
Review Article
Austin J Biotechnol Bioeng. 2023; 10(2): 1124.
Biotechnology Sustainability in Energy Development
Wisam M Kareem Al-Khazaali1,2; Seyed Ahmad Ataei1*
1Department of Chemical Engineering, Faculty of Engineering, Shahid Bahonar University of Kerman, Kerman, Iran
2Chemical Engineering Section, Design Department, Projects Division, Misan Oil Company, Misan, Iraq
*Corresponding author: Seyed Ahmad Ataei Department of Chemical Engineering, Faculty of Engineering, Shahid Bahonar University of Kerman, Kerman, Iran. Email: ataei@uk.ac.ir
Received: October 18, 2023 Accepted: November 17, 2023 Published: Novembet 24, 2023
Abstract
The application of biotechnology in the oil and environmental sectors plays a crucial role in promoting sustainability. Biotechnology offers innovative solutions for enhancing oil recovery, reducing environmental impacts, and advancing sustainable practices. In the oil sector, biotechnology enables the development of microbial-enhanced oil recovery techniques that improve oil extraction efficiency while minimizing environmental disturbances. Additionally, biotechnological approaches facilitate the remediation of oil-contaminated environments by harnessing the capabilities of microorganisms to degrade hydrocarbons. In the environmental sector, biotechnology aids in the development of renewable energy sources, such as biofuels, through the efficient conversion of biomass. Moreover, biotechnological advancements contribute to the sustainable management of waste, wastewater treatment, and the preservation of biodiversity. Embracing biotechnology in these sectors fosters a sustainable pathway by reducing reliance on fossil fuels, mitigating pollution, and promoting resource efficiency, ultimately supporting the long-term well-being of both ecosystems and human societies.
Keywords: Biotechnology; MEOR; Harmful emissions; Conversion; Removal; Biosensor
Introduction
Biotechnology has found significant applications in both the oil and environmental sectors, offering innovative solutions to address challenges in these industries. In the oil sector, biotechnology plays a crucial role in bioremediation, where microorganisms are used to break down and remove contaminants from oil spills and polluted sites. These microorganisms have the ability to degrade various hydrocarbon compounds, including crude oil, gasoline, and diesel, converting them into harmless byproducts. Bioremediation offers a more sustainable and environmentally friendly approach compared to traditional cleanup methods, such as mechanical removal or chemical dispersants. It has been successfully applied in large-scale cleanup operations, helping to restore polluted ecosystems and mitigate the ecological impact of oil spills.
In the environmental sector, biotechnology has revolutionized waste management practices. Microorganisms are employed in the treatment of various types of waste, including municipal solid waste, industrial effluents, and agricultural residues. Through processes like anaerobic digestion and composting, microorganisms break down organic matter and convert it into valuable products such as biogas and organic fertilizers. Biotechnology also plays a crucial role in the production of biofuels, where microorganisms are used to ferment biomass feedstocks and convert them into bioethanol, biodiesel, and biogas. These renewable fuels offer a sustainable alternative to fossil fuels, reducing greenhouse gas emissions and dependence on non-renewable resources. Furthermore, biotechnology enables the development of innovative techniques for water purification, air pollution control, and the remediation of contaminated sites, contributing to the preservation and restoration of the environment.
However, the application of biological oil recovery is highly dependent on the specific characteristics of the reservoir, such as temperature, pH, and nutrient availability. Extensive research and analysis are required to identify the most suitable microorganisms and optimize the conditions for their growth and activity within the reservoir.
Risk Analysis and Regulations
Low pressure and flow rate of crude oil from the oil wells of reservoirs.
High concentration of contaminants such as sulfur, nitrogen, oxygen, halogen, and aromatics.
Presence of emulsions of oil and water.
High concentrations of released emissions.
Due to the increasing negative effect of sulfur, nitorgen, and the other contaminants, EPA and EU organisation limited the contents of these compounds in the oil products [1].
Oil Recovery
Biological oil recovery, also known as Microbial Enhanced Oil Recovery (MEOR), is an innovative approach that utilizes the power of microorganisms to enhance oil production from reservoirs. This method harnesses the natural metabolic activities of certain microorganisms to modify the properties of the oil reservoir and improve oil recovery rates. The process of biological oil recovery typically involves the injection of selected microorganisms, such as bacteria or fungi, into the oil reservoir. These microorganisms grow under specific conditions within the reservoir, where they can interact with the oil and surrounding rock formations.
