
Research Article
Austin J Biotechnol Bioeng. 2024; 11(1): 1130.
Assessing the Bio-Efficacy of Trichoderma Asperellum (IT13) Against Rhizopus Stolonifer, Fungus Associated with Post-Harvest of Yam (Dioscorea Rotundata) Tubers Rot
Dikongue NJF¹, Ntah A Ayong M¹, Nguemnang MLC², Bedine BMA³, Jiogue ML¹, Kamsu NPF¹, Tchameni NS¹*, Sameza ML¹
1Department of Biochemistry, Laboratory of Biochemistry, Faculty of Science, University of Douala, Cameroon
2Department of Biochemistry, Faculty of Science, Antimicrobial Agents Unit, Laboratory of Phytobiochemistry and Medicinal Plant Study, University of Yaounde I, Cameroon
3Phytopathology and Agricultural Zoology Research Unit, Department of Agriculture, Faculty of Agronomy and Agronomic Sciences, University of Dschang, Cameroon
*Corresponding author: Tchameni NS Department of Biochemistry, Laboratory of Biochemistry, Faculty of Science, University of Douala, PO Box 24 157, Douala, Cameroon. Email: tchameni1@yahoo.fr
Received: March 04, 2024 Accepted: April 09, 2024 Published: April 16, 2024
Abstract
The aim of this findings was to evaluate the effect of Trichoderma asperellum (It-13) as bio-control agent against Rhizopus stolonifer the causative agent of yam tuber rot. The antagonism test was done by dual culture on Potato Dextrose Agar (PDA). Antibiosis was evaluated on PDA medium by the cellophane membrane method. The production of lytic enzymes (chitinase, cellulase, protease, and lipase) by T. asperellum was detected on PDA supplemented with specific substract. The organic extract was obtained by fermentation of T. asperellum on Potato Dextrose Broth. Total phenols and flavonoids were by spectrophotometer while, Volatile Organic Compounds (VOC) were analysed by Gas Chromatography coupled with Mass Spectrometry (GC-MS). The efficiency of the organic extract on Rhizopus stolonifer was evaluated by poisoning method. The ability of the antagonist to protect yam tuber was done before and after infected the tubers. The results showed that, in dual culture, T. asperellum significantly inhibited the growth of the Rhizopus stolonifer at 48.68 % and 96.42% respectively, in the same media and after growth on cellophane membrane. In the specific solid media, T. asperellum produced chitinase (58.7mm), cellulase (53.8mm), protease (70.0mm) and lipase (50.7mm). The organic extract produced by this strain content total phenol (70.0mg/ml) and flavonoid (30.0mg/ml). The GC-MS analysis of VOC reveal the presence of 17 components with 6-β-hydroxyfluoxymesterone (23.32%), 2-[5-chloro-8-hydroxy-3-methyl-1-oxoisochroman-7-carbonyl) amino]-3-phenyl propionate (18.44%) and acid-2-chloro-5-sulfoaniline (12.02%) as major components. At 75μg/μl, the organic extract had total inhibited (100%) the mycelial growth of Rhizopus stolonifer while, at 107conidia/ml, the spore of T. asperellum (It-13) significantly reduced the necrosis of yam tuber at 25% and 100% respectively, before and after infection with R. stolonifer. These works suggested that the use of Trichoderma asperellum could be serve as alternative to bio-control against post-harvest rot of the yam tubers.
Keywords: Trichoderma asperellum; Organic extract; lytic enzymes; Yam tuber rot; Rhizopus stolonifer
Introduction
Yam (Dioscorea spp.), is an annual herbaceous monocotyledon of Dioscoreaceae family. It is the 4th most consumed tuber in the world after sweet potato, potato and cassava [1]. Yam is cultivated on an area of 8.6 million hectares and its world production is estimated at 72 million tonnes per year, with 95% of the production which came from Africa [2]. In Cameroon, its production has estimated around 0.67 million tonnes, ranked 7th among the world and African producers [domesticated and cultivated species include Dioscorea rotundata, Dioscorea cayenensis, Dioscorea alata, Dioscorea esculenta and Dioscorea dumetorum [4,5]. Yam tubers are rich in starch, proteins, minerals, vitamins and fiber. It is a staple food in many subtropical regions of the world including Cameroon where it is regularly consumed in boiled water, roasted, fried, braised and pounded form [6].
Despite the socio-economic and nutritional importance of yam, its production is limited by pest and diseases. Among these, post-harvest tuber rot caused by Rhizospus stolonifer is one of the most devastating diseases [7].
