
Review Article
Austin J Biotechnol Bioeng. 2024; 11(2): 1133.
Promises and Pitfalls of microRNAs as Biomarkers of Heart Failure
Raissi-Dehkordi NA¹; Raissi-Dehkordi NE¹; Farjoo MH²*
1Department of Pharmacology, Shahid Beheshti University of Medical Sciences, Iran
2Department of Pharmacology, School of Medicine, Shahid Beheshti University of Medical Sciences, Iran
*Corresponding author: Farjoo MH Department of Pharmacology, School of Medicine, Shahid Beheshti University of Medical Sciences, Daneshjoo Blvd, Velenjak, P.O. Box: 19835355, Tehran-Iran. Tel: 23872539; Fax: 22439969 Email: m.farjoo@sbmu.ac.ir
Received: April 29, 2024 Accepted: May 24, 2024 Published: May 31, 2024
Abstract
Background: MicroRNAs (miRNAs) are important non-coding molecules with regulatory roles in gene expression. In the past few years, miRNAs have arisen as apt candidates to assist with the diagnosis, prognosis and monitoring of several diseases. Cardiovascular pathologies have emerged as a particularly interesting field for miRNAs as markers, as it appears that the pathophysiological changes following heart failure lead to measurable alterations in circulating miRNA levels.
Contents: In the present review, we explore the value, sensitivity and specificity of circulating miRNAs in diagnosis and prognosis of heart failure as reported in the literature, and discuss the underlying factors that currently prevent circulating miRNAs from becoming true clinical markers. In spite of the numerous attempts in identification and administration of cardiac miRNA markers, relatively small progress has been made in the application of these markers in clinical settings.
Small study populations and methodological differences, among other possible etiologies, have resulted in little reproducibility among studies.
Conclusion: Despite the encouraging preliminary results, considerable challenges, mainly concerning standardization, have thus far prevented miRNAs from approximating the validity of established biomarkers that are routinely used in clinics, let alone surpassing them. To conclude, rigorous methodology and universally standardized protocols prior to, and during miRNA analysis are required to warrant reproducible, validated and reliable results which will lead to clinical application of miRNA cardiac biomarkers.
Keywords: microRNA; Heart failure; Diagnosis; Prognosis; Biomarker
Abbreviations: miRNAs: MicroRNAs; RISC: RNA-Induced Silencing Complex; MI: myocardial infarction; HF: Heart Failure; BNP: B-type Natriuretic Peptide; NT-proBNP: N-Terminal pro–B-type Natriuretic Peptide; NYHA: New York Heart Association; RT-qPCR: Quantitative Reverse Transcription Polymerase Chain Reaction; LVEF: Left Ventricular Ejection Fraction; hs-cTnT: High-Sensitivity Cardiac Troponin T; AUC: Area Under the Curve; DOR: Diagnostic Odds Ratio; CI: Confidence Interval; HR: Hazard Ratio; RT-qPCR: Reverse Transcription Polymerase Chain Reaction; CHF: Congestive Heart Failure; ICM: Ischemic Cardiomyopathy; NICM: Non-Ischemic Cardiomyopathy; AHF: Acute HF; OR: Odds Ratio; CRT: Cardiac Resynchronization Therapy; SROC: Summary Receiver Operating Characteristic Curves Value; qPCR: quantitative PCR; NGS: Next Generation Sequencing.
Introduction
MicroRNAs (miRNAs) are short non-coding RNAs of around 22 nucleotides involved in post-transcriptional gene expression regulation. lin-4 miRNA, the first miRNA discovered in 1993 in a nematode, Caenorhabditis elegans, was involved in producing non-coding RNAs that controlled the development of C. elegans through regulating the expression of a protein, lin-14. Subsequently, another miRNA, let-7 was discovered in C. elegans, which was involved in developmental timing [1,2]. Let-7 was subsequently discovered in humans in 2000 [3] miRNA genes are transcribed by RNA polymerase II & III from primary miRNA into precursor miRNA and finally mature miRNA [4]. Primary miRNAs containing stem-loop structures are cleaved in the nucleus into precursor miRNAs by DGCR8, an RNase III enzyme. Precursor miRNAs are then exported into the cytoplasm by Exportin-5, where Dicer removes the terminal loop from the hairpin structure and creates a miRNA-miRNA duplex [5]. Following the unwinding of the duplex, Argonaute protein integrates one of the mature miRNA strands, the so-called guide strand, into RNA-Induced Silencing Complex (RISC), while the other strand, nominated passenger strand, is degraded in the cytoplasm. Subsequent binding of the guide strand and RISC to complementary mRNAs results in degradation or inhibition of translation [6]. A summary of miRNA biogenesis can be found in Figure 1.