There are different mechanisms through which microorganisms aid in oil recovery. One mechanism involves the production of biosurfactants, which are compounds that can reduce the surface tension between oil and water. Biosurfactants help to mobilize trapped oil, making it easier to recover from the reservoir. Another mechanism involves the production of gases, such as carbon dioxide or methane, by the microorganisms. The accumulation of these gases increases the reservoir pressure, leading to the displacement of oil towards production wells. In addition, some microorganisms can alter the viscosity of the oil by breaking down complex hydrocarbons into simpler compounds. This reduction in viscosity makes the oil more mobile, facilitating its flow through the reservoir and extraction.
Biological oil recovery has several advantages over traditional oil recovery methods. It is a more environmentally friendly approach as it reduces the need for harsh chemicals and energy-intensive processes. It can also target hard-to-reach oil pockets and enhance oil recovery from depleted reservoirs, thereby extending the lifespan of oil fields.
In conclusion, biological oil recovery is a promising technology that harnesses the power of microorganisms to improve oil recovery rates. By utilizing the metabolic activities of these microorganisms, it offers a more sustainable and efficient approach to oil extraction while minimizing environmental impact. Continued research and development in this field hold the potential for significant advancements in the oil industry.
Environmental Solutions
Quality Enhancement
There are many scientific studies about the evaluation of the biological treatment of contaminants in order to reduce the harmfull and hazardous cotents in oil such as sulfur, nitrogen, oxygen, halogen, and aromatics. Biodesulfurization (BDS) is one of the important methods contributing the enhancement of quality of oil. Until now BDS is considered as a complementary technology of HDS [2], and in order to reach rate of desulfurization 20+ μM/gcat.min [3], the development and improvement of BDS can be achieved by many methods and techniques:
Treated and targeted cuts
The studies aimed for Targeting whole crude oil. especially in order to treat the recalcitrant HCS on HDS and ODS. Biotreaters or Biocatalysts (recombinant and genes engineering)
Many improvements ways were reported destinate recombinant strain, DNA (primer, operon, enzyme, and promoter), thermophilic enzymes, increasing expression of key enzymes, expression of desulfurization enzymes in heterologous hosts, alternate cells for the expression of dsz genes, coexpression, dsz genes, copy number of desulfurization genes, change of the gene order in the dsz operon, accession of flavin reductase, promoter modification, altering of the promoter of dsz operon, rearranging the dsz gene cluster, altering of the translational sequences , and conducting multi ages or wild of some microorganisms [3-10].
1. Systems
- RD (Applier Equipment or incubators): RD has an effect on BDS efficiency through the reactor type affecting gas-liquid MT either stirred tank reactor or airlift reactor, the reactor size and geometry whether flask or bioreactor, the presence of water (aqueous phase) as shown above about both OWR and emulsions and also aeration or presence of air or oxygen, packed reactor or not, HT reactor with thermophilic bacteria like PAe, My. And Su. families, micro–channel reactor [11,12].Process design (PFD), Scale-up, and Combination of various methods units: The planned process package usually consists of microorganism generator or fermentor, bioreactor (multi staged or series), O/W/microorganism separation and precipitation of sulfates by lime or salts, biocatalysts recycling, purification of oil from residuals units. Then the unit effect is through: implementing the two-phase bioreactor system and de-emulsification steps, the product recovery step, Use of multiple-stage air-lift reactors, use of immobilized cells, use of membranes, the hydrophobicity of cell surface by incubation with hexadecane, the novel combination (integrated method) [3,4].
- Intensification: BDS can be affected by the intensification which can be achieved by applying ultrasonic irradiation (sonication) as a stirrer, field of electricity [5,13].
2. Environment
3. Medium
In 2019, Pacheco who studied the BDS of DBT with isolated bacterium Gordonia alkanivorans strain 1B, studied the experimental optimization of BDS with BSM-SFM culture medium used for cultivation of this microorganism. Whereas, the study was concentrated on the inorganic key compounds (N, Mg, TES ‘micronutrient’).This medium was supplemented with SF-TES (0.50 ml/l) and its final pH was adjusted to 7.5, before being autoclaved at (T 121 C, 1 atm, t 15 min). Pure carbon sources, namely glucose and fructose, were dissolved in Millipore water in concentrated solutions (50% w/v). Pure carbon sources and JAJ were filter sterilized before being added to the culture medium in aseptic conditions, to an initial concentration of about total reducing sugars (10 g/l). Then, to get the SFMM (SFM minimised) with less and minimized qualities of sources of nitrogen (NH4Cl), magnesium (MgCl2.6H2O), TES, and the qualities of source of carbon ‘fixed for comparing’ too. Whereas, their control or initial state was with amounts 1.22 g/l, 0.17 g/l, 0.50 ml/l, and commercial glucose/fructose. Then, it was found the minimized medium for these components had these modifications or optimization: 85 %, 25 %, 25 %, local source of carbon JAJ. Also, it was found that the specific rate of BDS had been duplicated from 2.17 to 3.39 and from 1.91 to 3.58 μmol/gDCW.h for commercial and local sources of carbon respectively. The production was 17μmolHBP/gDCW.Adjuvance by Controlling Mass Transfer (MT) limitation, reaction rate and pathway:
- Emulsifiers or Surfactants for MT improvement: Malani 2021 reported that the improvement of MT can be achieved by controlling OWR and emulsification [5,9,14].
