
Review Article
Austin J Biotechnol Bioeng. 2024; 11(1): 1131.
From Bronchial Asthma to Tuberculosis: Unravelling the Cytokine-Macrophage Axis in Diverse Diseases
Samim Ali1; Satyawart Sangwan2; Priyam Srivastava3; Shahzeb Khan4; Vikas Raghuvanshi5; Amarjeet Kumar7; Ankur Nawani6; Vishal Chandra3; Pramod Yadav3,6,7*
1ICMR- National Institute of Cancer Prevention and Research, Noida, Uttar Pradesh, India
2Kalpana Chawla Government Medical College, Karnal, Haryana, India
3School of Life Sciences and Biotechnology, Chhatrapati Shahu Ji Maharaj University Kanpur, Uttar Pradesh, India
4Department of Biotechnology, Himachal Pradesh University, Shimla, India
5School of Biotechnology, Madurai Kamaraj University, Tamil Nadu, India
6Department of AFAF, Amity University Uttar Pradesh, India
7Amity Institute of Neuropsychology and Neurosciences, Amity University Uttar Pradesh, India
*Corresponding author: Pramod Yadav Amity Institute of Neuropsychology and Neurosciences, Amity University Uttar Pradesh, Noida Campus, 201313, India. Email: pramodyadavk3@gmail.com, pyadav@amtiy.edu
Received: April 06, 2024 Accepted: May 02, 2024 Published: May 09, 2024
Abstract
Cytokines, tiny proteins secreted by cells, play a critical role in mediating communication and interactions between cells. They also function as immunomodulating agents, adjusting immune responses. When released into the bloodstream or tissues, cytokines bind to specific receptors on target immune cells, triggering cellular responses. Cytokines are implicated in various diseases, including asthma, COPD, HIV infection, and multiple sclerosis. This review delves into the intricate interplay between cytokines and macrophages, focusing on their roles in inflammation and the immune response. Macrophages, which are scavenger cells of the immune system, exhibit remarkable heterogeneity, reside in diverse tissues, and play crucial roles in both innate and acquired immunity. They can be activated for proinflammatory or anti-inflammatory functions, contributing to tissue destruction or regeneration. Cytokines influence macrophage activation and polarization, impacting the inflammatory process. Dysregulated cytokine production and macrophage activation are observed in various diseases. For example, in asthma, an imbalance in macrophage phenotypes contributes to airway hyperresponsiveness. Understanding the complex interplay between cytokines and macrophages is crucial for developing novel therapeutic strategies for inflammatory diseases. Future research directions include utilizing humanized animal models, single-cell sequencing, and immunomodulatory therapies to further decipher the intricate roles of cytokines and macrophages in health and disease. Ultimately, elucidating these interactions holds immense potential for improving human health outcomes.
Keywords: Chronic obstructive pulmonary disease (COPD); Atherosclerosis; Cardiovascular disease; Macrophage polarization; Multiple sclerosis; Immune response
Introduction
Cytokines, proteins secreted by cells, play pivotal roles in mediating intercellular communication and interactions. These proteins are also referred to as “lymphokines,” “monokines,” “chemokines,” and “interleukins” (when produced by one leukocyte and acting on other leukocytes). The term ‘cytokine’ originates from the Greek words ‘cyto’ (cell) and ‘kinos’ (movement) [23]. Recently, cytokines have been referred to as ‘immunomodulatory agents’ due to their ability to adjust immune responses. Cytokines are released into the bloodstream or directly into tissues, where they bind to their target immune cell receptors, triggering specific cellular responses. Inflammatory Pathogen-Associated Molecular Patterns (PAMPs), such as Heat Shock Proteins (HSPs), Peptidoglycans (PGNs), and lipopolysaccharides (LPS), as well as DAMPs, such as HMGB1 and adenosine triphosphate, are key players in this process [23]. These molecules, originally intracellular proteins or nucleic acids are not typically found outside the cell. Pattern Recognition Receptors (PRRs) recognize PAMPs and DAMPs. The primary PRRs involved in inflammatory pathways are TLR, NLR, and MBL [23]. Upon engagement, PRRs transmit signals within the cell, for instance, through MAP kinase signaling pathways to the nucleus, where various transcription factors are activated. Additionally, two polypeptide chains are present: a cytokine-specific a subunit and a signal-transducing β subunit. Upon binding to these receptors, cytokines can induce cellular and humoral immune responses and inflammatory responses, regulate hematopoiesis, control cell proliferation and differentiation, and promote wound healing [29]. In the context of bronchial asthma, research has indicated that cytokine production by T cells, rather than the eosinophil concentration or IgE synthesis, is typically associated with altered airway behaviour [23]. An increase in the number of CD4+ Th cells of the Th2 subtype has been observed in the airways. In Chronic Obstructive Pulmonary Disease (COPD), there is increased expression of IL-4 in Bronchoalveolar Lavage (BAL) fluid from patients, which is pivotal in the immune response and influences the differentiation of Th0 cells into Th2 cells, which could trigger allergen sensitivity [25]. IFN-γ, a key cytokine in patient inflammation, facilitates the lung infiltration of Th1 and Tc cells by enhancing the expression of the chemokine receptor CXCR3 on these cells and promoting the secretion of CXCR3-activating chemokines such as CCL9, CCL10, and CCL11 [18]. In the context of HIV infection, cytokines play a vital role in maintaining immune system homeostasis.