In the absence of any treatment, yield loss could reach 25-50%. To reduce these losses, farmers commonly used chemical pesticides [8]. Despite their effectiveness, their reapeat and used could have a negative impact on human, animal and environmental health[9]. Then, an alternative is the used of biological control agents especially the antagonists of the genus of Trichoderma [10,11]. Many species of Trichoderma are frequently used with succes for plant deseases control. Tchameni et al. [12] showed that Trichoderma asperellum (PR11) is considered potential eco-friendly biocontrol agents against cocoa black pod caused by Phytophthora megakarya. Recently, Mohaled et al. [13] showed that, T. harzianum, T. hamatum, T. asperellum, and T. atroviride are used to control some plant diseases caused by Alternaria alternata, B. sorokiniana, D. halodes and F. proliferatum. Gwa and Ekefan [14] recommended the use of T. harzianum in the management of rot causing pathogens (F. oxysporum f. sp. Melonganae, A. niger and B. theobromae) of yam tubers in storage caused. Mechanisms used by this fungal as biological control agents against plant pathogens include competition for space and nutrient, induced systemic resistance, myco-parasitism, production of antibiotics, and extracellular lytic enzymes [15;16]. In our Laboratory, many species of Trichoderma have been isolated and characterized. Among them, T. asperellum (It-13) had shown the ability to control many plant pathogens [17]. However, there is no information about this antagonist against Rhizopus stolonifer. So, the aim of this work was to assess the bioprotective potential of Trichoderma asperellum (It-13) against Rhizopus stolonifer associated with post-harvest rot of Dioscorea rotundata. It will specifically be to determine the in vitro and in vivo antifungal potential of T. asperellum (It-13) against pathogen responsible for yam rot in stock, determine presence of lytic enzyme, and evaluate the effect of organic extract of T. asperellum (It-13) on the mycelial growth of pathogen.
Materials and Methods
Rhizopus Stolonifer and Trichoderma Asperellum (It-13)
The strain of Trichoderma asperellum (It-13) and the isolate of Rhizopus stolonifer used in this study came from the culture collection of the mycotheque of the Phytobiochemistry Laboratory and Medicinal Plants Studies of University of Yaounde I (Cameroon). Information on the isolation and identification of the both microorganisms are given respectively, by Sameza et al. [18] and Bedine et al. [19]. The strain of Trichoderma asperellum (It-13) had already been characterized by light microscopy and molecular tools. Its nucleotide sequence was compared with NCBI (National Center Biotechnology Information) Genbank data (www.ncbi.nlm.nih.gov/BLAST) and its accession number was JN004173.
Antagonism Assay
Dual culture
The antagonistic potential of T. asperellum was evaluated against R. stolonifer using dual culture. Mycelial discs (6 mm diameter) were taken from 3 days old cultures of the antagonist and the pathogen. The discs were then paired on PDA plate in 90 mm Petri dishes supplemented with ampicillin (250 mg/L) and penicillin (250 mg/L). R. stolonifer was inoculated same time of T. asperellum strain. Control has made by plates inoculated only by the pathogen [19]. All culture plates were incubated at room temperature for 4 days. The inhibition percentage (%I) of R. stolonifer radial growth was done according the formula: % I = ((Do-Dx)/Do)×100; where, Do is the radial growth of pathogen in the control and Dx is the radial growth of pathogen in dual culture [20].
Effect of Non-Volatile Compounds
The eff ect of non-volatile compounds was evaluated according to the method used by Ntah et al. [20]. In this case, each Trichoderma was grown for 24 h on a sterile cellophane disc laying on PDA in 90 mm Petri dish.In the control plate, the antagonist was replaced by an agar disc. The cellophane with the mycelia was then removed and the test pathogen inoculated for an incubation period of 4 days at 27±2°C.
For both assays, each treatment consisted of three PDA plates and the experiments was repeated two time. At the end of incubation, colony diameter of the pathogen was measured and the inhibition percentage of mycelial growth was evaluated by the following formula: %I = ((Do-Dx)/Do)×100, where, Do is diameter growth of pathogen in the control and Dx diameter growth of pathogen in the presence of T. asperellum..
Lytic Enzyme Assay
Hydrolytic activities of chitinase, cellulase, lipase and protease were detected on specifi c solid media. T. asperellum strain was grown on a medium containing the enzyme substrate and the zone of degraded substrate (halos) formed around the colony was measured after 3 days of incubation.
Chitinase activity was performed according to the method described by Agrawal and Kotasthane[21]. The detection medium was prepared as follow: 4.5 g/L of colloidal chitin, 3.0 g/L (NH4)2SO4, 0.3 g/L MgSO4, 2.0 g/L K2HPO4, 1 g/L citric acid monohydrate, 15 g/L agar, 0.15 g/L bromocresol purple, 200 μL of tween 80, pH 4.7 and autoclaved at 121°C for 15 min. Cellulase activity was determined by using plate screening medium containing 1% carboxymethylcellulose (Sigma, USA) [22]. The inoculated plates were incubated at 28°C and thereafter stained with 0.1 % Congo red dye solution for 15 min; the solution was discarded and the culture washed with 1M NaCl for 15 min.