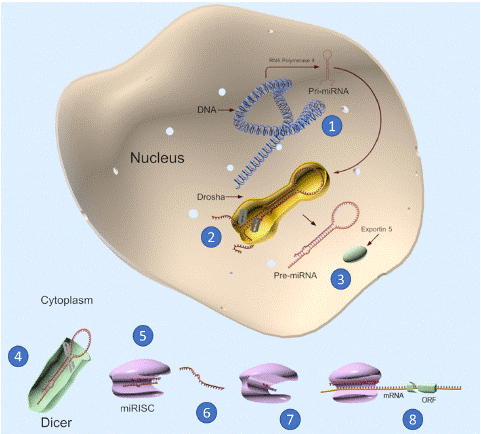
Figure 1: Overview of microRNA formation. Transcription of miRNA-coding-genes into pri-miRNA occurs inside the nucleus (1). Cleavage of pri-miRNA by the microprocessor complex of Drosha + DGCR8 creates precursor microRNAs (pre-miRNA) (2), which are then exported to cytoplasm by Exportin-5 (3). Dicer excises the precursor microRNAs into intermediary duplexes (4), one strand of each is subsequently loaded onto RNA-induced silencing complex (RISC) together with Argonaut (AGO) protein family to form a miRNA-induced silencing complex (miRISC) (5). Eventually, the duplex unwinds, releasing the complementary strand (6), while retaining the mature strand (7). The mature miRNA binds to target mRNAs and leads to microRNA-mediated gene regulation through several mechanisms (8).
miRNAs contribute to cardiac development. They function through regulation of cardiomyocyte differentiation and proliferation. miRNA dysregulation may lead to hypertrophy, arrhythmia and ischemia of the myocardium [7]. They are involved in developing and maintaining proper heart and vascular function by regulating pathways involved in cellular growth and communication, and cardiogenesis [8]. Through their regulatory roles, miRNAs are involved in a multitude of cardiovascular pathologies. These pathologies include congenital heart diseases, cardiomyopathies, cardiac hypertrophy, arrhythmias, Myocardial Infarction (MI) and Heart Failure (HF) [7-9]. Owing to their stability in plasma and protection from RNase activity [10], tissue specificity and presence in most extracellular fluids, miRNAs have emerged as possible biomarkers for a variety of pathologies including cardiovascular diseases [8,11]. In this review, we summarize the roles of miRNAs in diagnosis and prognosis of HF, focusing on the most recent findings of the field. We also delve into the challenges of getting cardiac miRNA biomarkers from bench to bedside.
Heart Failure
The current gold standard markers for HF, B-type Natriuretic Peptide (BNP) and N-terminal pro–B-type natriuretic peptide (NT-proBNP), are used in combination with other markers such as troponins to guide the management of HF patients. Nevertheless, new biomarkers are under investigation to help with the diagnosis, prognosis and evaluation of HF patients [12]. Previously mentioned characteristics of miRNAs, in addition to their involvement in HF pathophysiology including hypertrophy, fibrosis and apoptosis, make them possible candidate biomarkers for HF [13].
A preliminary study by Tijsen et al. offered an association between miRNAs and HF by proposing miR-423-5p as a biomarker for HF. Using miRNA array, miR-423-5p was reported as an HF specific marker capable to distinguish between 39 healthy subjects, 30 HF patients and 20 non-HF dyspneic patients (AUC 0.91 and p-value <0.001) and positively correlated to New York Heart Association (NYHA) score, ejection fraction and NT-proBNP levels. Ischemia and infarction cases were excluded to increase specificity [14]. A 2012 study Goren et al. evaluated the level of 186 miRNAs in the serum of 30 chronic systolic HF patients and 30 controls using quantitative Reverse Transcription Polymerase Chain Reaction (RT-qPCR) and discovered four miRNAs to be significantly elevated in the patients: miR-423-5p, miR-320a, miR-22, and miR-92b (p-value < 0.0005) [15]. A score based on these four miRNAs proved to be a sensitive and specific (both 90%) predictor of HF and furthermore, it was also strongly associated with HF prognostic markers, correlating with serum BNP levels (p-value = 0.002), wide QRS (p-value = 0.009), increased LV end-diastolic diameter (p-value = 0.03), and increased left atrial dimension (p-value = 0.01).
However, a prospective, multi-center cohort of 246 patients with a first anterior wall Q-wave MI found no relationship between miR-133a or miR-423-5p and LV end-diastolic volume, LV end-systolic volume, Left Ventricular Ejection Fraction (LVEF) and BNP [16], contradicting the results of previous studies [14,15]. Serial measurements and echocardiographic studies were obtained at discharge, followed by 1, 3 and 12 months to monitor disease progression. One possible explanation for this discrepancy is the treatment regimens, which in this cohort consisted of antiplatelet drugs, statins, angiotensin-converting enzyme inhibitors, and beta-blockers, but were different in the previous studies.