- Immobilizers for MT improvement: Either by nanotechnology: Such as addition of SPION, or natural materials like normal materials or particles such as PVA and inorganic materials like (alumina, Silica, Se) [15]. In 2021, Gunam studied BDS of DBT by Pseudomonas sp. strain KWN5 supplemented on MSSF. The cells immobilized by entrapping them with sodium alginate (SA) had high DBT BDS activity and could degrade 100 mg DBT/L in n-tetradecane of 46.76–100%, depended on concentrations of sodium alginate and cells at (T 37 C, Sr 160 rpm, t 24 h). The combination of SA concentration of 3% (w/v) with bacterial cells OD660 40 (25.52 mg DCW/mL) has an optimal BDS activity on 100 mg DBT/L in n-tetradecane, which is equal to 71.85 % BDS. The immobilized cells of Ps. sp. strain KWN5 in alginate beads were more efficient for the degradation of DBT and can be reused for five cycles (220 h) without any loss in their activity. The results of this study clearly show the role of the effects of cell immobilization in increasing the process of BDS.
- Chemical Catalysts for rate improvement: The development of catalysts as heterogeneous bio-nanocatalysts with canavalin, sulfonated polystyrene, phosphotungstic acid on MWW (matter twenty two) Zeolite, or Fe(II) porphyrin conjugated amino-modified HMS (FeC4Pc) as catalyst [5].
- Host Improver for Mechanism Pathway: That the known enzymes and genes related to the BDS are low rated in 4S pathway, then conduction of genes products of dszA,B,C of plasmid and cloning operon, then using middle host like E. coli to increase MT to genetic materials into non-entering gram negative bacteria. Whereas, the incompatible groups are transferred to conjugation methods. Also, increase in the activity of DszD of the limiting step can improve rate [3,16].
1. Simultaneous or combination treatment: such as Physical, chemical and biochemical as in addition to microorganism with hydrogen gas in hydrotreater bioreactor.
2. Factors experimental mechanism kinetics for modeling correlations, systems simulations, and simple classical optimization to select the optimum operation conditions of pre-, post-, and treatment processes
3. Many studies of optimization result in the optimum pretreatment such as the isolation method or the treatment of objective desulfurization such as the operation conditions of BDS and selectivity (for mixtures case studies) and tendency (availability or specificity for pure substrate or lumped mixtures case studies) of microorganism. Also, the intensification can be optimized simply through known range.
In the treatment step, the objective function is aimed to mathematically minimise the consumption of consumables and the operation costs; or maximise the product quality by application of the mathematical kinetic models. Also, it may be on empirical or experimental trials by application of statistical models in design of experiments.
In 2016, Adlakha investigated the BDS of crude oil and HDS diesel with high and low sulfur content using Gordonia sp. IITR100 in a shake flask and benchtop bioreactor. The study utilized an optimized MSM supplemented with sucrose as a carbon source and heavy crude oil as the only sulfur source. The BDS efficiency was determined to be 76.1% at 3 days, 98% at 7 days, and 70% at 3 days for the aforementioned fractions. Additionally, an upgrade of crude oil was observed as the viscosity of heavy crude oil decreased by 31%.
In 2019, Habibi AD studied the BDS of DBT by Ralstonia eutropha using SFMS in a conical flask. Glucose and DBT were added as the carbon and sulfur sources, respectively. The study found that the maximum specific growth rate and maximum cell concentration increased with the rise of initial pH from 6 to 9. The growth-associated and non-growth-associated 2-HBP formation constants were determined to be 3.82 mg2-HBP/gcell and 0.06 mg2-HBP/gcell.h, respectively, at an initial pH of 8.