There was a marked decrease in the release of Th1 cytokines, including IL-2 and IFN-γ, concurrent with an increase in the production of Th2 cytokines (IL-4 and IL-10) and proinflammatory cytokines (IL-1, IL-6, IL-8, and TNF-a) during HIV infection [18]. Moreover, cytokines such as TNF-a, TNF-β, IL-1, and IL-6 have been found to stimulate HIV replication in lymphocytes and Monocyte-Derived Macrophages (MDMs). In the case of Multiple Sclerosis (MS), neurodegeneration is a direct result of demyelination, leading to the formation of plaques in the white matter, a hallmark pathology of the disease. The cytokines IL-6 and IFN-γ instigate an inflammatory response in the brain’s white matter, contributing to plaque development [18]. Notably, the administration of IFN-γ exacerbates MS symptoms. However, IFN-γ treatment has demonstrated some efficacy in reducing the relapse rate among MS patients.
Considering the pivotal role of cytokines in these diseases, as corroborated by the referenced data and articles, it is essential to further explore the therapeutic potential of cytokines. Consequently, this study aimed to elucidate the role of cytokines and macrophages in several of the most common diseases. A comprehensive literature search was conducted using a variety of keywords, resulting in a plethora of articles. The most pertinent and relevant articles were meticulously selected for this systematic review.
Macrophage Heterogeneity and Functional Diversity
Macrophages
Macrophages, which are sentinels of the immune system, exhibit remarkable tissue tropism and strategically position themselves in lymphoid organs, mucosal interfaces, and other pivotal sites. These versatile cells act as resident custodians, wielding their phagocytic ability to maintain tissue homeostasis [10]. These cells diligently scavenge apoptotic and necrotic debris while simultaneously standing guard against invading pathogens. In addition to being mere scavengers, macrophages are key players in both innate and adaptive immunity [10]. The innate arsenal of these viruses includes immediate neutralization of foreign microorganisms and orchestration of leukocyte recruitment. These proteins meticulously regulate the delicate balance between antigen presentation and clearance through phagocytosis and subsequent degradation. This intricate interplay with T and B lymphocytes, facilitated by a diverse repertoire of cytokines, chemokines, and bioactive molecules, dictates the trajectory of the immune response [35]. Macrophage activation, a dynamic process influenced by contextual cues, can skew toward proinflammatory or anti-inflammatory functions. This versatility allows them to orchestrate tissue destruction during infection, followed by a switch toward regenerative and wound healing processes. Ultimately, macrophages act as crucial orchestrators, initiating, instructing, and even terminating the adaptive immune response, ensuring delicately balanced and dynamic defenses against diverse immunological challenges [6].
Classically Activated Macrophages
M1 macrophage polarization is orchestrated by the coordinated symphony of IFN-γ and microbial stimuli, particularly LPS. IFN-γ, the lone maestro of type II IFNs, interacts with a dedicated orchestrator conductor, IFNGR, a heterodimer formed by two ligand-capturing IFNGR1 subunits and two signal-transducing IFNGR2 subunits. This exquisite interplay triggers a cascade of molecular events directing macrophage differentiation toward the robust effector repertoire of M1 macrophages [34].
Tumor-Associated Macrophages
Environmental cues within the TME orchestrate intricate leukocyte infiltration, proliferation, and polarization, thereby dictating the functional repertoire of recruited macrophages. Among these, TAMs exhibit a protumorigenic phenotype and thus harbor a potent arsenal of proangiogenic and tumor-promoting chemokines, such as CCL2/MCP-1. Hypoxia, a defining hallmark of the TME, further sculpts TAM functionality, igniting a proangiogenic program that amplifies tumor-driven vascularization in an indirect yet potent manner [49]. However, not all macrophage contributions favor tumor progression. In a murine model, tumor-derived GM-CSF induced macrophage-mediated degradation of the extracellular matrix via upregulation of MMPs, concurrently stimulating angiostatin production, ultimately leading to suppressed metastatic growth [48].
Role of Cytokines and Macrophages in Inflammation
The intricate nature of inflammation relies on the delicate balance between proinflammatory and resolving signals. Disruption of this equilibrium leads to uncontrolled inflammation, causing cellular and tissue damage [39]. Macrophages, the stalwart foot soldiers of the mononuclear phagocyte system, orchestrate this intricate dance, playing a pivotal role in initiation, maintenance, and resolution. Elie Metchnikoff, a Nobel laureate, initially christened these phagocytic warriors as "white blood cells" for their frontline defenses against infection. In 1924, Aschoff refined the nomenclature, coining the term "macrophage" for this diverse lineage encompassing monocytes, macrophages, and histiocytes. During inflammation, macrophages carry out three essential steps: antigen presentation, phagocytosis, and immunomodulation through cytokine and growth factor orchestration [42]. Activated by a symphony of signals, including cytokines such as IFN-γ, GM-CSF, and TNF-a; bacterial lipopolysaccharide; and matrix cues, macrophages ignite the inflammatory response. Resolution, the counterpoint to inflammation, necessitates the silencing of these inflammatory conductors and their effector cells. Deactivation of macrophages, achieved through diverse mechanisms, allows tissue repair and restoration [8]. Neutrophilic granulocytes, the rapid responders of the acute inflammatory phase, extravasate through a fleeting set of endothelial adhesion molecules orchestrated by cytokine-induced upregulation. Intriguingly, the duration of inflammation appears to differ between preterm deliveries and term deliveries, with elevated proinflammatory cytokine and proteinase levels preceding cervical dilation. Similarly, chorioamnionitis patients exhibit elevated proinflammatory cytokines across various compartments, potentially contributing to preterm contractions. The role of inflammation in atherosclerosis is a captivating research avenue. Recent studies have investigated [46]. Ate interplay between proinflammatory cytokines such as IL-1, IL-18, and OPN and their anti-inflammatory counterparts, including IL-1 receptor antagonists, IL-10, and IL-18-binding proteins [48]. Additionally, the contribution of chronic infections such as Helicobacter pylori and Chlamydophila pneumoniae to persistent inflammation has garnered significant attention.
