
Special Article: Fermentation
Austin J Biotechnol Bioeng. 2024; 11(1): 1129.
Probiotic Flavored Fermented Goat Milk as An Adequate Vehicle for Beneficial Bacteria and Higher Total Phenolic and Antioxidant Activity
Livia Bordalo Tonucci1*, Bárbara Pereira da Silva2,4*, Maria Eliza de Castro Moreira2, Isabel Cristina Silva de Oliveira3, Mariana Rodrigues Carneiro1, Hércia Stampini Duarte Martino4, Karina Maria Olbrich dos Santos5
1UNINTA University. Rua Coronel Antônio Rodrigues Magalhães, Brazil
2Faculdade Dinamica do Vale do Piranga – FADIP Rua G, MG, Brazil
3Federal Institute of Ceará, Sobral, Brazil. Avenida Doutor Guarany, Derby Clube, Brazil
4Federal University of Viçosa. Department of Health and Nutrition, Viçosa, Minas Gerais, Brazil
5Brazilian Agricultural Research Corporation (Embrapa), Rio de Janeiro, RJ, Brazil
*Corresponding author: Livia Bordalo Tonucci Rua Coronel Antônio Rodrigues Magalhães, 359. Dom Expedito. Sobral, Ceará, Zip Code: 62.050-100. Postal code, 10, Brazil. Tel: +55 (88) 98809-9885 Email: livia_bordalo@hotmail.com
Received: January 25, 2024 Accepted: March 01, 2024 Published: March 08, 2024
Abstract
The study aimed to evaluate two grape flavored fermented goat milk produced with or without probiotics. Physicochemical characteristics, sensory analysis, antioxidant profile and probiotics viability of the dairy beverages were analyzed. The phenolic contents and antioxidant activity of probiotic milk were higher than conventional milk. A higher loss in cell viability was observed for L. acidophilus than for the B. animalis. The average sensory acceptability scores obtained by both dairy beverages were higher than 6.5. Therefore, the probiotic fermented milk showed an adequate vehicle for the probiotics with good viability, a higher total phenolic and antioxidant activity and good acceptability.
Keywords: Goat milk; Probiotic; Dairy beverage; Gastrointestinal simulation
Introduction
Probiotic functional foods are reported to provide several health benefits. Their efficacy are influenced by the selection of microbial cultures and the concentration of viable population in the product [32]. Lactobacillus and Bifidobacterium are associated with maintaining an optimum gut microbiota balance [35], decreasing the lactose intolerance symptoms [30], immune system stimulation [25], and presents antioxidative properties [26].
Probiotics have become an integral part of the complex world as biologics, pharmaceuticals, food and nutritional supplements due to their potential of providing health benefits. Currently, there is a notable increase in the consumption of non-bovine milk in substitution to conventional milk [40]. The specific nutritional composition of goat mill is related to higher protein digestibility, during digestion or technological processing, which can exert beneficial properties to the organism [37]. However, caprine milk products it is not widely accepted by consumers, mainly due to its typical flavor derived from their capric, caproic and caprylic acids content [16]. It is noted that the association of probiotic fermented milk with functional ingredients, such as juice, improves its nutritional profile as well as its sensory attributes [40]. In this way, purple grape juice stands out due to its pleasant flavor and flavonoids concentration, specifically anthocyanidins and resveratrol [13]. These compounds have important biological functions and health benefits, acting on the oxidative and inflammatory process [28].
Reactive Oxygen Species (ROS) mediated oxidative stress are known to play vital role in the development of chronic diseases such as cancer, diabetes, heart disease, stroke, Alzheimer's disease, rheumatoid arthritis, cataract and aging [3]. Thus, a novel approach is represented by the development of probiotic products exerting antioxidant activity. To neutralize the oxidant molecules, the human body synthesizes antioxidant enzymes and molecules that, together with the antioxidants contained in food, form a biological antioxidant barrier to chemicals that induce oxidative stress, either by generating ROS or by inhibiting antioxidant system [22]. Furthermore, the administration of probiotic lactic acid bacteria can modulate the gut microbiota composition and activity, influencing the metabolism of polyphenols and the release of bioactive metabolites at the intestinal level. However, there are a limited number of studies about the contribution of probiotic bacteria to the antioxidant activity of probiotic beverages [23].
The viability of probiotic microorganisms in food products and their resistance during the gastrointestinal transit is necessary to obtain health benefits related to activities exerted at the intestinal level [31]. In the production of flavored fermented milks, the addition of fruit juices or pulps may interfere in the survival of probiotic microorganisms [12], and should be investigated. Few publications on probiotic flavored fermented goat milk are available in literature [33,34,40]. Thus, the present study aimed to evaluate the viability and in vitro gastrointestinal tolerance of probiotics Bifidobacterium animalis and Lactobacillus acidophilus in flavored fermented milk produced with goat milk and grape juice, and their influence on the antioxidant activity, total phenolic content, texture and sensory features of the beverages during refrigerated storage.