In 2019, Chen S investigated the BDS of gas oils with different initial concentrations of sulfur using Gordonia sp. SC-10 with different OWR ranging from 2/5-1/12 in SFM supplemented with glucose. The study found a reduction in sulfur concentration and a broad range of HCS desulfurization activity against alkylated DBTs. The efficiency and rate of BDS increased as OWR decreased, with the remaining sulfur decreasing from 80 to 28 mg/l by decreasing OWR from to 1/12.
In 2020, Awadh M examined the tendency of the BDS consortium through restructuring the community. The study found that Klebsiella and Pseudomonas can be stimulated by various compounds, while Rh. grows better with DBT only, and Sp. grows with DBT and 4 MDBT. The study concluded that Ps. can consume the endproduct HBP and grow due to that.
A study conducted in 2020 by Saeed compared the desulfurization efficiency of crude oil from Yemen by Pseudomonas aeruginosa to its peer ATCC 27853. The results showed that after one day, the desulfurization efficiency was approximately 50%, and the amount of microorganism used had an impact on the remaining sulfur content, reducing it from 0.4% to 0.2% wt.
In another study conducted in 2021 by Chen S, the desulfurization activity of Gordonia sp. SC-10 on diesel oil, BT, DBT, and alkylated derivatives was investigated in SFM. The results showed that it had a preference for HCS and effectively desulfurized diesel oils, reducing the sulfur content from 167.7 mg/L to 19.7 mg/L in real-life diesel oil with 88.3% sulfur removal at T 30 C, Sr 160 rpm, and t 5 day.
8- Caring specificity of target HCS by biotreaters: The preference of each microorganism towards specific types of HCS should be taken into consideratio [17].
Water, Soil, and Air Pollution Treatment
1. The biodegradation is one of the famous methods which can decompose the total hydrocarbon in water and soChemical
Which is called biodegradation, in it,ome microorganisms have the abitlity on degradation of hydrocarbonic compounds of oil and plastic materials which are hazards on the environment [18]. Also, there are ones which have the ability to clean air from volatile organisc compounds (VOCs) [19].
2. Physical biotechnology
Which can be called bioseparation, whereas, some microorganisms have the ability on physical breaking the oil/water emulsions [20].
Production of Fuels
The biotechnology can produce some fuels such as biofuels and …
The application of biotechnology in the oil and environmental sectors plays a crucial role in promoting sustainability. Biotechnology offers innovative solutions for enhancing oil recovery, reducing environmental impacts, and advancing sustainable practices. In the oil sector, biotechnology enables the development of microbial-enhanced oil recovery techniques that improve oil extraction efficiency while minimizing environmental disturbances. Additionally, biotechnological approaches facilitate the remediation of oil-contaminated environments by harnessing the capabilities of microorganisms to degrade hydrocarbons. In the environmental sector, biotechnology aids in the development of renewable energy sources, such as biofuels, through the efficient conversion of biomass. Moreover, biotechnological advancements contribute to the sustainable management of waste, wastewater treatment, and the preservation of biodiversity. Embracing biotechnology in these sectors fosters a sustainable pathway by reducing reliance on fossil fuels, mitigating pollution, and promoting resource efficiency, ultimately supporting the long-term well-being of both ecosystems and human societies.
1. Bioethanol: Microorganisms such as yeast and bacteria are used to ferment sugars derived from biomass (such as corn, sugarcane, or cellulose) to produce bioethanol, which can be blended with gasoline as a renewable fuel source.
2. Biodiesel: Certain types of microorganisms, particularly algae, can produce oils that can be converted into biodiesel through a process called transesterification. Algae-based biodiesel is considered a promising alternative to fossil fuels due to its high lipid content and rapid growth rates.
3. Biogas: Anaerobic bacteria can break down organic matter in the absence of oxygen, producing biogas as a byproduct. Biogas primarily consists of methane and carbon dioxide and can be used as a renewable energy source for heating, electricity generation, or as a vehicle fuel.
4. Biohydrogen: Some microorganisms, such as certain types of bacteria and algae, have the ability to produce hydrogen gas through various metabolic pathways. Biohydrogen is considered a clean and sustainable alternative to conventional hydrogen production methods.
5. Biobutanol: Certain bacteria, such as Clostridium species, can produce biobutanol through a process called acetone-butanol-ethanol (ABE) fermentation. Biobutanol has similar properties to gasoline and can be used as a fuel additive or as a standalone fuel.
6. Methane: Methanogenic archaea are microorganisms that produce methane gas as a metabolic byproduct during the breakdown of organic matter in anaerobic conditions. Methane, also known as natural gas, is widely used as a fuel for heating, cooking, and electricity generation.
These are just a few examples of how microorganisms can be utilized to produce different types of fuels, showcasing the potential of biotechnology in the production of sustainable and renewable energy sources.