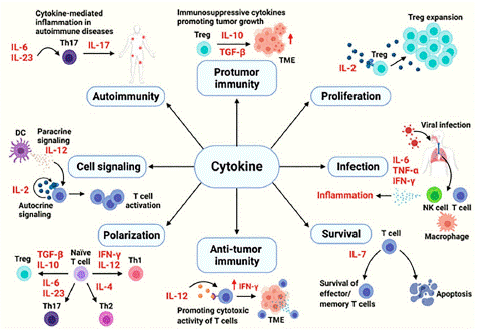
Figure 1: The cytokines in immune processes.
Source: (Kang et al., [22]).
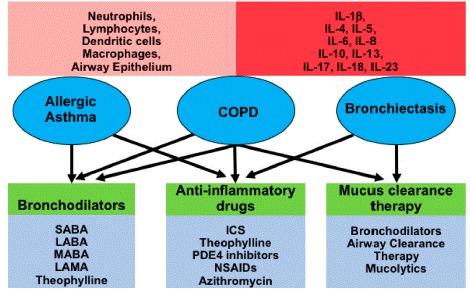
Figure 2: The diagram is summarizing the three chronic inflammatory airway diseases.
Source: (Garth et al., [16])
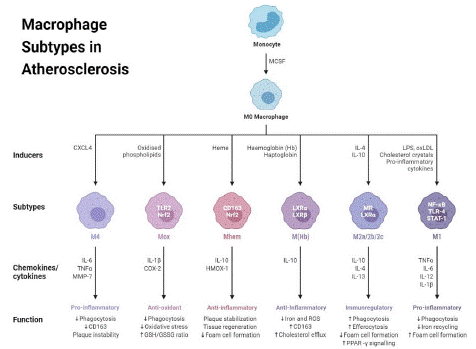
Figure 3: Macrophage Subtypes in Atherosclerosis.
Source: (Macrophages: Structure, Immunity, Types, Functions, n.d.)
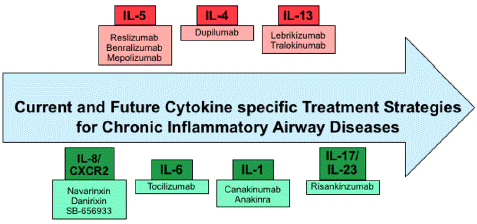
Figure 4: Current and future specific treatment strategies of cytokines.
Source: (Garth et al., [16]).
Cytokine Therapeutic Uses
IL-1 and IL-2 have demonstrated potential as consistent immunostimulants for combating AIDS. Empirical studies have substantiated the hypothesis that immunostimulatory cytokines can counteract the immunosuppressive characteristics of cancer. The synthesis of IL-10, TGF-β, and PGE2 by ovarian cancer and Cap (3) cells contributes to the comprehensive inhibition of antitumour activities [42]. IL-10 facilitates the differentiation of monocytes into mature macrophages and impedes their differentiation into dendritic cells. The alarming inflammatory response of humans to cytokines such as IL-1, IL-2, IL-3, IL-4, IL-6, IL-12, and TNF-a has resulted in adverse effects. The first cytokine approved for cancer treatment was IL-2, but its proinflammatory effects are poorly tolerated, thereby limiting its efficacy in conditions such as melanoma and renal cell carcinoma [9]. IL-10 has emerged as a promising candidate for various autoimmune diseases because it not only suppresses the production of IFN-γ, IL-1, TNF-a, and IL-6 but also has other anti-inflammatory effects [9]. However, several trials of recombinant human IL-10 have demonstrated limited effectiveness in treating psoriasis, rheumatoid arthritis, and Crohn’s disease, and this cytokine has not been approved for therapeutic use [13]. The FDA has approved the cytokines IL-2 and IFN-a, with IL-2 being used for the treatment of metastatic melanoma and renal cell carcinoma in high-dose boluses and IFN-a serving as an adjuvant treatment for stage III melanoma, hematologic malignancies, AIDS-related Kaposi’s sarcoma, and in combination with bevacizumab as an antiangiogenic agent for advanced renal cancer [18]. Recently, various cytokines, including IL-7, IL-12, IL-15, IL-18, IL-21, and GM-CSF, have been tested in clinical trials for advanced cancer [18].