Materials and Methods
Production of the Flavored Fermented Milk
The Probiotic Fermented Milk (PFM) and Conventional Fermented Milk (CFM) were produced using the commercial starter culture Streptococcus thermophilus TA-40 (Danisco, Sassenage, France; 0.003 g/100 g). The PFM was added by B. animalis subsp. lactis BB-12 and L. acidophilus La-5 (Chr. Hansen, Hørsholm, Denmark; 0.024 g/100 g). Goat milk provided by Embrapa Goats and Sheep (Sobral, Ceará, Brazil) was supplemented with 5% (w/v) sucrose and pasteurized at 90°C for 15 min, then cooled to 43±2°C for the addition of the starter and probiotic cultures. The fermentation process was conducted at 40±1°C until reaching pH 5.0±0.1. Next, the fermented milk temperature was decreased to 4°C up to the following day, and then the beverages were flavored with 20% (w/v) of purple grape juice obtained from Embrapa Grapes and Wine (Bento Gonçalves, Rio Grande do Sul, Brazil). All ingredients were mixed with a blender to form a homogeneous product. The final product was packed in polypropylene bottles and stored at 4±1°C for further analysis. The CFM was used for analysis of physicochemical properties and sensory quality in comparison with PFM. Total solids, total dietary fiber, ash, fat, and protein content were determined for PFM on the seventh day of storage (AOAC, 2012). All the analyses were performed in triplicate and were expressed as g/100g of whole matter.
Physicochemical Parameters and Instrumental Analysis
During fermentation and at 1, 7, 14, 21 e 28 days of storage at 4°C, PFM samples were taken to determine pH (pH meter Jenway 3510, Staffordshire, UK) and titratable acidity. Titratable acidity was determined according to standard methods and expressed as g/100 g lactic acid [21]. Firmness, consistency, cohesiveness and viscosity index were evaluated in CFM and PFM samples after 1, 14 and 21 days of storage using a back extrusion cell (A/BE) on a Texture Analyzer TA-XTPlus (Stable Micro Systems, Surrey, UK), as described by Buriti et al. (2014). The analyses were performed in quadruplicate.
Total Phenolic Content and Antioxidant Activity
The total phenolic compounds content in CFM and PFM were determined using the Folin-Ciocalteu method [45]. Aliquots of 0.5 mL of each fermented milk extract were added to 0.5 mL of Folin-Ciocalteu reagent (20%). After homogenization, 0.5 mL of sodium carbonate (7.5%) was added. The reaction mixture was homogenized by vortex (2865 g, 10 s) and incubated at room temperature (30 min). The reading of absorbance was performed in spectrophotometer (Thermo scientific, Evolution 606, USA) at 765 nm. Analytical curve of gallic acid (0.0 to 250 μg/mL, R2=0.999) was used to quantify the compounds. The results were expressed in mg of gallic acid equivalents/mL (mg GAE/mL). In a test tube, protected from light, aliquots of samples (PFM and CFM) were added to 1.5 mL of methanolic DPPH solution (1.1-diphenyl-2-picrylhydrazyl) and stirred by vortex (3000 rpm) for 30 s. After 30 min of standing, the absorbance of the solution was read in a spectrophotometer (Thermo scientific, 606 Evolution, USA) at 517 nm (Bloor, 2001). The scavenging activity was estimated based on the percentage of DPPH radical scavenged as the following equation: Scavenging ability (%) = [(control absorbance – sample absorbance) / (control absorbance)] x 100
Microbial Viability
Populations of S. thermophillus, B. animalis and L. acidophilus in PFM was determined after 1, 7, 14, 21 and 28 days of storage at 4 °C. The samples were serially diluted in sterile peptone water (1g/L) and subsequently plated, in duplicate. S. thermophilus enumeration was performed on M17 agar, containing lactose (Vetec, Duque de Caxias, Brazil; 5g/L), and incubated at 37°C for 48h. Populations of L. acidophilus were enumerated by pour plating 1 mL of adequate dilutions into MRS agar (Oxoid Basingstoke, UK), followed by incubation at 37°C for 72 h. For the selective enumeration of B. animalis, 1mL of adequate dilutions were pour plated in modified DeMan-Rogosa-Sharpe (MRS) agar (Oxoid, Basingstoke, UK), prepared with dicloxacillin (Sigma, St. Louis, US), cysteine hydrochloride (Cromoline, Diadema, Brazil), and lithium chloride (Cinética®, Jandira, Brazil) to reach a concentration of 0.5 mg/L, 0.5 g/L and 1 g/L, respectively, followed by incubation at 37°C for 72h. All bacteria were incubated in anaerobic jars (Anaerobic System Anaerogen, Oxoid, UK), except S. thermophilus, which was incubated under aerobic conditions. The results were expressed as of log colony forming units per gram (CFU/mL).
Resistance to Simulated Gastrointestinal Conditions
PFM samples were collected at 7 days of storage for the evaluation of L. acidophilus and B. animalis survival to gastric and enteric simulated conditions according to the method described by Liserre and Franco (2007), with modifications. Samples were decimally diluted in a sterile 0.85% (w/v) NaCl solution. For the gastric phase simulation, the pH was set at 2.5 with 1 N HCl solution. Pepsin (Sigma-Aldrich, St. Louis, USA) and lipase (Aldrich Chemical Company, Milwaukee, USA) solutions were added to reach final concentrations of 3.0 g/L and 0.9 g/L, respectively. The flasks were incubated at 37°C for 2 h under agitation (150 rpm). Subsequently, enteric conditions were simulated in two phases. In the enteric phase 1, the pH was increased to 5.0 with a sterile alkaline solution (150 mL of 1 N NaOH, 14 g of PO4H2Na.2H20 and distilled water up to one 1 L), and bovine bile and pancreatin (Sigma-Aldrich, St. Louis, USA) were added to reach a concentration of 10 g/L and of 1 g/L, respectively. After 2 h of incubation at the same conditions, the pH was adjusted to 6.5 - 7.0, and the respective bile and pancreatin concentrations were adjusted to 10 g/L and 1 g/L for the second enteric phase, followed by an additional incubation period of 2 h. In order to enumerate the viable L. acidophilus and B. animalis cells, aliquots were taken at the assay baseline (0 h) and after 2, 4 and 6 h and serially diluted in peptone water solution. Adequate dilutions (1 mL) were pour plated in acidified MRS agar, followed by anaerobic incubation at 37°C for 48 h. A survival ratio (SR%) was calculated based on the initial and final populations to estimate the relative resistance of each strain to the simulated TGI conditions. All results are presented as log CFU/mL.