Theorectical Foundations of Niotechnology
This section aims to analyze the literature on BDS from an engineering perspective, particularly in the areas of kinetics, modeling, simulation, and optimization. The model takes into account various factors such as the feedstock (single or multiple), transport phenomena (MT and HT), and metabolic reactions
Kinetic Mechanism Pathway
Table 1 and Figure 1 demonstrate that microorganisms have various pathways for cleaving bonds of C-S or C-C. However, it is advisable to selectively remove the sulfur atom from the HC structure (such as DBT) through the 4S-pathway, which retains the HC skeleton and fuel value while producing non-toxic or less toxic compounds depending on the strain, such as 2-HBP, 2,2'-bihydroxybiphenyl, 2-methoxybiphenyl, and 2,2'-dimethoxy-1,1'-biphenyl. Both these compounds are then directed to the HC phase (the fuel), while the sulfur is eliminated as inorganic sulfate in the aqueous phase containing the biocatalyst. This process is recommended as it results in the production of less or non-toxic compounds. Several studies have investigated this process, including Gray KA 1996, Nekudzuka 1997 (My.), Ma T 2010, Bhatia S 2012 (Kl.), Monticello DJ 2000, Bordoloi 2014, El-Gendy 2014, Nassar 2016, El-gendy 2018, Kilbane 2016, and Malani RS 2021. From an engineering perspective, the model for this process depends on the feedstock (single or multiple), transport phenomena (MT and HT), and metabolic reactions, which are represented in kinetics, modeling, simulation, and optimization.
Bond
Pathway, Microorganism, and References
Oxidative cleavage C-C bond
Kodama pathway
Pseudomonas Putida [5]
Reductive cleavage C-S bond
N/A because it requires a strict maintenance under anaerobic conditions - [5]
Oxidative cleavage C-S bond, generally, intermediates here are DBT, DBT sulfoxide, DBT sulfonate, 2,2-diHBP.
4S pathway, This is the desired pathway for the BDS because it keeps the value of energy of fuel
Agrobacterium strain MC501, Arthrobacter, Gordonia sp., Nocardia sp., Goordonia alkanivorans RIPI90A, Klebsiella, Mycobacterium strain G3y, Pseudomonas, Rhodococcus erythropolis, Rhodococcus erythropolis IGTS8, Rhodococcus sp. 309, Sphingomonas subarctica T7b, Stenotrophomonas sp.strain SA21, Xanthomonas, Bacillus subtilis, Bacillus strain Al 1-2, Mycobacterium sp., Paenibacillus sp. [2,3,28,29,30]
2HBP sulfate endproduct pathway
Corynebacteria sp [3]
2HBP+ sulfate endproducts pathway [3]
Table 1: Metabolism pathways.
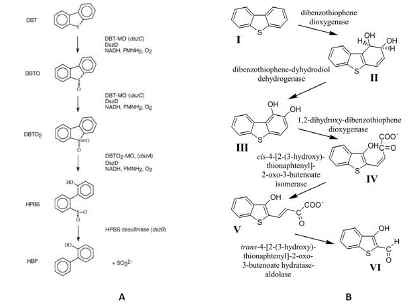
Figure 1: Mechanism of metabolic pathways. (a) BDS 4S pathway for quality improvement [5,27]. (b) BDG or Kodama pathway for environmental purposes [3].
Various microorganisms, including Rhodococcus erythropolis IGTS8, Gordonia, Nocardia, Microbacterium, Mycobacterium, Actinomycetales, Sphingobacterium, Pantoea agglomerans, Stenotrophomonas, and Brevibacillus species, have been reported to retain the 4S-pathway. Some moderate thermophiles and thermotolerant species have also been reported, which are beneficial for downstream Hydrodesulfurization (HDS) in real field operations, such as Paenibacillus, Bacillus subtilis, Mycobacterium, Thermobifida, Rubrobacter, and Klebsiella [3,9,21].
Malani 2021 reported a significant study on DBT BDS using resting cells of genetically modified microorganism, Pseudomonas Putida CECT 5279, in both whole and disrupted forms. The study demonstrated that the rate of BDS was not limited by the concentrations of reducing equivalents NADH and FMNH2 or the activity of Flavin-oxido-reductase enzyme inside the cells. The impact of transporting intermediate metabolites of the 4S pathway across the cell membrane on BDS kinetics was also investigated. It was discovered that there was no accumulation of any metabolite or final product of the 4S pathway inside the cells, and the transport of substrates and intermediate metabolites across the cell membrane did not limit the overall BDS rate [5].