Lipase activity was conducted according to Singh et al. [23] method. Trichoderma asperellum was grown on chromogenic substrate plates (pH7.4) containing for 1 L: phenol red (0.01 %), 2 % agar, 1 % lipidic substrate (olive oil) and 10 mM CaCl2.
Protease activity was evaluated in agar plate containing 2.0 g/L K2HPO4, 10 g/L glucose, 5 g/L peptone, 15 g/L gelatin and 15 g/L agar. After incubation, Petri plates were fl oated with sodium (10%) [24]. For each enzyme activity test, the bioassays were done in 4 replicates and the experiment repeated twice.
Liquid Culture and Extraction of Organic Extract
Four agar plugs from 3-old days culture of T. asperellum were introduced into 1 L Erlenmeyer containing 350 mL of PDB. The flask was incubated at 25±2°C in dark under stationary condition for 30 days [20]. Cultures were then filtered on vacuum using Whatman paper N°4. The obtained filtrate was extracted two times with ethyl acetate as solvent and evaporated under reduced pressure at 35°C. The organic residue obtained was stored at 4°C until use [25].
Total Phenols and Flavonoids Content
The total phenol content of the T. asperellum organic extract was evaluated by applying the Folin-Ciocalteu method [26]. This includes the alkaline reduction of the phosphotungstic and phosphomolybdic mixture of the Folin-ciocalteu reagent by reducing groups of phenolic compounds leading to the formation of blue-coloured products. The latter have a maximum absorption at 760 nm whose intensity is proportional to the amount of phenols present in the sample. In fact, at 125 μL of T. asperellum organic extract were added 500 μL of distilled water and then 125 μL of Folin-ciocalteu reactive. Six minutes later, 1.25 mL of 7 % sodium carbonate (Na2CO3) aqueous solution was added to the reaction medium and then the mixture fitted to 3 mL with distilled water. After 60 minutes of incubation at room temperature and darkness, the absorption was measured at 760 nm at the spectrophotometer against the control tube without extract. The quantification of phenolic compounds was made according to a linear calibration curve made using gallic acid and expressed in equivalent milligrams of gallic acid per 1000 mL of culture filter.
Evaluation of flavonoids content was carried out using the colour method of aluminium trichloride [27]. Indeed, in the presence of aluminium trichloride, flavonoids form yellow complexes. The latter have a maximum absorption at 430 nm whose intensity is proportional to the amount of flavonoids present in the sample. In practice, sample was prepared at the concentration of 1 mg/mL in distilled water. A 1 mL of sample was added 1 mL of Aluminium Trichloride Solution (AlCl3) to 2% prepared in methanol. Ten minutes later, the absorption was read at 430 nm. A standard range was established separately with quercetin to calculate the concentration of flavonoids in fungal extract. The results of the dosage were expressed in milligrams of quercetin equivalent per 1000 mL of culture filter (mgEQ/1000 mL).
GC-MS Assay of Volatile Organic Extract
To identify the volatile compounds contained in the organic extract of T. asperellum, Gas Chromatography-Mass Spectrometry (GC-MS) was used [19]. Briefly, the organic extract (1 mg/mL)was analyzed by an HP 6890 chromatogram equipped with a hair column model HP-5MS (5% phenylmethylsiloxane). The oven temperature of the GC was programmed as follows; 80°C for 2 min, followed by a first rise at 10°C/min to 180°C (stabilizing for 2 min) and a second rise at 5°C/min to 250°C (stabilizing for 15 min). The injector was programmed at a fixed temperature of 250°C and the carrier gas was helium. Ten microliters (10 μL) of extract were injected. Compounds were identified by coupling gas chromatography with HP5973 electron impact mass spectrometry. This was achieved by comparing retention times and spectral masses with those of the Wiley 275 and NIST08.L databases.
Antifungal Assay of Organic Extract
Antifungal activity of organic crude of T. asperellum was evaluated in the Petri dishes containing 10 mL of PDA on which on a mycelial disc (6 mm) of the pathogen was deposited. The extract solution was prepared in DMSO (10 %) at concentrations of 25; 50; 75 and 100 μg/μL. Then 10 μL of each solution taken separately was delicately deposited on top of the mycelial disc as described by Tchameni et al. [19]. For the control treatment, the extract has been replaced by DMSO 10 %. The dishes thus treated were left open in a laminar flow hood to allow the solvent to evaporate and then incubated at ambient laboratory temperature for 4 days. The mycelial growth of the pathogen was measured and the percentage of inhibition calculated by the following formula: %I = ((Dt-De)/De)×100, where, Dt, pathogen growth diameter on control and De pathogen growth diameter on treated plate. For each treatment, three replicates were used and the experiment repeated two times.