Sensory Analysis
The sensory evaluation of the flavored fermented goat milk was approved by the Federal University of Viçosa Human Ethics Research Committee, Brazil (Process No. 219.644; CAAE: 13380413.8.0000.5153) and was carried out at the Laboratory of Sensory Analysis of UNINTA College. Sensory evaluation was performed with CFM and PFM samples after 7 days of cold storage (4±1°C) through acceptability tests, using the hybrid hedonic scale (1 = disliked extremely, 5=neither liked nor disliked, 9=liked extremely) focusing on attributes of taste, flavor, color, consistency and overall acceptability [38]. The samples were maintained under refrigeration prior the tests and served, monadically, in individual disposable plastic cups (approximately 30 mL) codified with three random digits. The sensory test was carried out with 63 untrained panelists aged 19 – 40 years old recruited among potential consumers of the beverages, and performed in individual booths. Water and unsalted crackers were available during a 1 min rest period between sample sets to refresh the palate. The consumers were also instructed to report the sensory attributes that they liked and disliked most in the samples.
Statistical Analysis
The experimental data were analyzed by SPSS software version 20 (IBM, Armonk, NY) and the results were expressed as mean±Standard Deviation (SD). Values were the average of quadruplicate/triplicate/duplicate experiments. Before analysis, data were checked for the normality, homogeneity of variances and sphericity using the Shapiro-Wilk, Levene’s and Mauchly’s tests, respectively. Differences between trials (CFM and PFM) in a single moment were tested using unpaired t test or Mann-Whitney test. Differences between experimental storage periods were statistically analyzed using repeated measures Analysis of Variance (ANOVA), followed by the post hoc Bonferroni test, taking on p<0.05. When normality was not found, the equivalent non-parametric tests were applied. Differences at p<0.05 were considered to be significant.
Results and Discussion
Composition, Physicochemical and Instrumental Analysis
Probiotic Flavored Fermented goat Milk (PFM) had the following chemical composition: total solids 17.3±0.04 g/100g, protein 2.47±0.02 g/100g, fat 2.64±0.02 g/100g, ash 0.74±0.01 g/100g and total dietary fiber 0.14±0.01 g/100g. Total solids, protein and fat contents of PFM were found to be lower than other study [29], reflecting the higher moisture content in flavored fermented milk due to the addition of grape juice. Changes in these parameters, especially total solids and fat content may affect physical-chemical properties such as viscosity, syneresis and water holding capacity [39].
The texture parameters analyse of the dairy beverages are presented in Table 1. The measured firmness, consistency, cohesiveness and viscosity index of Conventional Fermented Milk (CFM) showed an increase during the 28 days of storage, but the values registered at 14 and 28 days did not differ significantly (p>0.05). Interestingly, these parameters values increased only until day 14 (p<0.05) for PFM, however, did not affect the sensory acceptability scores. PFM presented a lower viscosity index on the first day of storage (p=0.04) compared to CFM, and no significant difference was detected for firmness and consistency (p>0.05) when the two trials were compared at the same sampling period. However, little information exists about the influence of probiotic strains on physicochemical properties of dairy beverages. Fermented milk prepared using only probiotic strains, such as L. acidophilus and Bifidobacterium spp. are often characterized by the undesirable sensory proprieties and texture [40], whereas physical properties such as firmness and ability to retain water are important factors for quality assessment [18]. As we report below, these parameters were not affected in the present study, probable due to the presence of starter culture S. thermophilus in both beverages.
Beverages
Time
(days)Firmness
N x102Consistency
N x 102 sCohesiveness
N x102Viscosity index
N x 102 sCFM
1
12.19±0.18A
241.70±5.96A
8.06±0.16A
14.94±0.87A,a
14
15.99±0.40B
369.52±5.35B
10.75±0.30B
17.59±1.31B
28
16.41±0.77B
369.60±4.32B
11.71±0.59B,a
19.42±1.06B
Overall mean
14.87±2.03
326.60±62.88
10.17±1.64
17.31±2.16
PFM
1
12.06±0.23A
234.86±2.70A
8.01±0.13A
13.26±0.48A,b
14
15.99±0.20B
369.61±2.63B
10.75±0.15B
17.63±0.55B
28
16.15±0.29B
369.81±3.60B
10.95±0.20B,b
18.50±0.75B
Overall mean
14.73±2.10
324.76±66.61
9.90±1.43
16.46±2.43
Values are mean ± SD of four replicate determinations. A-C Different superscript capital letters in a column for a same beverage denote significant differences (p<0.05) between sampling days. a-b Different superscript letters in a column denote significant differences (p<0.05) between trials for a same moment. There was no significant difference between trials for overall mean. CFM: Conventional Fermented Milk; PFM: Probiotic Fermented Milk.