In contrast, a study on patients presenting with recent onset severe shortness of breath failed to corroborate the results of previous studies, as miR-423-5p levels could not differentiate HF from healthy controls (p-value = 0.07), chronic obstructive pulmonary disease (p-value = 0.57), and other patients with shortness of breath (p-value = 0.19) [17]. Nonetheless, adding miR-423-5p results to NT-proBNP resulted in a 3.2% increase in AUC (p-value = 0.030). A positive correlation was observed between miR-103, miR-142-3p, miR-342-3p, miR-199a-3p, miR-23a, miR-27b, and miR-324-5p with HF, however it was much weaker than past reports (max AUC 0.67 vs 0.91 as reported for miR-423-5p). Studies have linked miR-30 family to cardiac targets such as fibroblast activation protein, alpha cold shock domain protein A, catalase and interferon-γ; miR-199 family to hypoxia-induced cell death through regulation of hypoxia-inducible factor-1a; and miR-27b to hypoxia and angiogenesis, miR-324-5p to cardiac development and ischemia and miR-103 to hypoxia. However, none of the seven miRNAs were able to match the sensitivity and specificity of NT-proBNP or high-sensitivity cardiac troponin T (hs-cTnT).
A 2013 study on 53 patients and controls using RT-qPCR in whole blood samples found miR-558, miR-122, and miR-520d-5p to be predictors of systolic HF with miR-520d-5p showing a sensitivity of 64%, specificity of 74%, and accuracy of 68%. Interestingly, a panel of eight miRNAs including miRNA-520d-5p, miRNA-558, miRNA-122, miRNA-200b, miRNA-622, miRNA-519e, miRNA-1231, and miRNA-1228, had superior ability in diagnosing HF with an accuracy of 70%, a specificity of 66%, and a sensitivity of 74%. In comparison, NT-proBNP showed a sensitivity of 78% and specificity of 44%. Although no correlation was found between NYHA score and miRNA levels, six of the eight mentioned miRNAs showed a significant correlation with LVEF. In addition, with a median follow-up of 924 days, miR-519e showed a significant ability in predicting disease progression as was measured by event-free survival defined by the rate of hospitalization, heart transplantation, stroke and cardiovascular death [18]. Differences observed in this study compared to the previous ones could, in part, be explained by sample source (whole blood vs serum/plasma) as explained later. For example, miR-519, miR-622, and miR-1228 were highly upregulated in CD15+ granulocytes, possible inflammatory contributors to occurrence and progression of HF. The mechanisms leading to alterations in miRNA expression remain unknown, as the expression pattern of a large number of miRNAs is unknown. The eight-miRNA signature in this study could be involved in cardiac function, as was evident by a significant overexpression of MAPK signaling, cardiac muscle contraction, Notch pathway, and cardiac muscle fiber development.
Prognosis
A 2013 study on endothelial progenitor cells offered miR-126 and miR-508-5p as prognostic markers for Congestive Heart Failure (CHF) due to Ischemic Cardiomyopathy (ICM) and Non-Ischemic Cardiomyopathy (NICM), independently predictive of cardiovascular death and lower survival rates. In a study on 55 ICM, 51 NICM and 30 controls with a two-year follow-up period, miR-126 and miR-508-5p levels were independent prognosis markers of cardiovascular death (miR-126 for ICM: p-value = 0.003, HR 0.19; 95% CI 0.06–0.58, and miR-508-5p for NICM: p-value = 0.002; HR 2.292; 95% CI 1.37–3.84). Pathway enrichment studies revealed important angiogenesis regulators including PIK3R2, VEGFA, Glut1, and ARNT as targets of miR-126 and miR-508-5p. Abnormal neo-angiogenesis and endothelial function have been linked to prognosis of CHF patients and could be of prognostic and therapeutic value in CHF patients [19].
In a validation cohort of 711 acute HF (AHF) patients, miR-423-5p was a significant predictor of mortality (odds ratio (OR) 0.54 [0.36–0.82], p-value = 0.004) but failed to predict hospital readmission in a one-year follow-up period (OR 0.82 [0.47–1.42], p-value = 0.48) [20]. A test cohort of 294 patients with acute dyspnea (236 AHF, 58 non-AHF) and 44 patients with stable CHF had previously drawn strong correlations between admission levels of miR-423-5p and risk of rehospitalization (p-value = 0.0001). The discrepancy in results could be attributed to differences in patient population as the test cohort had aggravated HF and higher rehospitalization rates. However, a prospective observational cohort of 263 CHF patients found no association between miR-423-5p and HF hospitalization, cardiovascular mortality, cardiac transplantation, and left ventricular assist device implantation [21].