Dysregulated Immunity: Cytokines and Macrophage Links
Macrophages in Acute lung Disease
Acute Respiratory Distress Syndrome (ARDS) is a critical condition characterized by the rapid onset of diffuse alveolar damage and impaired gas exchange [26]. Diverse primary and secondary mechanisms can trigger ARDS, including shock, severe sepsis, pulmonary contusion, gastroesophageal reflux, pneumonia, drug toxicity, transfusion, and acute pancreatitis [3]. Iatrogenic or physical injury from mechanical ventilation can further contribute to ARDS development. The pathogenesis of this disease commences with the rupture of alveolar septa, disruption of the epithelium-capillary interface, release of protein-rich fluid, secretion of proinflammatory chemokines and cytokines, and infiltration of inflammatory cells such as monocytes and neutrophils. ALI is characterized by an amplified M1 response and impaired M2-mediated repair, while chronic diseases such as fibrosis and cancer are dominated by hyperresponsive, alternatively activated M2 cells [49].
Macrophages in Various Infections
During the initial phase of bacterial infection, macrophages exhibit an M1 phenotype. Delayed regulation of macrophage-mediated inflammation results in a cytokine storm, contributing to the pathogenesis of severe sepsis [48]. During inflammatory responses, macrophages exhibit two key strategies for resolution: programmed cell death (apoptosis) or phenotypic reprogramming toward an M2 state. These mechanisms safeguard the host from excessive tissue damage and initiate the critical transition from inflammation to tissue repair. Apoptosis eliminates potentially harmful proinflammatory macrophages, while the M2 phenotype promotes debris clearance, angiogenesis, and collagen deposition, ultimately facilitating wound healing [34].
Macrophage Counts in Bronchiectasis
In bronchiectasis, a chronic pulmonary pathology characterized by persistent inflammation, bacterial colonization, and specific commensal bacteria, such as Stromatosus mucilaginosus, exacerbates disease progression. S. mucilaginosus facilitates the establishment of opportunistic pathogens, notably Pseudomonas aeruginosa, by fostering a conducive microenvironment within compromised airways [44]. This bacterium specifically activates Toll-like receptor 2 (TLR2) on host immune cells, particularly M1 macrophages, triggering a proinflammatory cytokine cascade that perpetuates tissue damage and infection susceptibility [39].
Macrophages in Asthma
Asthmatic inflammation hinges on a precarious balance between proinflammatory and anti-inflammatory pulmonary macrophage functions. While M2 macrophages, champions of tissue repair and lung microenvironment homeostasis, normally serve as allies, their dysregulation unleashes a cascade of pathological events. This dysregulation manifests as airway hyperresponsiveness, fuelled by augmented cell recruitment and mucus hypersecretion, potentially orchestrated by aberrant miRNA expression [14].
Recent investigations have shed light on the intriguing nexus between specific miRNAs and alleviating asthmatic symptoms. For instance, miR-145 exhibits therapeutic potential by dampening the expression of proinflammatory cytokines (IL-13 and IL-5), thereby curbing inflammation [17]. Similarly, miR-146a orchestrates multiple processes by suppressing an array of proinflammatory cytokines and chemokines, effectively eliminating the inflammatory storm. Furthermore, miR-21 strategically neutralizes IL-21, a pivotal player in Th1 cell polarization, by disrupting a key pathogenic pathway. The intricate link between asthma and miRNAs is further underscored by the observation that let-7 downregulation amplifies the levels of IL-13, a pivotal orchestrator of allergic responses, potentially influencing Th2 skewing [19].
Macrophages in Chronic Obstructive Pulmonary Disease (COPD)
Progressive inflammatory and structural derangements orchestrate the pathogenesis of COPD. Chronically inhaled noxious stimuli trigger diverse responses in lung epithelial cells, culminating in a maladaptive cascade [45]. Epithelial senescence accelerates, the pulmonary capillary vasculature undergoes progressive destruction, and airway remodelling ensues. This architectural disarray manifests as decreased lung compliance, the hallmark functional signature of COPD. The inflammatory cascade in COPD involves a repertoire of key mediators. Cytokines such as TNF-a, IL-1β, and GM-CSF act in both autocrine and paracrine fashions, perpetuating the inflammatory milieu [32]. TGFβ orchestrates the nefarious transformation of fibroblasts into myofibroblasts, fueling the fibrotic crescendo of airway remodelling. Recent insights implicate miRNA dysregulation in this macabre dance, with downregulation of miR-152 liberating MMP12, a potent maestro of emphysematous destruction [4].
Macrophages in Tuberculosis (TB)
Mycobacterium tuberculosis (Mtb), the causative agent of the ubiquitous infectious disease Tuberculosis (TB), faces its initial barrier within the alveoli. Alveolar macrophages, the sentinels of pulmonary immunity, engulf Mtb droplets via phagocytosis. Within the phagolysosome, a potent arsenal of Reactive Oxygen Species (ROS) unleashes oxidative mayhem upon invaders [50]. This initial skirmish triggers the recruitment of mononuclear leukocytes, bolstering the immune response. These professional phagocytes constitute the first line of defense, actively engaging and eliminating mycobacterial threats. Among these leukocytes, classically activated macrophages (M1) are central to combating intracellular parasites [40]. Their potent microbicidal arsenal, fueled by enhanced antigen presentation and inflammatory cytokine production, spearheads the antimycobacterial response. Alternatively, activated M2 macrophages adopt a different paradigm. These versatile cells prioritize tissue repair, suppress inflammation, and promote wound healing, contributing to a more orchestrated immune response. This dynamic interplay between M1 and M2 macrophages underscores the complex immunological landscape of TB. Understanding these intricate orchestrations paves the way for novel therapeutic strategies aimed at tipping the balance toward pathogen eradication and tissue preservation [27].