Table 1: Texture analyses of fermented goat milk beverages during 28 days of storage at 4±1 °C.
The use of a combination of starter and probiotic cultures requires an adequate formulation that should guarantee rapid acidification during fermentation and reproducibility of the fermented milks features. The time needed to reach pH 5.0±0.1 during the fermentation was lower for PFM as compared to CFM (2h 30 min vs 5 h; p<0.001). In this way, we obtained a PFM with adequate technological parameters, with time of fermentation lower than others study developed with non-flavored fermented goat milk containing lactobacillus cultures (48 h to reach pH 3.06) [36] or S. thermophilus ST-20Y, L. acidophilus La-5 and B. animalis BB-12 (6 h to reach pH 5.0) [29].
During refrigerated storage, the pH of PFM ranged from 4.10 to 4.19 (p< 0.001), indicating no further acidification, probably due to the presence of three bacteria that show a lower proteolytic activity [43]. Values of pH below 4.0 are generally considered detrimental to the survival of probiotic organisms [47]. Titratable acidity remained stable during the storage period, ranged from 0.82 to 0.85 mg lactic acid g-1. Others studies have been reported small reductions in the pH and increase in the titratable acidity for goat’s milk beverages [12,39].
Total Phenolic Content and Antioxidant Activity of Dairy Beverages
The Total Phenolic (TP) contents and antioxidant activity of PFM were significantly higher (p<0.01) than CFM. The TP of fermented milk was 0.179±0.001 mg GAE/mL in CFM and 0.264±0.01 mg GAE/mL in PFM. The antioxidant activity ranged from 65.17±0.24 for CFM to 83.30±0.68 % for PFM (Figure 1). Studies have shown that selected strains of bifidobacteria and lactobacilli present antioxidants properties and can be used to elaborate fermented dairy beverages that improve total antioxidant status and decrease markers of oxidative stress [10].
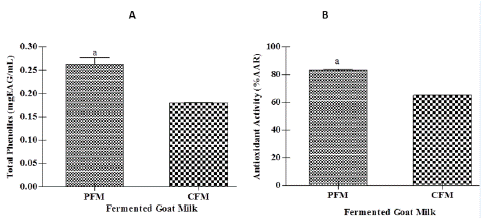
Figure 1: Total phenolic contents (A) and antioxidant activity (B) of Conventional Fermented Milk (CFM) and Probiotic Fermented Milk (PFM). Values are means and errors bars indicate standard deviations (n=3). a p<0.01 from unpaired t test.
A high proportion of polyphenols from the diet are not directly absorbed and the transformation these compounds in the gut depends on microbial esterase and glucosidase activities [20]. In this way, gut bacteria may play a major role in the production of new phenolic compounds “in situ”, which could have better bioavailability and higher biological activity than their parent compounds [41]. Besides, the lactic acid fermentation can increase concentrations of total phenols, flavonoids and anthocyanins in food [24]. This can explain, partially, the higher TP contents and antioxidant activity observed in PFM in this study.
Interestingly, Balakrishnan and Agrawal (2014) reported a higher antioxidant activity in probiotic fermented milk obtained from goat milk (93%) followed by a product from camel milk (86%) and then a product from cow milk (79%), suggesting that probiotic bacteria are able to utilize the nutrients in goat and camel milk more efficiently, in comparison to cow milk. Several studies have highlighted the association between the consumption of foods with high antioxidant activity and the development and progression of chronic low-grade inflammation and metabolic diseases [7,8].
Microbiological Analysis
The S. thermophilus population of the PFM remained stable during the entire period of cold storage, presenting a viability of 8.91 log CFU/mL at day 1 and 9.2 log CFU/mL at day 28. On the other hand, L. acidophilus and B. animalis subsp. lactis populations reduced significantly during the storage (p<0.001), reaching concentrations of 6.10 and 7.98 log CFU/mL at day 28, respectively. L. acidophilus maintained viable cell counts =7 log CFU/mL for 2 weeks, whereas S. thermophilus and B. animalis subsp. lactis maintained this concentration throughout the 28 days. The viability values of bacteria in PFM during the cold storage period are shown in Table 2.
Microorganism (log CFU/mL)
1 day
7 days
14 days
21 days
28 days
S. thermophilus
8.92±0.01
8.39±0.42
8.90±0.02
8.88±0.02
9.20±0.49
L. acidophilus
7.89±0.02A
7.76±0.10A
6.18±0.05B
6.16±0.03B
6.11±0.02B
B. animalis
8.65±0.02A
7.26±0.01B
7.19±0.01C
7.19±0.01C
7.98±0.02D
Values are mean ± SD of three batches, in duplicate, at each sampling day. A-E Different superscript capital letters in a same row denote significant differences (p<0.01) between sampling days.
Table 2: Viability of S. thermophilus TA-40, L. acidophilus La-5 and B. animalis subsp. lactis BB-12 in the Probiotic Fermented Milk (PFM) during 28 days of storage at 4±1°C.