In a cohort of 100 AHF patients, additional decreases in plasma levels of seven miRNAs 48 hours after admission was associated with 180-day mortality and a further validation cohort established miR-18a-5p and miR-652-3p as independent prognostic markers [22].
In a 2017 study on 96 AHF patients, lower serum miR-30d levels were predictive of greater one year mortality (AUC 0.806) and elevated serum miR-30d levels were linked with lower mortality (p-value =0.001) [23]. Baselines levels of miR-30d had been previously linked to better cardiac remodeling response following Cardiac Resynchronization Therapy (CRT) possibly through protective anti-inflammatory properties as measured 6 months after CRT [24]. miR-30d gain of function through genetic, lentivirus or Agomir has been shown to increase function in cardiac cells, decrease fibrosis in myocardium and apoptosis in cardiomyocytes, whereas decreased miR-30d expression leads to left ventricular remodeling, fibrosis, and death of cardiomyocytes. miR-30d seems to reduce apoptosis by targeting mitogen-associated protein kinase 4 and hinders fibroblast proliferation/activation by targeting integrin a5 through paracrine signaling in the acute ischemic remodeling phase and is linked to fibrosis and inflammation in the chronic phase [25].
A 2019 study on 54 post-MI HF patients, 59 post-MI non-HF patients, and 59 healthy controls found significantly lower levels of circulating miR-150 level in the serum of post-MI HF group compared to the post-MI non-HF group (p-value < 0.001) with miR-150 levels showing close associations with LVEF one year post discharge (p-value < 0.001). Furthermore, miR-150 was better at predicting post-MI HF compared to BNP [AUC for BNP was 0.616 (95% CI 0.511-0.721, p-value = 0.034) and AUC for miR-150 was 0.764 (95% CI 0.674-0.855, p-value < 0.001)] while combining the two markers revealed the best results with an AUC of 0.807 (95% CI 0.727-0.886, p-value < 0.001) [26]. miR-150-5p levels were previously correlated to symptom severity, adverse tissue remodeling, worse clinical outcomes resulting from heart pump failure and rehospitalization due to worsening of symptoms [27].
miRNAs may possibly be strong predictors of cardiovascular-related re-hospitalization. For instance, let-7i-5p had a HR of 2.06 (95% CI 1.29-3.28) [28], miR-132 had a HR of 0.79, (95% CI 0.66–0.95, p-value = 0.01) [29] and miR-423-5p had an adjusted OR of 0.70 (95% CI 0.53-0.93, p-value = 0.01) [20]. Repeatedly measured levels of miR-1306-5p were correlated with all-cause mortality and HF rehospitalization in a prospective cohort of 496 AHF patients with a one-year follow-up period (HR 4.69, 95% CI 2.18–10.06), independent from NT-proBNP, however, miR-1306-5p failed to improve discriminatory value of NT-proBNP [30]. A large study of two independent cohorts (2203 total CHF patients) found that higher levels of miR-1254 and miR-1306-5p were significantly associated with risk of all-cause mortality and HF hospitalization (HR ranging from 1.11 to 1.21 per log increase and p-values 0.004 to 0.009). However, these miRNAs failed to add prognostic value to NT-proBNP [31].
Despite the increasing interest in miRNA markers of HF, up to now no miRNAs fulfill the requirements to act as a clinical marker, as demonstrated in a 2020 systematic review on 20 studies in HF patients and 72 differentially expressed miRNAs which found inadequate support for any of the studied miRNAs, with only 5 miRNAs being differentially expressed in at least two studies [32]. Another systematic review and meta-analysis on 10 studies concluded that miRNAs still lack superiority to BNP as markers of HF and among the studied miRNAs, only miRNA-423-5p was a potential valuable biomarker [33] (Total mixed miRNAs had a pooled sensitivity of 0.74 (95% CI 0.72 to 0.75), a pooled specificity of 0.69 (95% CI 0.67 to 0.71) and area under the summary receiver operating characteristic curves value (SROC) of 0.7991. Whereas, miRNA-423-5p had a pooled sensitivity of 0.81 (95% CI 0.76 to 0.85), a pooled specificity of 0.67 (95% CI 0.61 to 0.73), and SROC of 0.8600, compared to BNP (SROC 0.9291 as extracted from the same studies). The possible reasons for the noted variability in results are explored below in the Challenges section.