In Vivo Assay
Preparation of conidial suspension of T. asperellum
Conidial suspension of biocontrol agent was obtained by liquid culture multiplication. Four agar plugs from 3 days pre-culture of T. asperellum strain were introduced into 1 L Erlenmeyer containing 350 mL of potatoes dextrose broth (PDB). The flask was incubated at 25±2°C in the dark under stationary condition for 15 days. After fermentation, the culture was ground using a robot mixer. The resulting solution was evaluated with a Malassez Cellular and adjusted at 107, 106 and 105conidia/mL [20].
Preventive and Curative Test
It was carried out on yam rings about 90 mm in diameter and 40 mm thick, obtained from whole tubers apparently healthy and disinfected. These washers were sprayed with 20 mL of each spore concentration of T. asperellum prepared. After 48 hours, a mycelial disc (Ø=06 mm) of the pathogen taken from the margin of a 3-days culture was placed on one of the faces of the washer and this, in a well (10 x 10 mm) previously dug using the cookie cutter. Regarding the curative test, the mycelial disc of the pathogen was deposited on the tubers as before, 48 hours before treatment with the spore solution of T. asperellum.
In both cases the negative control tubers were treated with PDB. No solution and no pathogen were applied to the neutral control tubers. The tests were carried out in triplicate and the tubers incubated in ambient temperature of Laboratory. After 14 days of incubation, the tubers were split and the diameters of the necrotic areas were measured using a graduated ruler. In vivo bioprotective activity of the spore solution of T-. asperellum at different concentrations was evaluated by calculating the percent inhibition of necrosis according to the formula of . I% = ((Dt-De)/Dt)×100where I%: percentage inhibition of necrosis; De: radial distance from necrosis of the test tuber; Dt: radial distance of necrosis of the negative control [18]
Statistical Analysis
Data were entered into an Excel spreadsheet (Microsoft Office, USA, 2013) and subsequently analysed using STATIGRAPHICS Centurion version 17.1.12. Quantitative and qualitative data were presented as mean ± standard deviation (SD) and percent, respectively. The data analysis of the variance (ANOVA) one factor was used to compare the averages. Each treatment was compared by using LSD test at P=0.05 significance level.
Results
In vitro Antagonism of Trichoderma Asperellum
Trichoderma asperellum (It-13) was tested for its ability to inhibit the mycelial growth of R. stolonifer in vitro by dual culture. On control plates, colony of R. stolonifer covered the 9 cm PDA plate in 2 days (Figure 1). In paired cultures, the radial extension of this pathogen stopped abruptly few millimeter away from the colonies of T. asperellum strain, while the latter continued their normal growth all over the culture. The inhibition percentage of the mycelia growth of the pathogen was 48.68%. With increasing incubation days, the antagonist overgrew the colony of R. stolonifer (Figure 1).
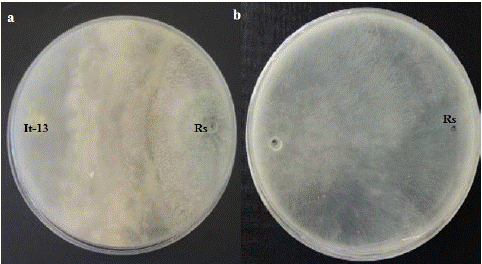
Figure 1: Dual culture test of T. asperellum against R. stolonifer on PDA plate after 4 days. (Rs: R. stolonifer; It-13: T. asperellum. a: dual culture test; b: Control plate).
Eff ect of Non-Volatiles Compounds of T. Asperellum against R. Stolonifer
Trichoderma asperellum (It-13) was tested in vitro for the production of non-volatiles bioactive compounds by cellophane membrane method. The results (Figure 2) indicated that, the non-volatile compounds released by T. asperellum (It-13) have signifi cantly (P=0.05) inhibited the mycelial growth of R. stolonifer. The inhibition rate was 96.42 %.
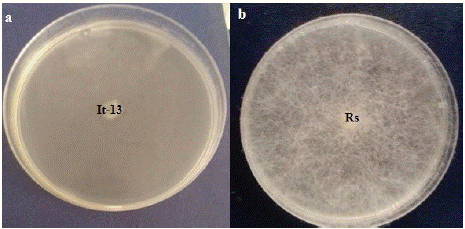
Figure 2: Effect of non-volatiles compounds of Trichoderma asperellum (It-13) against R. stolonifer (Rs: R. stolonifer; It-13: T. asperellum. a: Non volatile compound test; b: Control plate).
Production of Lytic Enzymes
The production of different lytic enzymes by T. asperellum was manifested by the formation of specific colouring halos around the colonies. The highest enzymes released was 70.0 mm of protease following by 58.7mm of chitinase, 53.8mm of cellulase and 50.7mm of lipase.
Total Phenols and Flavonoids
Total phenols and flavonoids content from organic extract of T. asperellum was performed by spectrophotometry method. The results show that, among of total phenol was 77.0 mg/ml while, the total flavonoid was 30.0mg/ml.