The health benefit of fermented milk containing probiotics depends on the viability of the probiotic microorganisms in the refrigerated product [44]. In spite of having no agreement about the effective dose, many authors have been suggesting a minimum dose between 106 - 109 CFU/day to assure the therapeutic effect [50]. The Codex Alimentarius (2010) states that the minimum viable quantity of probiotic culture should be 106 CFU/mL and 107 CFU/mL for starter culture. In the present study, despite the registered variations in viability during cold storage, probiotic bacteria maintained suitable amounts during the completely studied period. In agreement with other studies, a higher loss in cell viability was observed for L. acidophilus La-5 than for the bifidobacteria strain [39,51]. However, L. acidophilus La-5 was able to maintain the minimum therapeutic level (>106 CFU/mL) up to 4 weeks storage, in contrast to Ranadheera et al. (2012) in flavored goat`s milk yogurts.
The viability of the probiotic strain depends on several factors, being the drop in pH the most important cause of the decrease in the viability of the probiotic culture [31]. However, the pH values registered for PFM in the present study did not affect the viability of L. acidophilus La-5 and B. animalis BB-12, and there was no pH reduction during the storage period. In addition, it has been supposed that mixed cultures of probiotics in fermented milk may result in poor growth and subsequently poor viability in storage compared to pure cultures, most probably due to competition for nutrients [48]. Thus, it seems that B. animalis subsp. lactis has an advantage compared to the L. acidophilus [4.39]. Additionally, other study has reported that food polyphenols are able to selectively modify the growth of susceptible microorganisms. L. acidophilus and B. animals subsp. lactis BB-12 showed an inhibition of growth by the phenolic extracts. On the other hand, L. plantarum, L. casei, and L. bulgaricus strains reached maximal growth in the presence of polyphenol extracts (343 mg/g of total phenolic content), being most appropriate for use in fermented beverages containing high proportion of polyphenols [46].
Probiotic Resistance to Simulated Gastrointestinal Conditions
The survival of a probiotic bacteria during the gastrointestinal transit should be investigated in each food matrix, complementing the study of probiotic viability, since most probiotic effects depends on the viable cells action at intestinal level and the food matrix might exert an important role in probiotic protection or inhibition. Overall, moderate reductions in L. acidophilus and B. animalis subsp. lactis counts were observed throughout exposition to simulated gastrointestinal conditions. In the present study, L. acidophilus and B. animalis populations were within the detection limits throughout the entire assay, in contrast to previous studies [9,11,15].
During the simulated gastric phase, the populations of L. acidophilus and B. animalis subsp. lactis decreased significantly (p<0.001 and p<0.01, respectively). At gastric conditions, populations of L. acidophilus reduced 1.2 log CFU/mL, in average, after 2 h of exposure, whereas B. animalis subsp. lactis populations reduced 1.8 log CFU/mL. Similarly to the present study, Fávaro-Trindade and Grosso (2002) reported a reduction of only 1 log cycle for L. acidophilus La-5 after 2 h of exposure to pH 2. Population reductions varying from 0.1 to 2.5 log cycles was reported for 6 strains of L. acidophilus isolated from commercial probiotic yoghurts, after 90 min in simulated gastric juice containing pepsin at pH 2.0. However, strains of L. acidophilus were more tolerant to the low pH 2.0 than strains of L. paracasei and L. rhamnosus, which rapidly lost viability (Schillinger, Guigas, Heinrich 2005). B. animalis subsp. lactis has also exhibited a higher sensitivity to simulated gastric conditions [4,11,19].
During the simulated enteric phase, the L. acidophilus La-5 survived well, maintaining almost the same population. On the other hand, populations of B. animalis BB-12 reduced significantly (Table 3). It is possible that the presence of bile salts affects the phospholipids and proteins of bacterial cell membranes, and gram-positive bacteria seem to be more susceptible to the deleterious effects of bile than Gram-negative. However, the tolerance to the bile is a strain-dependent characteristic that should not be generalized in terms of species [5]. In the present study, B. animalis BB-12 showed a higher sensitivity to the simulated enteric conditions.
Microorganism
(log CFU/mL)0 h
2 h
4 h
6 h
L. acidophilus
6.95±0.01A,a
5.75±0.08B,a
5.55±0.02B,a
5.25±0.15B,a
B. animalis
8.75±0.20A,b
6.94±0.01B,b
5.68±0.03C,b
5.12±0.02D,a
Values are mean ± SD of three replicate determinations. A-D Different superscript capital letters in a same row denote significant differences (p<0.01) between different sampling periods of the in vitro assay. a-b Different superscript letters in a column denote significant differences (p<0.05) between probiotics for a same moment. pH at 0, 2, 4 and 6h=4.39; 2.46; 5.0 and 6.3, respectively.
Table 3: Survival of L. acidophilus La-5 and B. animalis BB-12 under simulated gastrointestinal conditions in the fermented milk at 7 days of storage at 4±1 °C.
Overall, the minimum populations of probiotic bacteria registered after the in vitro digestion assay remained above 5 log CFU/mL, for both bacteria. However, the survival rate of L. acidophilus (75.71%) was higher than B. animalis (58.55%) considering the entire assay (p<0.01). A different study reported that L. acidophilus La-5 exhibited greater survival rates than B. animalis supsp. lactis BB-12 in the more acidic stirred fruit yogurts, confirming their capacity for acid tolerance [39]. Thus, the microenvironments produced by the food matrix or ingredients on the intestine level may protect the probiotic microorganism from the harsh conditions of the gastrointestinal tract. Moreover, food components could bind to bile acids, reducing their toxic effect on probiotic cells [5].