Kidney Diseases
In Chronic Kidney Disease (CKD), infiltrating immune cells transcend passive bystanders, morphing into detrimental actors within the pathogenic orchestration [43]. These inflammatory elements actively propel disease progression, orchestrating a nefarious symphony of nephron attrition and fibrotic encasement. This insidious immune-mediated assault elevates CKD to the ominous ranks of chronic inflammatory diseases, demanding novel therapeutic strategies that not only eliminate inflammation but also disarm the nefarious orchestrators of renal devastation [15]. Anti-inflammatory strategies have emerged as common therapeutic targets for renal disorders, given that patients with early-stage CKD exhibit subclinical inflammation and activation of circulating immune cells. Recent investigations have illuminated the versatility and complexity of immune cell roles [5]. Monocytes/macrophages, a critical type of immune cell, are innate immune system phagocytes found across various organs.
Autoimmune Disease
Interleukin-6 (IL-6) is a pleiotropic cytokine that critically influences diverse pathological processes, encompassing autoimmune disorders, bacterial infections, and metabolic dysregulations. Composed of four a-helices, this 184-amino acid protein has multiple functions, transcending its initial characterization as a B-cell stimulatory factor (Kishimoto et al., 1986). Notably, IL-6 has potent immunomodulatory effects, promoting CD4+ T-cell expansion via IL-21 induction and guiding CD4+ T-cell differentiation toward the Th2 and Th17 lineages [37,38,50,51].
Cardiovascular Disease
Cytokines and macrophages are instrumental in the onset and progression of CVD. Cytokines, signaling molecules produced by various cells, including immune cells such as macrophages, regulate immune responses, inflammation, and cell growth and differentiation [1,21]. In CVD, cytokines such as TNF-alpha, IL-6, and IL-1beta contribute to the development of atherosclerosis, a primary cause of CVD. Macrophages, a type of immune cell, play a pivotal role in atherosclerosis. In its early stages, macrophages infiltrate the arterial wall and take up modified lipids such as oxidized LDL to form foam cells. These foam cells contribute to the formation of fatty streaks, the earliest visible signs of atherosclerosis [29]. Macrophages also secrete cytokines and other inflammatory mediators that promote inflammation and atherosclerosis progression. In advanced atherosclerosis, macrophages can form a necrotic core by undergoing apoptosis and releasing their contents, including cholesterol and proinflammatory cytokines. This can lead to plaque instability and rupture, triggering acute cardiovascular events such as myocardial infarction or stroke [47].
Cancer
In cancer, cytokines can exhibit both protumor and antitumour effects. Some cytokines, such as IL-6 and TNF-alpha, promote tumor growth by stimulating cell proliferation, inhibiting cell death, and promoting angiogenesis [7]. Conversely, other cytokines, such as IFN-gamma and tumor necrosis factor-beta (TNF-beta), exhibit antitumour effects by promoting cell death and activating immune cells to attack cancer cells. Macrophages play a key role in the early stages of cancer and help recognize and eliminate cancer cells. However, as the tumor grows, macrophages can become “reprogrammed” to adopt a protumor phenotype. These “Tumor-Associated Macrophages” (TAMs) promote tumor growth by secreting cytokines and growth factors that stimulate cell proliferation and angiogenesis, suppress the immune response, and remodel the extracellular matrix to promote metastasis [41]. Targeting cytokines and macrophages is a promising strategy for cancer therapy. For instance, drugs that block cytokine signaling, such as anti-IL-6 or anti-TNF-alpha antibodies, are currently undergoing clinical trials for various types of cancer. Additionally, there is growing interest in developing therapies that target TAMs, either by depleting them or reprogramming them to adopt an antitumour phenotype [47].
Current Challenges
Investigating the complex interplay between cytokines and macrophages in inflammation poses several significant challenges. First, the sheer diversity of cytokines involved complicates the identification of specific actors in individual cases. Overlapping and sometimes redundant functions of certain cytokines further enhance this complexity, demanding sophisticated tools for discerning their unique contributions [12]. Determination of the concentrations of many cytokines in biological samples necessitates highly sensitive assays, such as flow cytometry or ELISA, for accurate quantification. Macrophages, a heterogeneous cell population, present another critical hurdle. The diverse tissue-specific phenotypes of these viruses exhibit distinct gene expression profiles and functional properties, necessitating tailored isolation techniques. Fluorescence-Activated Cell Sorting (FACS) or Magnetic-Activated Cell Sorting (MACS) have become essential tools for differentiating these nuanced subsets [9]. Maintaining macrophage viability throughout an experiment, especially during cytokine stimulation studies, can be delicate due to the high sensitivity of macrophages to environmental changes and the tendency for rapid activation or apoptosis. Deciphering the intricate signaling pathways activated by cytokines within macrophages is yet another challenge. Western blotting and gene expression profiling have become crucial tools for revealing the molecular cascades triggered by specific cytokines, allowing for a deeper understanding of their downstream effects [33]. Both cytokines and macrophages exhibit pronounced heterogeneity, further adding layers of complexity to their roles in inflammation [28]. Understanding the tissue-specific nuances of different macrophage subsets and the diverse effects of cytokine combinations is crucial for dissecting their precise contributions to the inflammatory process. Additionally, cytokine storms, characterized by a hyperactive immune response with excessive cytokine production, pose a potentially fatal threat, leading to widespread tissue damage and organ failure. Elucidating the mechanisms triggering such events holds immense clinical significance [20]. Finally, immunosenescence, the age-related decline in immune function, further complicates the picture. Understanding how these changes impact cytokine profiles and macrophage polarization patterns is vital for developing effective strategies to combat age-related infections, cancers, and autoimmune diseases. By addressing these significant challenges, researchers can elucidate the intricacies of cytokine–macrophage interactions in inflammation, paving the way for novel therapeutic interventions and improved human health outcomes [24].