Challenges
Despite the advances made in recent years and numerous studies published in the field of miRNA as cardiac biomarkers, clinical use of miRNAs in this field remains elusive at least for the near future as the published results are often neither reproduced nor validated in further studies. The etiology of the observed incongruence can be traced back to a number of technical challenges that remain unsolved and need to be addressed before any miRNAs equate currently used cardiac markers. In this section, we explore the main challenges.
miRNA Sample Choice
The fraction of blood sample used for detecting miRNAs could be responsible for disparate results across studies as miRNAs are not similarly expressed in serum and plasma. A study on expression of six main CVD-associated miRNAs, reported variances in expression of all studied miRNAs in serum vs plasma [34]. Serum and plasma are processed via different procedures. In the process of coagulation, serum is acquired following fibrin clot formation, but plasma is acquired following the addition of an anticoagulant, thus they affect coagulation cascade in different ways. The varied amounts of miRNA in serum versus plasma can further be explained by quantitative PCR (qPCR) inhibition, hemolysis, platelet contamination, and rupture of white, and red blood cells. Activated platelets release microparticles containing miRNAs and complexes of miRNA-Argonaute 2, a gene regulatory protein which guides gene silencing [35]. The process of coagulation itself may release microparticle miRNAs from platelets and centrifuged plasma may still contain leftover platelets [34]. Considering the active role that platelets play in MI, special attention should be paid to sample standardization and analysis to avoid sample choice as a confounding factor in interpreting miRNA expression results.
microRNA Detection and Quantification
A number of methods are employed to detect and quantify miRNAs in circulation, including reverse transcription quantitative PCR (RT-qPCR), microarray, and Next-Generation Sequencing (NGS). One of the most widely used methods, RT-qPCR is highly sensitive and specific, highly automated, cost-efficient, quick and works well with small samples, making it the method of choice in miRNA studies [36]. The main limitations of this method are the inability to detect new miRNAs and relatively low throughput which limits the number of miRNAs analyzed [37,38]. In comparison, microarrays are high throughput with a large capacity for miRNA analysis in a day and are relatively cost-effective, but are not as sensitive, or specific with an inability to identify new miRNAs or to measure absolute miRNA levels. Microarrays also depend on large amounts of miRNA starting material, which is problematic in cases where only small amounts of miRNA are available, and the required pre-amplification prior to analysis might influence the true miRNA concentration. In contrast, NGS is a high throughput method with small starting material requirements and excellent sensitivity and accuracy [39], which is not limited by cross-hybridization or background noise. Prolonged processing time and high costs still hamper their widespread use. NGS is superior to other methods in its ability to detect novel and unknown miRNAs since it does not need known target miRNAs or distinct probes/primers. Other common laboratory methods such as northern blotting and in-situ hybridization are not exploited very commonly because they are limited by low throughput, low sensitivity and slow processing [37]. Although useful in research, miRNA quantification methods are still too time-consuming to be used in practice, an issue that needs to be addressed before a bench-to-bedside transition for miRNA markers becomes feasible [40]. In addition, the heterogeneity in detection and quantification methods may be responsible for some of the heterogeneity observed across studies.
miRNA Data Normalization
To accurately measure miRNA expression across various samples, normalization methods through reference genes are often employed. By ranking selected genes in terms of expression stability in a mathematical process, normalization decreases variance and helps increase reliability and reproducibility of the results. A number of normalization strategies exist, but none are universally agreed upon, which invariably leads to incomparable results across studies. It is shown that different normalization methods can lead to different results [41]. Relative or absolute quantification methods are employed in most studies, as discussed below.
Absolute quantification relies on associating PCR signals to a standard synthetic curve created by synthetic miRNAs but it is reliant on high quality inputs and is not able to quantify exact miRNA levels, which limits its widespread application. Therefore, most studies use some form of relative quantification, either to an exogenous or endogenous reference gene. Synthetic oligonucleotide miRNAs, such as Caenorhabditis elegans miRNAs cel-miR-39-3p, cel-miR-54-3p, and cel-miR-238-3p are added as exogenous controls prior to RNA isolation, helping to control for variances observed during extraction, amplification and reverse transcription of samples. This method faces a number of challenges; active RNases degrade miRNAs and therefore it is necessary to carefully monitor the timing of the addition of spike-ins to the samples [38]. Also, any sampling errors remain uncorrected and it is not possible to review and correct the quality of samples as may be caused by preparation, storage and collection of tissues and samples [42].