Interestingly, the observed patterns of survival after exposure to simulated gastrointestinal conditions were in contrast with the trends observed in the viable counts recorded during the cold storage of PFM, since B. animalis subsp lactis showed higher viability than L. acidophilus. However, considering the usual portion of dairy beverages consumed, at least 100 mL, the results obtained in vitro suggests that a relevant number of probiotic viable cells, ensures in the beverages a minimum dose of 106 CFU/mL [14]. Furthermore, the population of B. animalis subsp lactis was higher than L. acidophilus (p<0.01) until 4h at pH 5.0 of assay.
Sensory Evaluation of Dairy Beverages
The results of the sensory evaluation of the dairy beverages are shown in Table 4. Sixty-three panelists (41 females and 22 males; mean age = 24.2±4.40 years old) participated in the study. The average sensory acceptability scores obtained by CFM and PFM for flavor, appearance, texture, color, and overall impression were higher than 6.5 in a 9-point hedonic scale. There was no significant difference between the average sensory acceptability scores attributed to PFM and CFM, although the overall acceptability score of PFM was slightly higher than CFM (7.0 vs 6.6). Among the tested sensory attributes, flavor received the lowest scores for both fermented milks; meanwhile the color was the most appreciated.
Fermented milk
Flavor
Appearance
Texture
Color
Overall acceptability
Conventional
6.76±1.99
6.87±1.46
7.08±1.41
7.43±1.34
6.65±1.79
Probiotic
6.86±1.89
7.14±1.67
7.06±1.50
7.27±1.73
7.00±1.53
Values are mean ± SD. Scores vary between 1 (dislike extremely) and 9 (like extremely). There were no significant difference
Table 4: Sensory evaluation scores of the fermented goat milk.
It has been suggested that probiotic bacteria addition creates sensory advantages in dairy products [17], especially, the L. acidophilus La-5 may produce flavor compounds, such as acetaldehyde, which are recognized as important flavor components. However, in this study, despite the good flavor score, no increase in consumer’s acceptance was observed for PFM compared to CFM, probably reflecting the contribution of the grape juice flavor compounds. Martín-Diana et al. (2003) obtained lower scores for all sensory attributes for a non-flavored fermented goat’s milk containing L. acidophilus La-5 and B. animalis BB-12. The incorporation of natural sugars into the dairy beverages base through addition of fruit juice has been proposed a key factor to achieve higher consumer acceptability for goat’s milk beverages [39,49], besides contributing with nutrients and bioactive compounds not found in milk, particularly, polyphenols.
The general comments by the panelists regarding sensory attributes were also evaluated. The most common criticisms were related to the semi-liquid texture of the beverages and the “goaty” flavor. Ranadheera et al. (2012) related that complaints regarding the characteristic unpleasant ‘‘goaty’’ taste were not recorded for the 10% and 15% stirred fruit yogurts.
Conclusion
The probiotic flavored fermented goat milk evaluated in the present study showed to be an adequate vehicle for the probiotics L. acidophilus La-5 and B. animalis subsp. lactis BB-12 and exhibited a good sensory quality. Furthermore, the antioxidant activity of the probiotic fermented milk was higher than conventional fermented milk. Thus, this study presents relevant information on physicochemical, sensory, microbial and antioxidant properties of a probiotic flavored fermented goat milk, which could be exploited to formulate novel probiotic foods that can exert a role in the prevention of oxidative stress and related diseases.
Author Statements
Acknowledgments
This study was supported by Brazilian Agricultural Research Corporation (EMBRAPA) and the Brazilian funding agencies: Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), and Conselho Nacional de Desenvolvimento Cientifico e Tecnológico (CNPq) for financial support with the research and postdoctorate, researcher’s fellowships. We would like to thank Etianne Lopes (UNINTA) and Tabosa Santos (EMBRAPA) for technical assistance, and Adriano Lima (EMBRAPA) for providing data management support. The authors also thank Embrapa Goats and Sheep for generous support.
Conflict of Interest
The authors declare no conflict of interest.
References
- AC. Official methods of analysis of the AOAC International. Gaithersburg, MD, USA: AOAC International. 2012; 19.
- Balakrishnan G, Agrawal R. Antioxidant activity and fatty acid profile of fermented milk prepared by Pediococcus pentosaceus. Journal of Food Science and Technology. 2014; 51: 4138-4142.
- Bardaweel SK, Gul M, Alzweiri M, Ishaqat A, ALSalamat HA, Bashatwah RM. Reactive oxygen species: The dual role in physiological and pathological conditions of the human body. The Eurasian Journal of Medicine. 2018; 50: 193-201.
- Bedani R, Rossi EA, Isay Saad SM. Impact of inulin and okara on Lactobacillus acidophilus La-5 and Bifidobacterium animalis Bb-12 viability in a fermented soy product and probiotic survival under in vitro simulated gastrointestinal conditions. Food Microbiology. 2013; 34: 382–389.
- Begley M, Gahan CGM, Hill C. The interaction between bacteria and bile. Microbiological Reviews. 2005; 29: 625–651.
- Blois MS. Antioxidant Determinations by the use of a Stable Free Radical. Nature. 1958; 181: 1199–1200.
- Bonaccio M, Pounis G, Cerletti C, Donati M B, Iacoviello L, de Gaetano G. Mediterranean diet, dietary polyphenols and low-grade inflammation: results from the MOLI-SANI study. British Journal of Clinical Pharmacology. 2017; 83: 107-113.