Future Strategies
The emergence of humanized animal models constructed by engrafting human cells or tissues into nonhuman hosts has revolutionized biomedical research and drug discovery [11]. These models offer unparalleled advantages over traditional in vitro or in silico approaches by enabling the investigation of human pathophysiology in vivo, evaluating potential therapeutic efficacy, and providing a more accurate representation of disease progression than conventional methods. By serving as a preliminary testing ground for new drugs, humanized models can streamline the drug development process by identifying potential safety or efficacy concerns early on. Continued advancements in technology hold promise for the creation of even more sophisticated and precise humanized models, potentially paving the way for the development of more effective disease treatments [30].
Single-cell sequencing, while a powerful tool for understanding cellular heterogeneity, faces several challenges. Isolation of individual cells from complex tissues or rare cell populations can be difficult, impacting subsequent analysis. The quality and quantity of isolated cells significantly influence the accuracy and reliability of the generated data. Additionally, the high-dimensional nature of single-cell sequencing data necessitates a meticulously designed analysis pipeline to manage technical variability and biological heterogeneity. Amplification, required to generate sufficient DNA or RNA for sequencing, introduces potential biases that can distort results, highlighting the importance of robust quality control measures. Macrophage polarization plays a pivotal role in regulating inflammation. Inducing M2 polarization through IL-4 and IL-13 has potential for mitigating inflammation in diseases such as asthma [2]. Emerging immunomodulatory therapies, such as senolytics and immune checkpoint inhibitors, offer promising avenues for combating immunosenescence. Senolytics selectively eliminate senescent cells, which contribute to age-associated inflammation, while immune checkpoint inhibitors enhance immune function by negating immunosuppressive signals. In the context of COVID-19, cytokine inhibitors such as tocilizumab and baricitinib have shown efficacy in managing cytokine storms by targeting specific proinflammatory cytokines. Despite the identification and study of numerous cytokines and macrophage subsets, the landscape has not been fully elucidated. Future research should focus on discovering novel entities and elucidating their roles in inflammation and the immune response [36]. Dysregulated cytokine production is a hallmark of chronic inflammatory diseases. Deciphering the regulatory mechanisms governing cytokine production in macrophages and other immune cells provides fertile ground for identifying novel therapeutic targets. Similarly, the diverse spectrum of macrophage polarization states warrants further investigation to identify specific targets for modulating inflammation in various diseases. While the pivotal roles of cytokines and macrophages in inflammation and the immune response are well established, their specific contributions to many diseases remain unclear. Current imaging techniques provide limited information about cytokine and macrophage activity in vivo [36]. The development of novel imaging modalities with the potential to visualize these activities in real time holds immense promise for advancing our understanding of inflammation and the immune response, paving the way for the development of more targeted and effective therapeutic strategies.
Conclusion
In conclusion, macrophages exhibit remarkable heterogeneity and play a crucial role in both innate and adaptive immunity. The diverse activation states of M1 and M2 macrophages contribute to tissue repair, inflammation resolution, and disease progression depending on the specific context. Cytokines, signaling molecules produced by macrophages and other immune cells, further orchestrate these complex processes. Understanding the intricate interplay between cytokines and macrophages in inflammation holds immense potential for developing novel therapeutic interventions for various diseases, including cancer, autoimmune disorders, and chronic inflammatory conditions.
Future research efforts should focus on deciphering the regulatory mechanisms governing cytokine production and macrophage polarization, identifying novel therapeutic targets, and developing advanced imaging modalities to visualize these activities in vivo. By addressing these challenges and revealing the secrets of cytokine–macrophage interplay, we can pave the way for a healthier future.
Author Statements
Funding
This research was unfunded by any public, commercial, or not-for-profit agency.
Guarantor
The article’s full responsibility lies with PY, who is the corresponding author and the third author on the list.
Author Contributions
SA, PS, SS, AK, and VR were responsible for manuscript conceptualization, writing - original draft, consent, and sample collection. PY participated in the writing, review and editing.
Acknowledgements
Due to space constraints, some pertinent publications could not be found.
Availability of Data and Materials
All the data have been truly cited in the articles.
Consent for Publication
All the authors consented to the publication of this manuscript.
References
- Amin MN, Siddiqui SA, Ibrahim M, Hakim ML, Ahammed MS, Kabir A, et al. Inflammatory cytokines in the pathogenesis of cardiovascular disease and cancer. SAGE Open Medicine. 2020: 8.
- Apeku E, Tantuoyir MM, Zheng R, Tanye N. Exploring the polarization of M1 and M2 macrophages in the context of skin diseases. Molecular Biology Reports. 2024; 51: 269.