GC-MS Analysis
The volatile organic extract of T. asperellum was performed using GC-MS. Seventeen (17) compounds representing 97.07% of total components were identified (Table 1). The major componenents were 6-β-hydroxyfluoxymesterone (23.32 %), 2-[5-chloro-8-hydroxy-3-methyl-1-oxoisochroman-7-carbonyl) amino]-3-phenyl propionate (18.44 %) and acid-2-chloro-5-sulfoaniline (12.02 %) (Table 1).
N°
RT (min)
MW (g.mol-1)
Relative Percentage
Components
1
8.52
279.3
3.2
(4Z)-4-(2-methoxybenzyliden)-2-phenyl-1.3-oxazol-5-(4H)-on
2
10.53
522.9
0.54
2-octadec-1-enoxyethyltetradecanoate
3
10.92
417.8
18.44
2-[(5-chloro-8-hydroxy-3-methyl-1-oxoisochroman-7-carbonyl)amino]-3-phenylpropionate
4
12.14
208.3
12.02
2-chloro-5-sulfoanilin acid
5
18.04
166.1
3.5
Phthalic acid
6
29.70
283.3
3.2
2-bromo-1.3.5-triisopropylbenzen
7
31.09
326.4
2.6
Gliotoxin
8
32.55
488.6
1.65
11.16.22-triacetoxyandrost-4-ene-3.20-dion
9
34.25
451.1
9.45
Hirsutellon B
10
35.44
430.7
2.93
Cholesta-7.22-dien-3b.5a.6b-triol
11
37.02
352.4
23.32
6β-hydroxyfluoxymesteron
12
37.83
436.6
1.59
Ethyl iso-allocholate
13
38.00
262.4
4.20
2,6-bis(1,1-dimethylethyl)-4-(1-oxopropyl)phenol
14
38.79
3.59
17-ethylenedioxy-5.19-cycloandrost-6-en-3-on
15
40.44
/
0.20
Unknown
16
41.14
426.7
1.43
15.17.19-nonacosatriynoic acid
17
44.12
5.21
1.3-dioxolane-2-(1-hydroxyethyl)-methylate
Total
97.07
Table 1: Volatile organic compounds identified in T. asperellum organic extract by GC-MS.
Eff ect of Organic Extract of T. Asperellum on Mycelial Growth of R. Stolonifer
The organic extract of T. asperellum significantly (P=0.05) inhibited the radial growth of R. stolonifer (Figure 2). The inhibition percentage increased with the extract concentration. The total inhibition (100 %) of mycelial growth of R. stolonifer occurred at 75 μg/μL. A significant correlation was observed between inhibitory effect of organic extract and the production of total phenols (p=0.001; r=0.87= and flavonoids (p=0.001; r=0.85).
Inhibitions of Necrosis of Yam Tuber rot by T. Asperellum
Postharvest inhibition of yam tuber rot due to R. stolonifer was evaluated. Results showed that the inhibition of tuber rot increased signifificantly (P=0.05) when using conidia of T. asperellum for both preventive and curative tests. The reduction of necrosis increased with the concentration of conidia. At 107 conidia/ml, the reduction was 25 % for curative test while, for preventive test, total inhibition (100%) of necrosis were obtained (Figure 3).
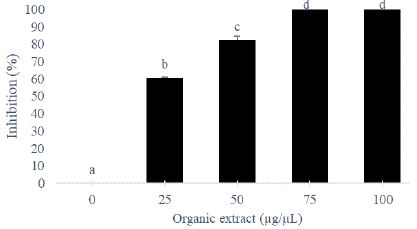
Figure 3: Mycelial growth inhibition of R. stolonifer by organic extract of T. asperelum (Histograms with the same letter represent the mean ± standard deviation of percentage inhibition. Each treatment was made in triplicate, the LSD test was used and the significance level was p = 0.05).
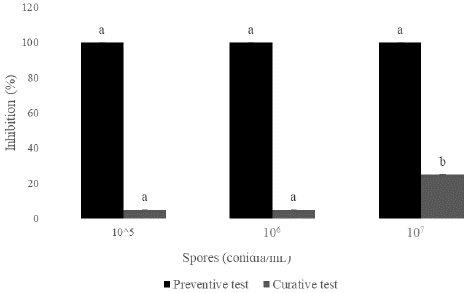
Figure 4: Inhibition of rot on yam rings by T. asperellum (It-13) in preventive and curative test. (Histograms with the same letter represent the mean ± standard deviation of percentage inhibition. Each treatment was made in triplicate, the LSD test was used and the significance level was p = 0.05).