Several endogenous reference genes have been suggested for normalizing miRNA data, none of which are yet agreed upon as standard. Small non-coding RNAs including small nucleolar RNAs such as RNU6 and RNU6B and the ribosomal RNAs 5S and 18S are traditional choices but seem to be unstable and variable in serum/plasma and fail to correctly represent the original miRNA sample in regard to expression, processing and transcription style [42]. It seems that normalizing miRNAs with miRNA endogenous controls may yield better results compared to other reference genes, and in this regard miR-16, miR-93, combination of miR-221, miR-26a and miR-191 among others are employed [43], highlighting the heterogeneity observed in choosing a reference gene. In addition, choosing the reference genes does not follow any guidelines but instead seems to be based on previous research, researcher choice, or by software [41]. To date, a standard normalization method is not available yet. Creating standardized normalization processes instead of a single, widely accepted normalization method or reference gene may bring us closer to accurate comparison of study results. Any real comparison among various studies is dependent on universally accepted guidelines on normalization strategies, which is not achieved yet.
Extraction Method
Three main methods exist for extracting RNAs; phenol-based, column-based, and phenol and column-based. Many studies have compared these methods to determine the optimized method of extraction but inter-laboratory variations still exist among studies [44]. Isolation and quantification of miRNAs from serum and plasma is challenging. Isolation efficacy is reduced due to sample contamination with proteins/inhibitors and lipoproteins/RNA-binding proteins and other protein complexes confound the process of isolation and quantification. In addition, miRNA samples are small to begin with, and therefore prone to contamination with RNase, albumin, and globulins [45]. Choice of extraction method is based on starting sample volume, method of analysis, and number of miRNAs species, among other factors. Therefore, there is a lot of variability among studies, and depending on their goal makes a comparison among studies difficult [46].
Medication
In addition to the technical challenges discussed, several other factors influence the study results. For example, heparin [47], aspirin [48] and statins [49] are shown to alter the expression of circulating miRNA levels. Therefore, careful study design is required to ensure differences in miRNA expression are not due to the drug regimen of patients.
Conclusions
In the past decade, a growing number of miRNAs have shown potential as possible biomarkers for diagnosis and prognosis of cardiovascular diseases, notably for HF. MiRNA-423-5p may be of potential value in determining the prognosis of CHF patients. However, a large gap remains between study results and clinical application of miRNA biomarkers as the study results are often incongruous and not validated. The discrepancy observed among studies could in part be explained by technical challenges including non-standardized methods for choosing sample source of miRNAs, detection and quantification of miRNAs, normalization of miRNA data and method of extraction. Population-based differences such as medication regimen used by patients also need to be addressed. In addition, despite their success in diagnosis and prognosis of HF, miRNA markers still lag behind traditional markers such as cardiac troponins and none of the studied miRNAs has been validated to match current markers. Therefore, large-scale, standardized cohorts are needed to validate the results of current studies and truly unleash the potential benefits of miRNA markers.
Author Statements
Compet?ing Interests
The authors have no competing interests to declare.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Authorís Contributions
NaRD had the idea for the article, NaRD and NeRD performed the literature search, NaRD and MHF drafted the manuscript and MHF critically revised the work and created the 3D picture. All authors have approved the final article.
References
- Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004; 116: 281–97.
- Ambros V. The functions of animal microRNAs. Nature. 2004; 431: 350–5.
- Pasquinelli AE, Reinhart BJ, Slack F, Martindale MQ, Kuroda MI, Maller B, et al. Conservation of the sequence and temporal expression of let-7 heterochronic regulatory RNA. Nature. 2000; 408: 86–9.
- Lee Y, Kim M, Han J, Yeom K-H, Lee S, Baek SH, et al. MicroRNA genes are transcribed by RNA polymerase II. EMBO J. 2004; 23: 4051–60.
- Yi R, Qin Y, Macara IG, Cullen BR. Exportin-5 mediates the nuclear export of pre-microRNAs and short hairpin RNAs. Genes Dev. 2003; 17: 3011–6.
- Okamura K, Ishizuka A, Siomi H, Siomi MC. Distinct roles for Argonaute proteins in small RNA-directed RNA cleavage pathways. Genes Dev. 2004; 18: 1655–66.
- Tian J, An X, Niu L. Role of microRNAs in cardiac development and disease. Exp Ther Med. 2017; 13: 3–8.
- Çakmak HA, Demir M. MicroRNA and Cardiovascular Diseases. Balkan Med J. 2020; 37: 60–71.
- Siasos G, Bletsa E, Stampouloglou PK, Oikonomou E, Tsigkou V, Paschou SA, et al. MicroRNAs in cardiovascular disease. Hellenic J Cardiol. 2020; 61: 165–73.
- Mitchell PS, Parkin RK, Kroh EM, Fritz BR, Wyman SK, Pogosova-Agadjanyan EL, et al. Circulating microRNAs as stable blood-based markers for cancer detection. Proc Natl Acad Sci USA. 2008; 105: 10513–8.