Chen (2021) conducted a study on the desulfurization pathway of Gordonia sp. SC-10. The study found that this pathway is sulfur-specific, producing hydroxylated products in polyphasic systems. In addition, the microorganism produced mycolic acids with 47 and 58 carbon atom chains on the cell surface and inclusion bodies inside the cells, which may enhance substrate bioavailability and adaptability to polyphasic systems. Interestingly, this is the first known instance of inclusion body formation during BDS [22].
Mathematical Modeling, Simulation, and Optimization
There have been numerous attempts to model the BDS process, which can be categorized based on the type of fluid used (single or multiple substrate) or based on the system or phenomena (design-based or metabolism-based models). For single-substrate systems, mathematical models such as Monod, Haldane, or similar models can be used. These models are useful for initial evaluation and comparison of desulfurization efficiencies of microbial cultures. However, they must be refined due to the complex interactions and inhibitions that can occur [5,23].
Various attempts have been made to model the BDS phenomenon, which can be categorized based on the pretreated fluid (single substrate or multiple substrate) or the system or phenomena (design-based models, metabolism-based models). Gross mathematical models like Monod, Haldan, or similar models can be applied when using a single substrate. These models are useful for initial evaluation and comparison of desulfurization efficiencies of microbial cultures. However, the models need to be developed further to take into account the competence and inhibition interactions [5,23].
In multiple substrate models, gross kinetic models were used to study the interactions among substrates. The presence of multiple substrates in the organic phase reduces the degradation rate of all HCS. Inhibition tends to increase with alkyl substituents on DBT, and the interaction among different HCS is mostly competitive inhibition [5].
Mathematical models that incorporate the design features of reactor design have mainly focused on the mass transfer issues of the BDS. The accessibility of HCS present in the organic phase (diesel, gasoline, or crude oil) to the microbial cells in the aqueous phase is a critical factor in determining the process kinetics. This factor is influenced by the interfacial area in the reactor, which is a function of energy input to the process for mixing the phases. Other important factors highlighted by the models include the volume ratio of the aqueous/organic phases and the dissolved oxygen concentration in the aqueous medium. In essence, these mathematical models provide practical insights into the process and can form guidelines for optimization [24].
The kinetic models based on metabolic pathways are mostly suitable for fundamental studies on various new microbial species or their recombinant/genetically modified versions, as shown in Fig. 1 [25].
There have been relatively few studies on this topic. One such study was conducted by Alvarez in 1999, which focused on modeling and optimizing BDS. The models were validated through a comparison of experimental and theoretical data, and optimal quantities of various factors were determined. The study also involved the development of theoretical models for glucose consumption, which were found to be consistent with experimental results and confirmed the accuracy of the assumptions and mathematical representations of metabolic and process phenomena. Optimal oil/water ratios were also derived as part of the overall optimization, and a general framework was proposed for incorporating all key process parameters in a comprehensive process optimization.
In addition to BDS the microorganisms can remove the nitrogen from various oils such as HY9 and R. erythropolis ATCC 4277 [26].
Analysis Methods
1. Biotechnology can indeed be used in laboratory testing of oil to provide valuable insights and analysis. Here's how it can be appliedMicrobial Analysis: Biotechnology enables the identification and characterization of microorganisms present in oil samples. Microbes can have a significant impact on the quality and degradation of oil, so understanding their composition and activity is crucial. Techniques such as Polymerase Chain Reaction (PCR) and Next-Generation Sequencing (NGS) allow researchers to analyze the genetic material of microorganisms in the oil. This information helps determine the types of microbes present, their potential impact on the oil, and their ability to degrade hydrocarbons.
2. Enzyme Studies: Biotechnology facilitates the study of enzymes involved in the breakdown of oil components. Certain bacteria and fungi produce enzymes, such as lipases and oxidases, which can degrade hydrocarbons. In the lab, researchers can isolate and characterize these enzymes, studying their properties and activities. This knowledge helps in developing enzyme-based assays for the detection and quantification of specific oil components. Enzyme studies also contribute to understanding the potential for biodegradation and the optimization of bioremediation processes.
3. Biomarker Analysis: Biotechnology techniques allow the analysis of biomarkers, which are specific compounds found in oil that can provide information about its origin, maturity, and composition. Biomarker analysis involves the identification and quantification of these compounds using methods like gas chromatography-mass spectrometry (GC-MS) or liquid chromatography-mass spectrometry (LC-MS). By analyzing biomarkers, researchers can determine the type of oil, assess its quality, and track its migration or degradation in the environment.