Discussion
In this study, in vitro evaluation of the potential antagonistic of T. asperellum (IT-13) has demonstrated its effectiveness to different degrees. The inhibition observed during pair culture could be explained by the fact that T. asperellum can produce antimicrobial compounds and hydrolytic enzymes such as chitinases, cellulases, proteases and lipases to lyse the walls of pathogens [28,29]. Enzymes produced by Trichoderma are involved in several biological processes such as autolysis, morphogenesis and nutrition. They play a role in the relationships between organisms including: plant-fungi, insects-fungi, and fungi-fungi [30]. Mushrooms of the genus Trichoderma are known for their invasiveness which makes them good competitors for space and nutrients [31,32]. Diffusible substances (non-volatile compounds) reduced the mycelial growth of the pathogen. This activity could be due to the quantity and nature of the compounds secreted by the antagonist. Syed Ab Rahman et al. [33] have proved the ability of Trichoderma strains to inhibit the mycelial growth of telluric pathogens by the release of substances with antimicrobial proporties. Evaluation of the effect of the organic extract on mycelial growth made it possible to demonstrate the ability to reduce the mycelial growth of the pathogen in vitro. This result obtained could be due to the presence in the extract of this antagonist the antimicrobial metabolites (phenols, flavonoids) who they can establish interactions with the cell membrane and lead to impaired membrane permeability and loss of homeostasis, destruction of the wall followed by death of the pathogen [34]. The groups of compounds (17) identified by GC-MS for T. asperellum: hirsutellon B (9.45%), phthalic acid (3.5%), gliotoxin (2.6%), 11.16.22-triacetoxy androst-4-en-3.20-dion (1.65%) and ethyl iso-allocholate (1.59%) are well known to the compound’s characteristic with mushrooms of the genus Trichoderma, associated with their specific molecular footprint [35]. This result is different of Nitish and Kumar [36] having detected 43 compounds in the T.asperellum strain including many important volatile secondary metabolites such as 1.2-benzenedicarboxylic acid, 2-butoxy-2-oxoethyl butyl ester (3.59%); 1.2-benzenedicarboxylic acid dibutyl ester (2.02%); Phthalic acid, 5-methylhex-2-yl butyl ester (0.19%). This variance could be explained by the culture conditions involving, among other things, light, aeration, pH and temperature. In addition, the identification of volatile organic compounds depends on the extraction solvent used and the capillary column used when performing the GC-MS.
Inoculation of Trichoderma spores on yam rings have been shown to reduce rot. This reduction varied depending on the nature of the test. The results showed total inhibition (100%) caused by Rhizopus stolonifer for the preventive test, and variable inhibitions in the curative test. The reducing effect for preventive test would result from the germination of the Trichoderma spores on the surface of the tuber which would probably inhibit or hinder the germination of the spores of the pathogen [37]. This germination capacity of Trichoderma spores on the tuber could strengthen the mechanisms of resistance to the penetration of the parasite. The results of the inetraction between the antagonist and the rot fungus on healthy yam tuber revealed that, T. asperellum was able to significant inhibite the growth of R. stolonifer and reduce the yam rot. T. asperellum could be acted by the production of non volatile and volatile antibiotics and lytic enzymes that inhibited the growth of the patogen. These substances produced by T. asperellum (It-13) may be responsible in the biocontrol of postharvest yam tuber rot. These results are simillar to many previous studies which showed that, members of the genus of Trichoderma were exploited in the control of rot fungi of tubers, fruits and vegetable diseases [38]. Accordindly, Gwa and Ekefan [41] used T. harzianum to control postharvest yam tuber rot for up to 5 months.
In this study, the main mechanism of action of T. asperellum (It-13) against R. stolonifer could be nutrient competition and antibiosis. In fact, T. asperellum grew rapidly on the culture medium to the detriment of R. stolonifer and produced toxic metabolites like hirsutellon B, phthalic acid and gliotoxin. These metabolites may have contribuated to the inhibition of mycelial growth of the pathogen during in vitro and in vivo trials. Trichoderma spp could released toxic metabolites such as harzianic acid, alamethicin, tricholin, antibiotics and viridian which prevent infection [25]. The metabolites produced by Trichoderma contained various secondary metabolites like peptailbols, which may also act as elicitors of plant defence mechanisms against pathogens [17]. The actions of T. asperellum may be also due to possible role of lytic enzymes such chitinase, cellulase, lipase and protease which could break down the polysaccharides, lipids and proteines that are responsible for the rigidity of fungal cell walls, thereby destroying the cell wall and limiting the growth of the pathogen [39,40]. According to the results of this work, the application of T. asperellum spores on the yams tuber may protect them from the pathogens responsible for postharvest losses.
Conclusion
The present findings has shown that T. asperellum (It-13) significantly inhibited mycelial growth of R. stolonifer. Futhermore, the antagonist could reduced the occurence of yam tuber rot. Results also demonstrated the potential of T. asperellum to be further exploited to develope natural biocontrol agent for yam preservation. In the future, formulation and mass production of a biofungicide for large scale use is needed.
Author Statements
Acknowledgement
The authors are grateful the Laboratory of Biochemistry, Faculty of Science, University of Douala, for providing research facilities
Funding
No funding was received for conducting this study.