- Bordoni L, Fedeli D, Fiorini D, Gabbianelli R. Extra virgin olive oil and Nigella sativa oil produced in central Italy: A comparison of the nutrigenomic effects of two Mediterranean oils in a low-grade inflammation model. Antioxidants. 2020; 9: 20.
- Botes M, Van Reenen CA, Dicks L MT. Evaluation of Enterococcus mundtii ST4SA and Lactobacillus plantarum 423 as probiotics by using a gastro-intestinal model with infant milk formulations as substrate. International Journal of Food Microbiology. 2008; 128: 362–370.
- Bujna E, Farkas NA, Tran AM, Sao Dam M, Nguyen QD. Lactic acid fermentation of apricot juice by mono-and mixed cultures of probiotic Lactobacillus and Bifidobacterium strains. Food Science and Biotechnology. 2018; 27: 547-554.
- Buriti FCA, Castro IA, Saad SMI. Viability of Lactobacillus acidophilus in synbiotic guava mousses and its survival under in vitro simulated gastrointestinal conditions. International Journal of Food Microbiology 2010; 137: 121–129.
- Buriti FCA, Freitas SC, Egito AS, dos Santos KMO. Effects of tropical fruit pulps and partially hydrolysed galactomannan from Caesalpinia pulcherrima seeds on the dietary fibre content, probiotic viability, texture and sensory features of goat dairy beverages. Food Science and Technology. 2014; 59: 196–203.
- Chavan RF, Sakhale BK. Grape Polyphenolics. Plant Antioxidants and Health. 2020; 1-16.
- Codex Alimentarius. Codex standard for fermented milks. 2nd ed. Codex Alimentarius Commission. Brussels, Belgium: Codex standard 243-2003 in Codex Alimentarius: Milk and Milk Products. 2010.
- Corsetti A, Caldini G, Mastrangelo M, Trotta F, Valmorri S, Cenci G. Raw milk traditional Italian ewe cheeses as a source of Lactobacillus casei strains with acid-bile resistance and antigenotoxic properties. International Journal of Food Microbiology. 2008; 125: 330–335.
- Costa MP, Balthazar CF, Franco RM, Mársico ET, Cruz AG, Conte Junior CA. Changes on expected taste perception of probiotic and conventional yogurts made from goat milk after rapidly repeated exposure. Journal of Dairy Science. 2014; 97: 2610–2618.
- Esmaeilnejad Moghadam B, Keivaninahr F, Fouladi M, Rezaei Mokarram R, Nazemi A. Inulin addition to yoghurt: Prebiotic activity, health effects and sensory properties. International Journal of Dairy Technology. 2019; 72: 183-198.
- El Khaled D, Novas N, Gazquez JA, Garcia RM, Manzano-Agugliaro F. Fruit and Vegetable Quality Assessment via Dielectric Sensing. Sensors. 2015; 15: 15363–15397.
- Fávaro-Trindade CS, Grosso CRF. Microencapsulation of L. acidophilus (La-05) and B. lactis (Bb-12) and evaluation of their survival at the pH values of the stomach and in bile. Journal of Microencapsulation. 2002; 19: 485–494.
- Feng S, Yi J, Li X, Wu X, Zhao Y, Ma Y, Bi J. Systematic Review of Phenolic Compounds in Apple Fruits: Compositions, Distribution, Absorption, Metabolism, and Processing Stability. Journal of Agricultural and Food Chemistry. 2021; 69: 7-27.
- IAL. Métodos físico-químicos para análise de alimentos. Lutz IA, editor. Sao Paulo, Brazil: Instituto Adolfo Lutz. 2008.
- Incalza MA, D’Oria R, Natalicchio A, Perrini S, Laviola L, Giorgino F. Oxidative stress and reactive oxygen species in endothelial dysfunction associated with cardiovascular and metabolic diseases. Vascular pharmacology. 2018; 100: 1-19.
- Khanniri E, Sohrabvandi S, Mortazavian AM, Khorshidian N, Malganji S. Effect of fermentation, cold storage and carbonation on the antioxidant activity of probiotic grape beverage. Current Nutrition & Food Science. 2018; 14: 335-340.
- Kwaw E, Ma Y, Tchabo W, Apaliya MT, Wu M, Sackey AS, Tahir HE. Effect of lactobacillus strains on phenolic profile, color attributes and antioxidant activities of lactic-acid-fermented mulberry juice. Food Chemistry. 2018; 250: 148-154.
- Lehtoranta L, Latvala S, Lehtinen MJ. Role of probiotics in stimulating the immune system in viral respiratory tract infections: A narrative review. Nutrients. 2020; 12: 3163.
- Li SC, Hsu WF, Chang JS, Shih CK. Combination of Lactobacillus acidophilus and Bifidobacterium animalis subsp. lactis shows a stronger anti-inflammatory effect than individual strains in HT-29 cells. Nutrients. 2019; 11: 969.
- Liserre AM, Ré MI, Franco BDGM. Microencapsulation of Bifidobacterium animalis subsp. lactis in Modified Alginate-chitosan Beads and Evaluation of Survival in Simulated Gastrointestinal Conditions. Food Biotechnology. 2007; 21: 1–16.