- Sohel MH. Extracellular/circulating microRNAs: release mechanisms, functions and challenges. Achievements in the Life Sciences. 2016: 10.
- Ibrahim NE, Januzzi JL Jr. Established and Emerging Roles of Biomarkers in Heart Failure. Circ Res. 2018; 123: 614–29.
- Wang H, Cai J. The role of microRNAs in heart failure. Biochim Biophys Acta Mol Basis Dis. 2017; 1863: 2019–30.
- Tijsen AJ, Creemers EE, Moerland PD, de Windt LJ, van der Wal AC, Kok WE, et al. MiR423-5p as a circulating biomarker for heart failure. Circ Res. 2010; 106: 1035–9.
- Goren Y, Kushnir M, Zafrir B, Tabak S, Lewis BS, Amir O. Serum levels of microRNAs in patients with heart failure. Eur J Heart Fail. 2012; 14: 147–54.
- Bauters C, Kumarswamy R, Holzmann A, Bretthauer J, Anker SD, Pinet F, et al. Circulating miR-133a and miR-423-5p fail as biomarkers for left ventricular remodeling after myocardial infarction. Int J Cardiol. 2013; 168: 1837–40.
- Ellis KL, Cameron VA, Troughton RW, Frampton CM, Ellmers LJ, Richards AM. Circulating microRNAs as candidate markers to distinguish heart failure in breathless patients. Eur J Heart Fail. 2013; 15: 1138–47.
- Vogel B, Keller A, Frese KS, Leidinger P, Sedaghat-Hamedani F, Kayvanpour E, et al. Multivariate miRNA signatures as biomarkers for non-ischaemic systolic heart failure. Eur Heart J. 2013; 34: 2812–22.
- Qiang L, Hong L, Ningfu W, Huaihong C, Jing W. Expression of miR-126 and miR-508-5p in endothelial progenitor cells is associated with the prognosis of chronic heart failure patients. Int J Cardiol. 2013; 168: 2082–8.
- Seronde M-F, Vausort M, Gayat E, Goretti E, Ng LL, Squire IB, et al. Circulating microRNAs and Outcome in Patients with Acute Heart Failure. PLoS One. 2015; 10: e0142237.
- van Boven N, Akkerhuis KM, Anroedh SS, Rizopoulos D, Pinto Y, Battes LC, et al. Serially measured circulating miR-22-3p is a biomarker for adverse clinical outcome in patients with chronic heart failure: The Bio-SHiFT study. Int J Cardiol. 2017; 235: 124–32.
- Ovchinnikova ES, Schmitter D, Vegter EL, Ter Maaten JM, Valente MAE, Liu LCY, et al. Signature of circulating microRNAs in patients with acute heart failure. Eur J Heart Fail. 2016; 18: 414–23.
- Xiao J, Gao R, Bei Y, Zhou Q, Zhou Y, Zhang H, et al. Circulating miR-30d Predicts Survival in Patients with Acute Heart Failure. Cell Physiol Biochem. 2017; 41: 865–74.
- Melman YF, Shah R, Danielson K, Xiao J, Simonson B, Barth A, et al. Circulating MicroRNA-30d Is Associated With Response to Cardiac Resynchronization Therapy in Heart Failure and Regulates Cardiomyocyte Apoptosis: A Translational Pilot Study. Circulation. 2015; 131: 2202–16.
- Li J, Salvador AM, Li G, Valkov N, Ziegler O, Yeri A, et al. Mir-30d Regulates Cardiac Remodeling by Intracellular and Paracrine Signaling. Circ Res. 2021; 128: e1–23.
- Lin X, Zhang S, Huo Z. Serum Circulating miR-150 is a Predictor of Post-Acute Myocardial Infarction Heart Failure. Int Heart J. 2019; 60: 280–6.
- Scrutinio D, Conserva F, Passantino A, Iacoviello M, Lagioia R, Gesualdo L. Circulating microRNA-150-5p as a novel biomarker for advanced heart failure: A genome-wide prospective study. J Heart Lung Transplant. 2017; 36: 616–24.
- Vegter EL, Ovchinnikova ES, van Veldhuisen DJ, Jaarsma T, Berezikov E, van der Meer P, et al. Low circulating microRNA levels in heart failure patients are associated with atherosclerotic disease and cardiovascular-related rehospitalizations. Clin Res Cardiol. 2017; 106: 598–609.
- Masson S, Batkai S, Beermann J, Bär C, Pfanne A, Thum S, et al. Circulating microRNA-132 levels improve risk prediction for heart failure hospitalization in patients with chronic heart failure. Eur J Heart Fail. 2018; 20: 78–85.