Ethics Approval and Consent
Ethical approval This article does not contain any studies involving human or animals participants performed by any of the authors.
Conflict of Interest
No conflicts of interest were declared by the authors.
References
- Padhan B, Panda D. Potential of Neglected and Underutilized Yams (Dioscorea spp.) for Improving Nutritional Security and Health Benefits. Front Pharmacol. 2020; 11: 496.
- Azeteh NI, Hanna R, Sakwe NP, Njukeng PA, Kumar LP. Yam (Dioscorea spp.) production trends in Cameroon: A review. Afri J Agri Res. 2017; 14: 1097–110.
- FAO. Food and Agriculture Organization of the United Nations. 2020.
- Mignouna HD, Dansi A, Zok S. Morphological and isozymic diversity of the cultivated yams (Dioscorea cayenensis/ Dioscorea rotundata complex) of Cameroon. Genetic Resource and Crop Evolution. 2020; 49: 21–9.
- Dumont R, Zoundjihekpon J, Vernier P. Origine et diversité des ignames Dioscorea rotundata Poir. Cahiers Agri. 2010; 19: 61–255.
- Maziya-Dixon M, Oladeji AE, Faustina DWM, Asiedu R. Retention of iron and zinc in yam flour and boiled yam processed from white yam (D. rotundata) varieties. Food Sci Nutrition. 2017; 5: 662–8.
- Ogunleye AO, Ayansola OT. Studies of Some Isolated Rot-Causing Mycoflora of Yams (Dioscorea spp. Am J Microbiol Biotechnol. 2014; 1: 9–20.
- Möhring N, Ingold K, Kudsk P, Martin-Laurent F, Niggli U, Siegrist M, et al. Pathways for Advancing Pesticide Policies. Natural Food. 2020; 1: 535–40.
- Jepson PC, Murray K, Bach O, Bonilla AM, Neumeister L. “Selection of Pesticides to Reduce Human and Environmental Health Risks: A Global Guideline and Minimum Pesticides List.” Lancet Planet Health. 2020; 4: 56–63.
- Poveda J. Trichoderma as biocontrol agent against pests: new uses for a mycoparasite. Biol Cont. 2021; 159: 104634.
- Admasu W, Sintayehu A, Gezahgne A. Terefework Z In vitro bioefficacy of Trichoderma species against two Botryosphaeriaceae fungi causing Eucalyptus stem canker disease in Ethiopia. J Nat Pes Res. 2023; 4: 100037.
- Tchameni NS, Sameza ML, O’donovan A, Fokom R, Ngonkeu MEL, Nana WL, et al. Antagonism of Trichoderma asperellum against Phytophthora megakarya and its potential to promote cacao growth and induce biochemical defense. Mycology. 2017; 8: 84–92.
- Mohamed Y, Mostafa AA, Al-Askar A. In vitro antagonistic activity of Trichoderma spp against fungal pathogens causing black point disease of wheat. Journal of Taibah University of Science. 2022; 16: 57–65.
- Gwa VI, Ekefan E. Potential for biological control of postharvest fungal rot of white yam (Dioscorea rotundata Poir) tubers in Storage with Trichoderma harzianum. Virol Mycol. 2021; 10: 210.
- Blaszczyk L, Siwulski M, Sobieralski K, Lisiecka J, Marek JM. Trichoderma spp. – application and prospects for use in organic farming and industry. J Plant Prot Res. 2021; 54: 309–17.
- Meena M, Swapnil P, Zehra A, Manish Kumar Dubey MK, Upadhyay RS. Antagonistic assessment of Trichoderma spp. by producing volatile and nonvolatile compounds against different fungal pathogens. Arch Phytopathol Plant Prot. 2017; 50: 629–41.
- Bedine BMA, Sameza ML, Iacomi B, Tchameni NS, Fekam BF. Screening, identification and evaluation of Trichoderma spp. for biocontrol potential of common bean damping-off pathogens. Biocontrol Sci Technol. 2020; 30: 228–42.
- Sameza ML, Mabou L, Tchameni NS, Bedine BMA, Tchoumbougnang F, Jazet P, et al. Evaluation of clove essential oil as a mycobiocide against Rhizopus stolonifer and Fusarium solani, tuber rot causing fungi in yam (Dioscorea rotundata Poir. J Phytopathol. 2016; 164: 1–8.
- Tchameni NS, Cotârlet M, Ghinea OI, Bedine BMA, Sameza ML, Borda G, et al. Involvement of lytic enzymes and secondary metabolites produced by Trichoderma spp. in the biological control of Pythium myriotylum. Int Microbiol. 2020; 23: 179–88.
- Ntah A, Tchameni NS, Siebatcheu C, Ambata AT, Sameza ML, Wansi JD. Efficacy of Trichoderma harzianum (Edtm) and Trichoderma aureoviride (T4) as potential bio-control agent of taro leaf blight caused by Phytophthora colocasiae. Int J Appl Microbiol Res. 2018; 6: 115–26.