4. Biosensor Development: Biotechnology plays a role in developing biosensors for oil analysis. Biosensors utilize biological components, such as enzymes or antibodies, to detect and measure specific substances in a sample. In the case of oil testing, biosensors can be designed to detect and quantify contaminants, such as heavy metals or organic pollutants, present in the oil. These biosensors offer rapid and sensitive detection methods, enabling real-time monitoring and analysis of oil samples in the laboratory.
By applying biotechnology techniques and tools, laboratory testing of oil can provide valuable information about its microbial composition, enzymatic activity, biomarkers, and contaminant levels. These insights help in assessing oil quality, understanding its behavior in the environment, and developing strategies for oil spill response, cleanup, and mitigation. Ultimately, biotechnology enhances our understanding of oil samples, enabling more informed decision-making in the oil industry and environmental management.
Operation Monitoring
Biotechnology can be used to develop sensor technologies for oil analysis. Biosensors, which incorporate biological components, can be designed to detect and measure specific substances or properties in oil samples. These sensors utilize the interaction between biological molecules, such as enzymes, antibodies, or DNA, and the target analytes present in the oil. In the context of oil analysis, biosensors can be used to detect various parameters, including the presence of specific hydrocarbons, contaminants, or pollutants. They can provide real-time, on-site monitoring capabilities, making them valuable tools for oil spill response, environmental monitoring, and industrial applications.
The development of biosensors involves immobilizing the biological components onto a transducer surface, which converts the biochemical signal into a measurable output, such as an electrical or optical signal. The transducer then detects and quantifies the target analytes in the oil sample. Biosensors offer several advantages in oil analysis, including high sensitivity, rapid response times, and potential for miniaturization and portability. They can be designed for specific analytes of interest and tailored for different applications within the oil industry, such as monitoring oil quality, detecting contaminants, or assessing the effectiveness of remediation processes.
By harnessing biotechnology, biosensors provide a promising avenue for improving the efficiency and accuracy of laboratory testing in the oil sector. They enable real-time monitoring, early detection of issues, and informed decision-making, ultimately contributing to more effective management of oil resources and environmental protection.
References
- Al-Khazaali WM, Ataei SA. Risk assessment of oily energy resources: current challenges and prospective opportunities. J Namibian Stud Hist Pol Cult. 2023; 33: 858-74.
- Alkhalili BE, Yahya A, Abrahim N, Ganapathy B. Biodesulfurization of sour crude oil. Mod Appl Sci. 2017; 11: 104.
- Nazari F, Kefayati ME, Raheb J. The study of biological technologies for the removal of sulfur compounds. J Sci Islamic Repub Iran. 2017; 28: 205-19.
- El-Gendy NS, Nassar HN. Biosynthesized magnetite nanoparticles as an environmental opulence and sustainable wastewater treatment. Sci Total Environ. 2021; 774: 145610.
- Malani RS, Batghare AH, Bhasarkar JB, Moholkar VS. Kinetic modelling and process engineering aspects of biodesulfurization of liquid fuels: review and analysis. Bioresour Technol Rep. 2021; 14: 100668.
- Li L, Shen X, Zhao C, Liu Q, Liu X, Wu Y. Biodegradation of dibenzothiophene by efficient Pseudomonas sp. LKY-5 with the production of a biosurfactant. Ecotoxicol Environ Saf. 2019; 176: 50-7.
- Yaqoub Z. Biocatalyst development for biodesulfurization. United Kingdom: University of Manchester. 2013.
- Chauhan AK, Ahmad A, Singh SP, Kumar A. Biodesulfurization of benzonaphthothiophene by an isolated Gordonia sp. IITR100. Int Biodeterior Biodegrad. 2015; 104: 105-11.
- Mohebali G, Ball AS. Biodesulfurization of diesel fuels–past, present and future perspectives. Int Biodeterior Biodegrad. 2016; 110: 163-80.
- Kilbane JJ, Stark B. Biodesulfurization: a model system for microbial physiology research. World J Microbiol Biotechnol. 2016; 32: 137.
- Martinez I, Santos VE, Alcon A, Garcia-Ochoa F. Enhancement of the biodesulfurization capacity of Pseudomonas putida CECT5279 by co-substrate addition. Process Biochem. 2015; 50: 119-24.
- Chen S, Zhao C, Liu Q, Zang M, Liu C, Zhang Y. Thermophilic biodesulfurization and its application in oil desulfurization. Appl Microbiol Biotechnol. 2018; 102: 9089-103.
