kinase C (PKC) [42]. It is reported that macrophages stimulated with Hz produces pro-inflammatory cytokine TNF-α. Moreover, it is also observed that severity of malaria correlates with the elevated level of TNF-α and again this secretion gets elevated when glutathione or lipopolysaccharide were added together with Hz [43]. It is reported that Hz activates the inflammasome intracellular protein complex to produce IL-1β [44,45]. In addition, Hz induces the expression of chemokines such as MIP-1α, MIP-1β, MIP-2, MCP-1, and chemokine receptors such as CCR1, CCR2, CCR5, CXCR2 and CXCR4 [44]. Generation of ROS and NO is another pro-inflammatory effect of Hz. It was reported that, generation of ROS from murine macrophage takes place in early phase of stimulation. But the mechanism by which Hz stimulates ROS generation is still unclear [46,47]. Thus literature gives a clear confirmation that Hz is pro-inflammatory in nature and contributes to the immunopathology of malaria.
Glycosylphosphatidylinositol (GPI) of Plasmodium falciparum acts as a PAMP [pathogen-associated molecular patterns) and is considered as a toxin during malaria infection. GPIs are glycolipids found abundantly in all eukaryotes and are known to anchor a varied range of proteins to the surface of Plasmodium falciparum. GPI functions as a potential endotoxin which contributes to pathogenesis of severe malaria. GPIs anchor increases the production of pro-inflammatory responses by the innate immune system of mammalian hosts [48-50].
The GPIs show a wide array of activities, which is similar to malaria pathology. They have been proposed as the major factors involved with the production of pro-inflammatory cytokines TNF-α, IL-1 and IFN-γ in macrophages [51]. GPI has insulin-like activity, causing hypoglycemia, triglyceride lipogenesis in adipocytes and expression of inducible nitric oxide synthase in macrophages and endothelial cells. They also shows an increase endothelial cell expression of intercellular-adhesion molecule 1, vascular cell-adhesion molecule 1 and E-selectin in leukocytes and endothelial cells [52,53]. GPI of Plasmodium falciparum is able to stimulate monocytes and macrophages, causing activation of signal transduction and expression of pro-inflammatory cytokines, chemokines, and nitric oxide [NO) by inducing downstream signaling pathway nuclear factor kappa B (NF-κB) [51].
It has been reported that the presence of GPI anchors also impairs the T-cell responses and development of antibody when present in sporozoite protein vaccines [54-56].
Anti-malarial IgE-antigen complexes and IgE-anti-IgE complexes are also malaria toxins, which induce TNF-α released from peripheral blood mononuclear cells. These complexes cross-link with the macrophage Fc receptors and activate NF-κB transcription factors [57].
During malaria and various hemolytic condition there is a disturbed homeostasis condition. The aim of the review is to understand the effect of various proxidants which is responsible for the various pathophysiology. Malaria infected RBC releases mainly MetHb, hemin and haemozoin in the circulation. These proxidants molecules are toxic in nature. These molecules exhibit toxicity towards different cells, tissue and organs which results in serious pathological consequences. Mostly these molecules exhibit toxicity through the generation of ROS or free electron species. Therefore anti-oxidant therapy and non-toxic free ion chelator can be also used as adjuvant in the treatment of malaria.
Rupture of malaria infected RBC releases various pro-oxidants molecules in circulation to disturb homeostasis.
Pro-inflammatory effect of hemin.
Schematic representation of processes involved in Hb ingestion, catabolism and Hz formation in the malaria parasite Plasmodium falciparum. Hb present in RBC cytoplasm is ingested into the parasite (Pf) via cytostome and transported to Food Vacuole (FV) in transport vesicles. Here Hb is digested by plasmepsins, falcipains and falcilysin to small peptides and finally degraded to aminoacids. This process releases hemin (Fe(II)PPIX) which gets oxidized by molecular dioxygen generating haematin (containing Fe[III]). Haematin is removed by incorporation into microcrystalline haemozoin.