- Agrawal T, Kotasthane A. Chitinolytic assay of indigenous Trichoderma isolates collected from different geographical locations of Chattisgarh in central India. 2012; 1: 73.
- Ferreira FV, Musumeci MA. Trichoderma as biological control agent: Scope and prospects to improve efficacy. World J Microbiol Biotechnol. 2021; 37: 90.
- Singh R, Gupta N, Goswami V, Gupta R. A simple activity staining protocol for lipases and esterases. Appl Microbiol Biotechnol. 2006; 70: 679–82.
- Berg G, Krechel A, Ditz M, Sikora R, Ulrich A, Hallmann J. Endophytic and ectophytic potato-associated bacterial communities differ in structure and antagonistic function against plant pathogenic fungi. FEMS Microbiol Ecol. 2005; 51: 215–29.
- Siebatcheu EC, Wetadieu D, Youassi YO, Bedine Boat AM, Kibrom GB, Tchameni NS, et al. Secondary metabolites from an endophytic fungus Trichoderma erinaceum with antimicrobial activity towards Pythium ultimum. Nat Prod Res. 2023; 4: 657–652.
- Singleton V, Arossi J. Colorimetry of total phenolics with phosphomolypdic-phosphotungstic acid reagents. Am J Technol Viticulture. 1965; 16: 144–53.
- Chang C, Yang M, Wen H, Chern J. Estimation of total flavonoid content in propolis by two complementary colorimetric methods. J food Drug Analysis. 2002; 10: 178–82.
- Wonglom P, Daengsuwan W, Ito SI, Sunpapa A. Biological control of Sclerotium fruit rot of snake fruit and stem rot of lettuce by Trichoderma sp. T76-12/2 and the mechanisms involved. Physiol Mol Plant Pathol. 2019; 107: 1–7.
- Loc NH, Huy ND, Quang HT, Lan TT, Thu Ha TT. Characterisation and antifungal activity of extracellular chitinase from a biocontrol fungus, Trichoderma asperellum PQ34. Mycology. 2020; 11: 38–48.
- Win TT, Bo BO, Malec P, Khan S, Fu P. Newly isolated strain of Trichoderma asperellum from disease suppressive soil is a potential bio control agent to suppress Fusarium soil borne fungal phytopathogens. J Plant Pathol. 2020; 03: 549–61.
- Gugliuzzo A, Aiello D, Biondi A, Giurdanella G, Siscaro G, Zappalà L, et al. Microbial mutualism suppression by Trichoderma and Bacillus species for controlling the invasive ambrosia beetle Xylosandrus compactus. Biol Cont. 2020; 170: 104929.
- Poveda J, Baptista P. Filamentous fungi as biocontrol agents in olive (Olea europaea L.) diseases: Mycorrhizal and endophytic fungi. Crop Prot. 2021; 146: 105672.
- Syed AB, Rahman SF, Singh E, Pieterse CMJ, Schenk PM. Emerging microbial biocontrol strategies for plant pathogens. Plant Sci. 2018; 267: 102–11.
- Ferreira FV, Musumeci MA. Trichoderma as biological control agent: Scope and prospects to improve efficacy. World J Microbiol Biotechnol. 2021; 37: 90.
- Mutawila C, Vinale F, Halleen F, Lorito M, Mostert L. Isolation, production and in vitro effects of the major secondary metabolite produced by Trichoderma species used for the control of grapevine trunk diseases. Plant Pathol. 2016; 65: 104–13.
- Nitish R, Kumar J. Characterization of volatile secondary metabolites from Trichoderma asperellum. J Appl Nat Sci. 2017; 9: 954–9.
- Moya P, Girotti T Jr, Av S, M.n. Antifungal activity of Trichoderma VOCs against Pyrenophora teres, the causal agent of barley net blotch. J Plant Prot Res. 2018; 58: 45–53.
- Okigbo RN, Ikediugwu FEO. Studies on Biological Control of Post Harvest Rot in Yams (Dioscorea rotundata) using Trichoderrma viride. J Phytopathol. 2000; 148: 351–5.
- Okigbo RN, Emeka AN. Biological control of rot-inducing fungi of water yam (Dioscorea alata) with Trichoderma harzianum, Pseudomonas syringe and Pseudomonas chlororaphis. J Stored Prod Res. 2010; 1: 18–23.
- Swehla A, Pandey AK, Nair RM. Bioactivity of Trichoderma harzianum isolates against the fungal root rot pathogens with special reference to Macrophomina phaseolina causing dry root rot of mungbean. Indian Phytopathol. 2020; 73: 787–92.
- Gwa E VI, E. Potential for biological control of postharvest fungal rot of white yam (Dioscorea rotundata Poir) tubers in Storage with Trichoderma harzianum. Virol Mycol. 2021; 10: 210.