- Yi Z, Ma X, Song J, Yang X, Tang Q. Investigations in enhancement biodesulfurization of model compounds by ultrasound pre-oxidation. Ultrason Sonochem. 2019; 54: 110-20.
- Martínez I, El-Said Mohamed MES, Santos VE, García JL, García-Ochoa F, Díaz E. Metabolic and process engineering for biodesulfurization in Gram-negative bacteria. J Biotechnol. 2017; 262: 47-55.
- Nassar HN, Abu Amr SS, El-Gendy NS. Biodesulfurization of refractory sulfur compounds in petro-diesel by a novel hydrocarbon tolerable strain Paenibacillus glucanolyticus HN4. Environ Sci Pollut Res Int. 2021; 28: 8102-16.
- Sousa SF, Sousa JF, Barbosa AC, Ferreira CE, Neves RP, Ribeiro AJ, et al. Improving the biodesulfurization of crude oil and derivatives: a QM/MM investigation of the catalytic mechanism of NADH-FMN oxidoreductase (DszD). J Phys Chem A. 2016; 120: 5300-6.
- Awadh M, Mahmoud H, Abed RMM, El Nayal AM, Abotalib N, Ismail W. Diesel-born organosulfur compounds stimulate community re-structuring in a diesel-biodesulfurizing consortium. Biotechnol Rep (Amst). 2020; 28: e00572.
- Bacha A, Nabi I, Zaheer M, Jin W, Yang L. Biodegradation of macro- and micro-plastics in environment: a review on mechanism, toxicity, and future perspectives. Science of The Total Environment. 2023; 858: 160108.
- Dhamodharan K, Varma VS, Veluchamy C, Pugazhendhi A, Rajendran K. Emission of volatile organic compounds from composting: a review on assessment, treatment and perspectives. Sci Total Environ. 2019; 695: 133725.
- Maruthapandi M, Saravanan A, Manoj S, Luong JHT, Gedanken A. Facile ultrasonic preparation of a polypyrrole membrane as an absorbent for efficient oil-water separation and as an antimicrobial agent. Ultrason Sonochem. 2021; 78: 105746.
- Capecchi E, Piccinino D, Bizzarri BM, Botta L, Crucianelli M, Saladino R. Oxidative bio-desulfurization by nanostructured peroxidase mediator system. Catalysts. 2020; 10: 313.
- Chen S, Zang M, Li L, Chen J, Liu Q, Feng X, et al. Efficient biodesulfurization of diesel oil by Gordonia sp. SC-10 with highly hydrophobic cell surfaces. Biochem Eng J. 2021; 174: 108094.
- Habibi DA, A, Vahabzadeh F, Akbari E. Bioenergetic aspects of dibenzothiophene desulfurization by growing cells of Ralstonia eutropha. Pollution. 2019; 5: 709-19.
- Abin-Fuentes A, Leung JC, Mohamed MES, Wang DI, Prather KL. Rate-limiting step analysis of the microbial desulfurization of dibenzothiophene in a model oil system. Biotechnol Bioeng. 2014; 111: 876-84.
- Martinez I, Santos VE, Gomez E, Garcia-Ochoa F. Biodesulfurization of dibenzothiophene by resting cells of Pseudomonas putida CECT5279: influence of the oxygen transfer rate in the scale-up from shaken flask to stirred tank reactor. J Chem Technol Biotechnol. 2016; 91: 184-9.
- Prado GHC, Rao Y, de Klerk A. Nitrogen removal from oil: a review. Energy Fuels. 2017; 31: 14-36.
- Nazari F, Kefayati ME, Raheb J. The study of biological technologies for the removal of sulfur compounds. J Sci Islamic Repub Iran. 2017; 28: 205-19.
- Ismail W, El-Sayed WS, Abdul Raheem AS, Mohamed ME, El Nayal AM. Biocatalytic desulfurization capabilities of a mixed culture during non-destructive utilization of recalcitrant organosulfur compounds. Front Microbiol. 2016; 7: 266.
- Shahaby AF, Essam-el-din, KM. Desulfurization of crude oil and oil products by local isolated bacterial strains. Int J Curr Microbiol Appl Sci. 2017; 6: 2695-711.
- Mohamed MES, Al-Yacoub ZH, Vedakumar JV. Biocatalytic desulfurization of thiophenic compounds and crude oil by newly isolated bacteria. Front Microbiol. 2015; 6: 112.
- Pacheco M., S.M. Paixão, T.P. Silva, L. Alves (2019). “On the Road for Cost-Effective Fossil Fuel Desulfurization by Gordonia alkanivorans Strain 1B”. RSC Advances 9 (44): 25405-25413.