- Magrone T, Magrone M, Russo MA, Jirillo E. Recent advances on the anti-inflammatory and antioxidant properties of red grape polyphenols: In vitro and in vivo studies. Antioxidants. 2020; 9: 35.
- Martin-Diana AB, Janer C, Peláez C, Requena T. Development of a fermented goat’s milk containing probiotic bacteria. International Dairy Journal. 2003; 13: 827–33.
- Masoumi SJ, Mehrabani D, Saberifiroozi M, Fattahi MR, Moradi F, Najafi M. The effect of yogurt fortified with Lactobacillus acidophilus and Bifidobacterium sp. probiotic in patients with lactose intolerance. Food Science & Nutrition. 2021; 9: 1704-1711.
- Meybodi NM, Mortazavian AM, Arab M, Nematollahi A. Probiotic viability in yoghurt: A review of influential factors. International Dairy Journal. 2020; 109: 104793.
- Min M, Bunt CR, Mason SL, Hussain MA. Non-dairy probiotic food products: An emerging group of functional foods. Critical Reviews in Food Science and Nutrition. 2019; 59: 2626-2641.
- Mituniewicz–Malek A, Zielinska D, Ziarno M. Probiotic monocultures in fermented goat milk beverages–sensory quality of final product. International Journal of Dairy Technology. 2019; 72: 240-247.
- Moreno-Montoro M, Navarro-Alarcón M, Bergillos-Meca T, Giménez-Martínez R, Sánchez-Hernández S, Olalla-Herrera M. Physicochemical, nutritional, and organoleptic characterization of a skimmed goat milk fermented with the probiotic strain lactobacillus plantarum C4. Nutrients. 2018; 10: 633.
- Nogacka AM, Oddi S, Salazar N, Reinheimer JA, Gueimonde M, et al. Intestinal immunomodulation and shifts on the gut microbiota of BALB/c mice promoted by two Bifidobacterium and Lactobacillus strains isolated from human samples. BioMed Research International. 2019; 2019: 2323540.
- Panchal G, Hati S, Darji VB, Prajapati JB. Antioxidant activities, proteolytic activity and growth behavior of Lactobacillus cultures during fermentation of goat milk. Indian Journal of Dairy Science. 2020; 73: 57-66.
- Park YW, Juárez M, Ramos M, Haenlein GFW. Physico-chemical characteristics of goat and sheep milk. Small Ruminant Research. 2007; 68: 88–113.
- Peryam DR, Pilgrim FJ. Hedonic scale method of measuring food preferences. Food Technology. 1957; 11: 9–14.
- Ranadheera CS, Evans CA, Adams MC, Baines SK. Probiotic viability and physico-chemical and sensory properties of plain and stirred fruit yogurts made from goat’s milk. Food Chemistry. 2012; 135: 1411–1418.
- Ranadheera CS, Evans CA, Baines SK, Balthazar CF, Cruz AG, et al. Probiotics in goat milk products: Delivery capacity and ability to improve sensory attributes. Comprehensive Reviews in Food Science and Food Safety. 2019; 18: 867-882.
- Requena T, Monagas M, Pozo-Bayón MA, Martín-álvarez PJ, Bartolomé B, et al. Perspectives of the potential implications of wine polyphenols on human oral and gut microbiota. Trends Food Science and Technology. 2010; 21: 332–344.
- Schillinger U, Guigas C, Heinrich HW. In vitro adherence and other properties of lactobacilli used in probiotic yoghurt-like products. International Dairy Journal. 2005; 15: 1289–1297.
- Shihata A, Shah NP. Proteolytic profiles of yogurt and probiotic bacteria. International Dairy Journal. 2000; 10: 401–408.
- Shori AB. Application of Bifidobacterium spp in beverages and dairy food products: an overview of survival during refrigerated storage. Food Science and Technology. 2021; 42.
- Singleton VL, Orthofer R, Lamuela-Raventos RM. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent. Packer Oxidants and Antioxidants, Part A. L editor. Methods Enzymology. 1999; 299: 152–78.
- Tabasco R, Sánchez-Patán F, Monagas M, Bartolomé B, Victoria Moreno-Arribas M, Peláez C. Effect of grape polyphenols on lactic acid bacteria and bifidobacteria growth: Resistance and metabolism. Food Microbiology. 2011; 28: 1345–1352.
- Terpou A, Papadaki A, Lappa IK, Kachrimanidou V, Bosnea LA, Kopsahelis N. Probiotics in food systems: Significance and emerging strategies towards improved viability and delivery of enhanced beneficial value. Nutrients. 2019; 11: 1591.
- Timmerman HM, Koning CJM, Mulder L, Rombouts FM, Beynen AC. Monostrain, multistrain and multispecies probiotics - A comparison of functionality and efficacy. International Journal of Food Microbiology. 2004; 96: 219–233.
- Tranjan Bár C, Cruz AG, Walter EHM, Faria JAF, Bolini HMA, Moura MRL. Development of goat cheese whey-flavoured beverages. International Journal of Dairy Technology. 2009; 62: 438–443.
- Vasiljevic T, Shah NP. Probiotics - From Metchnikoff to bioactives. International Dairy Journal. 2008; 18: 714–728.
- Vinderola CG, Costa GA, Regenhardt S, Reinheimer JA. Influence of compounds associated with fermented dairy products on the growth of lactic acid starter and probiotic bacteria. International Dairy Journal. 2002; 12: 579–589.