- van Boven N, Kardys I, van Vark LC, Akkerhuis KM, de Ronde MWJ, Khan MAF, et al. Serially measured circulating microRNAs and adverse clinical outcomes in patients with acute heart failure. Eur J Heart Fail. 2018; 20: 89–96.
- Bayés-Genis A, Lanfear DE, de Ronde MWJ, Lupón J, Leenders JJ, Liu Z, et al. Prognostic value of circulating microRNAs on heart failure-related morbidity and mortality in two large diverse cohorts of general heart failure patients. Eur J Heart Fail. 2018; 20: 67–75.
- Peterlin A, Pocivavšek K, Petrovic D, Peterlin B. The Role of microRNAs in Heart Failure: A Systematic Review. Front Cardiovasc Med. 2020; 7: 161.
- Yan H, Ma F, Zhang Y, Wang C, Qiu D, Zhou K, et al. miRNAs as biomarkers for diagnosis of heart failure: A systematic review and meta-analysis. Medicine. 2017; 96: e6825.
- Mompeón A, Ortega-Paz L, Vidal-Gómez X, Costa TJ, Pérez-Cremades D, Garcia-Blas S, et al. Disparate miRNA expression in serum and plasma of patients with acute myocardial infarction: a systematic and paired comparative analysis. Sci Rep. 2020; 10: 5373.
- Laffont B, Corduan A, Plé H, Duchez A-C, Cloutier N, Boilard E, et al. Activated platelets can deliver mRNA regulatory Ago2•microRNA complexes to endothelial cells via microparticles. Blood. 2013; 122: 253–61.
- Faraldi M, Gomarasca M, Banfi G, Lombardi G. Chapter Four - Free Circulating miRNAs Measurement in Clinical Settings: The Still Unsolved Issue of the Normalization. In: Makowski GS, editor. Advances in Clinical Chemistry. Elsevier. 2018; 87: 113–39.
- Dave VP, Ngo TA, Pernestig A-K, Tilevik D, Kant K, Nguyen T, et al. MicroRNA amplification and detection technologies: opportunities and challenges for point of care diagnostics. Lab Invest. 2019; 99: 452–69.
- Felekkis K, Papaneophytou C. Challenges in Using Circulating Micro-RNAs as Biomarkers for Cardiovascular Diseases. Int J Mol Sci. 2020; 21: 561.
- Ouyang T, Liu Z, Han Z, Ge Q. MicroRNA Detection Specificity: Recent Advances and Future Perspective. Anal Chem. 2019; 91: 3179–86.
- Paiva S, Agbulut O. MiRroring the Multiple Potentials of MicroRNAs in Acute Myocardial Infarction. Front Cardiovasc Med. 2017; 4: 73.
- Faraldi M, Gomarasca M, Sansoni V, Perego S, Banfi G, Lombardi G. Normalization strategies differently affect circulating miRNA profile associated with the training status. Sci Rep. 2019; 9: 1584.
- Schwarzenbach H, da Silva AM, Calin G, Pantel K. Data Normalization Strategies for MicroRNA Quantification. Clin Chem. 2015; 61: 1333–42.
- Donati S, Ciuffi S, Brandi ML. Human Circulating miRNAs Real-time qRT-PCR-based Analysis: An Overview of Endogenous Reference Genes Used for Data Normalization. Int J Mol Sci. 2019; 20: 4353.
- El-Khoury V, Pierson S, Kaoma T, Bernardin F, Berchem G. Assessing cellular and circulating miRNA recovery: the impact of the RNA isolation method and the quantity of input material. Sci Rep. 2016; 6: 19529.
- Wright K, de Silva K, Purdie AC, Plain KM. Comparison of methods for miRNA isolation and quantification from ovine plasma. Sci Rep. 2020; 10: 825.
- Moldovan L, Batte KE, Trgovcich J, Wisler J, Marsh CB, Piper M. Methodological challenges in utilizing miRNAs as circulating biomarkers. J Cell Mol Med. 2014; 18: 371–90.
- Boeckel J-N, Thomé CE, Leistner D, Zeiher AM, Fichtlscherer S, Dimmeler S. Heparin selectively affects the quantification of microRNAs in human blood samples. Clin Chem. 2013; 59: 1125–7.
- de Boer HC, van Solingen C, Prins J, Duijs JMGJ, Huisman MV, Rabelink TJ, et al. Aspirin treatment hampers the use of plasma microRNA-126 as a biomarker for the progression of vascular disease. Eur Heart J. 2013; 34: 3451–7.
- Li J, Chen H, Ren J, Song J, Zhang F, Zhang J, et al. Effects of statin on circulating microRNAome and predicted function regulatory network in patients with unstable angina. BMC Med Genomics. 2015; 8: 12.