- Arora S, Dev K, Agarwal B, Das P, Syed MA. Macrophages: Their role, activation and polarization in pulmonary diseases. Immunobiology. 2018; 223: 383–396.
- Birch J, Anderson RK, Correia-Melo C, Jurk D, Hewitt G, Marques FM, et al. Airway Epithelium Senescence as a Driving Mechanism in COPD Pathogenesis. Biomedicines. 2023; 11: 2072.
- Cantero-Navarro E, Rayego-Mateos S, Orejudo M, Tejedor-Santamaria L, Tejera-Muñoz A, Sanz AB, et al. Role of Macrophages and Related Cytokines in Kidney Disease. Frontiers in Medicine. 2021; 8: 688060.
- Catalfamo M, Le Saout C, Lane HC. The Role of Cytokines in the Pathogenesis and treatment of HIV Infection. Cytokine & Growth Factor Reviews. 2012; 23: 207-214.
- Cendrowicz E, Sas Z, Bremer E, Rygiel TP. The Role of Macrophages in Cancer Development and Therapy. Cancers. 2021; 13: 1946.
- Chen L, Deng H, Cui H, Fang J, Zuo Z, Deng J, et al. Inflammatory responses and inflammation-associated diseases in organs. Oncotarget. 2018; 9: 7204-7218.
- Chen S, Saeed AFUH, Liu Q, Jiang Q, Xu H, Xiao GG, et al. Macrophages in immunoregulation and therapeutics. Signal Transduction and Targeted Therapy. 2023; 8: 1–35.
- Chung KF. Inflammatory mediators in chronic obstructive pulmonary disease. Current Drug Targets: Inflammation and Allergy. 2005; 4: 619–625.
- Chuprin J, Buettner H, Seedhom MO, Greiner DL, Keck JG, Ishikawa F, et al. Humanized mouse models for immuno-oncology research. Nature Reviews Clinical Oncology. 2023; 20: 192–206.
- Cortés M, Brischetto A, Martinez-Campanario MC, Ninfali C, Domínguez V, Fernández S, et al. Inflammatory macrophages reprogram to immunosuppression by reducing mitochondrial translation. Nature Communications. 2023; 14: 1–18.
- Dinarello CA. Historical insights into cytokines. European Journal of Immunology. 2007; 37: S34–S45.
- Duan W, Huang J, Wasti B, Chen Z, Yuan Y, He Y, et al. miR-146a-3p as a potential novel therapeutic by targeting MBD2 to mediate Th17 differentiation in Th17 predominant neutrophilic severe asthma. Clinical and Experimental Medicine. 2023; 23: 2839-2854.
- Fattah H, Layton A, Vallon V. How Do Kidneys Adapt to a Deficit or Loss in Nephron Number?. Physiology. 2019; 34: 189-197.
- Garth J, Barnes JW, Krick S. Targeting Cytokines as Evolving Treatment Strategies in Chronic Inflammatory Airway Diseases. International Journal of Molecular Sciences. 2018; 19: 3402.
- Girodet PO, Nguyen D, Mancini JD, Hundal M, Zhou X, Israel E, et al. Alternative macrophage activation is increased in asthma. American Journal of Respiratory Cell and Molecular Biology. 2016; 55: 467–475.
- Gulati K, Guhathakurta S, Joshi J, Rai N, Ray A. Cytokines and their Role in Health and Disease: A Brief Overview. MOJ Immunol. 2016; 4: 00121.
- Habib N, Pasha MA, Tang DD. Current Understanding of Asthma Pathogenesis and Biomarkers. Cells. 2022; 11: 2764.
- Han X, Ding S, Jiang H, Liu G. Roles of Macrophages in the Development and Treatment of Gut Inflammation. Frontiers in Cell and Developmental Biology. 2021; 9: 625423.
- Haybar H, Bandar B, Torfi E, Mohebbi A, Saki N. Cytokines and their role in cardiovascular diseases. Cytokine. 2023; 169: 156261.
- Kang JH, Bluestone JA, Young A. Predicting and Preventing Immune Checkpoint Inhibitor Toxicity: Targeting Cytokines. Trends in Immunology. 2021; 42: 293–311.
- Kany S, Vollrath JT, Relja B. Cytokines in Inflammatory Disease. International Journal of Molecular Sciences. 2019a; 20: 6008.
- Kany S, Vollrath JT, Relja B. Cytokines in Inflammatory Disease. International Journal of Molecular Sciences. 2019b; 20: 6008.
- Kedzierska K, Crowe SM. Cytokines and HIV-1: interactions and clinical implications. Antiviral Chemistry & Chemotherapy. 2001; 12: 133–150.
- Kim E, Yang IV. Selective regulation of the airway mucin MUC5B in the distal airway. American Journal of Respiratory and Critical Care Medicine. 2019; 200: 129–131.
- Lavalett L, Ortega H, Barrera LF. Infection of Monocytes from Tuberculosis Patients with Two Virulent Clinical Isolates of Mycobacterium tuberculosis Induces Alterations in Myeloid Effector Functions. Frontiers in Cellular and Infection Microbiology. 2020; 10: 163.
- Leone GM, Mangano K, Petralia MC, Nicoletti F, Fagone P. Past, Present and (Foreseeable) Future of Biological Anti-TNF Alpha Therapy. Journal of Clinical Medicine. 2023; 12: 1630.
- Lv Z, Zhao B, Hu L, Pan J, Li X, Li M, et al. Identification of Macrophage-related Gene Signature Associated with Unstable Atherosclerotic Plaques through Integrative Multi- omics Analysis. 2023.
- Ma M, Ge JY, Nie YZ, Li YM, Zheng YW. Developing Humanized Animal Models with Transplantable Human iPSC-Derived Cells. Frontiers in Bioscience (Landmark Edition). 2024; 29: 34.
- Macrophages: Structure, Immunity, Types, Functions. (n.d.). 2024.
- McCormick TS, Hejal RB, Leal LO, Ghannoum MA. GM-CSF: Orchestrating the Pulmonary Response to Infection. Frontiers in Pharmacology. 2021: 12.
- Oishi Y, Manabe I. Macrophages in inflammation, repair and regeneration. International Immunology. 2018; 30: 511–528.
- Orecchioni M, Ghosheh Y, Pramod AB, Ley K. Macrophage polarization: Different gene signatures in M1(Lps+) vs. Classically and M2(LPS-) vs. Alternatively activated macrophages. Frontiers in Immunology. 2019; 10: 1084.
- Osuji FN, Onyenekwe CC, Ahaneku JE, Ukibe NR. The effects of highly active antiretroviral therapy on the serum levels of pro-inflammatory and anti-inflammatory cytokines in HIV infected subjects. Journal of Biomedical Science. 2018; 25: 88.
- Pérez S, Rius-Pérez S. Macrophage Polarization and Reprogramming in Acute Inflammation: A Redox Perspective. Antioxidants. 2022; 11: 1394.
- Pramod Y, Vishal C, Vikas R, Amarjeet Y, Adhishree Y, Samim A, et al. Interferons as a Potential Therapeutic Drug for COVID-19: A Literature Review of Mechanisms, Current Clinical Trials, and Challenges. Journal of Community Medicine and Health Solutions. 2023; 4: 048–056.
- Raghuvanshi V, Yadav P, Ali S. Interferon production by Viral, Bacterial & Yeast system: A comparative overview in 2023. International Immunopharmacology. 2023; 120: 110340.
- Romero R, Espinoza J, Gonçalves LF, Kusanovic JP, Friel L, Hassan S. The role of inflammation and infection in preterm birth. Seminars in Reproductive Medicine. 2007; 25: 21-39.
- Sampath P, Moideen K, Ranganathan UD, Bethunaickan R. Monocyte Subsets: Phenotypes and Function in Tuberculosis Infection. Frontiers in Immunology. 2018; 9: 1726.
- Shen J, Xiao Z, Zhao Q, Li M, Wu X, Zhang L, et al. Anti-cancer therapy with TNFa and IFNγ: A comprehensive review. Cell Proliferation. 2018; 51.
- Soehnlein O, Libby P. Targeting inflammation in atherosclerosis – from experimental insights to the clinic. Nature Reviews Drug Discovery. 2021; 20: 589–610.
- Stenvinkel P, Chertow GM, Devarajan P, Levin A, Andreoli SP, Bangalore S, et al. Chronic Inflammation in Chronic Kidney Disease Progression: Role of Nrf2. Kidney International Reports. 2021; 6: 1775.
- Vidaillac C, Chotirmall SH. Pseudomonas aeruginosa in bronchiectasis: infection, inflammation, and therapies. Expert Review of Respiratory Medicine. 2021; 15: 649–662.
- Wang Y, Xu J, Meng Y, Adcock IM, Yao X. Role of inflammatory cells in airway remodeling in COPD. International Journal of Chronic Obstructive Pulmonary Disease. 2018; 13: 3341–3348.
- Watanabe S, Alexander M, Misharin AV, Budinger GRS. The role of macrophages in the resolution of inflammation. The Journal of Clinical Investigation. 2019; 129: 2619.
- Wei T, Zhu Z, Liu L, Liu B, Wu M, Zhang W, et al. Circulating levels of cytokines and risk of cardiovascular disease: a Mendelian randomization study. Frontiers in Immunology. 2023; 14: 1175421.
- Xia T, Fu S, Yang R, Yang K, Lei W, Yang Y, et al. Advances in the study of macrophage polarization in inflammatory immune skin diseases. Journal of Inflammation (United Kingdom). 2023; 20: 33.
- Xie C, Liu C, Wu B, Lin Y, Ma T, Xiong H, et al. Effects of IRF1 and IFN-β interaction on the M1 polarization of macrophages and its antitumor function. International Journal of Molecular Medicine. 2016; 38: 148–160.
- Yadav P. Challenges & Solutions for Recent Advancements in Multi-Drugs Resistance Tuberculosis: A Review. Microbiology Insights. 2023; 16: 1–9.
- Yadav P, Chandra V, Raghuvanshi V, Yadav A, Yadav A, Ali S, et al. Interferons for Covid-19: A Literature Review on Their Therapeutic Potential, Clinical Data and Challenges. Preprints. 2023; 4: 048-056.
- Yadav P, Yadav A. Interferon Production and Potentiality as a therapeutic drug for SARS-CoV-2. Advances in Pharmacoepidemiology and Drug Safety. 2023; 12: 1–5.