
Research Article
Austin J Biotechnol Bioeng. 2024; 11(2): 1134.
N-acetyl-L Tryptophan Provides Protection Against Gamma Radiation-Induced Inflammation and Apoptosis in the Irradiated IEC-6 Cells and Mouse Intestine via Neurokinin-1 Receptor/Substance P Expression Inhibition
Pratibha Kumari; Ravi Kumar; Neelanshu Gaurav; Vimal Kumar; Shravan Kumar Singh; Raj Kumar*
Department of Radiation Biotechnology, Institute of Nuclear Medicine and Allied Sciences, Delhi-110054, India
*Corresponding author: Dr Raj Kumar Scientist-F, Head, Department of Radiation Biotechnology, Institute of Nuclear Medicine and Allied Sciences (DRDO), Brig. S.K. Mazumdar Road, Timarpur, Delhi-110054, India. Email: rajkumar790@yahoo.com
Received: June 13, 2024 Accepted: July 09, 2024 Published: July 16, 2024
Abstract
Radiation stimulates the neurokinin-1 receptor (NK-1R) via its agonist Substance P (SP) and elicits a cascade of inflammatory events that ultimately culminate in apoptotic cell death. Therefore, the present study was performed to find out the effects of NK-1R antagonist CP99994 and N-Acetyl-L-Tryptophan (L-NAT) on inflammatory and apoptotic pathway inhibition in irradiated IEC-6 cells and mice using cytotoxicity and proliferation assays. Radiation induced cellular Ca2+ accumulation and its modulation by L-NAT pre-treatment was quantified using flow cytometry. Gastrointestinal system radioprotection offered by L-NAT pre-treatment in irradiated mice was evaluated in terms of intestinal histology, NK-1R, substance P, and pro-inflammatory cyokines expression analysis in the jejunum section of irradiated mice. Pre-treatment with either L-NAT (-30 min) or CP99994 on irradiated IEC-6 cells provides significant protection (~80%) against radiation induced cell death. Furthermore, radiation induced over-expression of pro-apoptotic proteins, i.e., Apaf-1, cytochrome C, caspase-9, and NFkB, was found to be down-regulated upon L-NAT-pretreatment. Conversely, L-NAT pre-treatment of irradiated cells was observed to enhance pro-survival proteins like pERK1/2, pAKT, and Bcl-2. Interestingly, radiation-induced NK-1R, substance P, and other pro-inflammatory cytokine expressions such as NFkB, IL-6, IL-10, TGF-β1, IL-4, were also found to be significantly regressed in the jejunum of the irradiated mice upon L-NAT pre-treatment. Conclusively, L-NAT provides radioprotection to IEC-6 cells and mice’s small intestines by modulating the NK-1R/substance P interaction, inhibiting pro-inflammatory cytokines, up-regulating pro-survival proteins, and preserving intestinal crypt stem cells against radiation-induced cell death.
Keywords: Radiotoxicity; Neurokinin-1 Receptor (NK-1R); N-Acetyl L-Tryptophan (L-NAT); Apoptosis; Intestinal epithelial cells (IEC-6); Inflammation; Radioprotection
Introduction
Radiation exposure, whether occupational or accidental, is known to elicit major health complications such as oxidative stress, DNA damage, protein oxidation, and cell death. The Gastrointestinal System (GI) is considered the second-most radiosensitive system after the hematopoietic system. Radiation exposure to the GI system induces intestinal epithelial damage and chronic inflammation [1]. Radiation exposure also reduced the number and quality of villi and crypt stem cells, impacted intestinal motility, leading to diarrhea, compromised nutrient absorption, and electrolyte imbalances that ultimately led to intestinal functional impairment. High radiation doses induce “Acute Gastrointestinal Syndrome (AGIS)”, which is characterized by apoptosis in the intestinal epithelium, intestinal hemorrhoids, sepsis, electrolyte imbalance, and fluid imbalance resulting in death [2]. Therefore, inhibition of apoptosis and inflammation in irradiated intestinal cells is essential for recovery and survival against radiation-induced AGIS. Intestinal epithelial cells are bound to renew within 3-5 days [3] via intestinal crypt stem cell’s continuous division and differentiation.
The gastrointestinal system has mutual communication with the brain via the CNS and ENS. Brain-gut signaling controls gut motility, gastric secretion, and immune function regulation [4]. Furthermore, radiation-induced Substance P (SP) and Neurokinin-1 receptor (NK-1R) expressions affect nociceptive perception and visceral hypersensitivity [5]. Substance P is an excitatory neuropeptide that interacts with NK-1R in the gut's enteric nervous system [6]. Radiation-induced SP/NK-1R interaction induces an inflammatory and emesis response that makes the intestine more radiosensitive [7]. It is important to mention here that radiation induced emesis (i.e., the prominent qualitative signature of radiation overexposure) involved SP/NK-1R interaction. Radiation exposure initiates the GI system’s inflammation via activations of pro-inflammatory cytokine expressions such as IL-6, IL-12, NFkB, and TNFa [8,9], also linked with SP/NK-1R interactions. Besides cytokines, some chemokines like ICAM-1, MCP-1, MIP-2, and MIP-1a are reported to play a significant role in radiation mediated intestinal inflammation [10]. Intestinal crypt stem cells’ markers expressions are the prime indicator of the recovery of intestinal epithelial injury in an irradiated intestine. Intestinal stem cell markers, i.e., Lgr5, act as front-line indicators, while Bmi1, Msi1, and Dclk1 also act as reserve or quiescent crypt stem cell markers that contribute to maintaining intestinal crypt homeostasis [11]. Therefore, radiation induced intestinal toxicity can be minimized by modulating intestinal crypt stem cell marker’s expression using potential drug candidates. Although, numerous reports have been documented on diverse strategies to protect or minimize intestinal radiation injuries [12,13]. GI specific radioprotective drugs have yet to be identified. Interestingly, in previous studies, we have reported radioprotective activities of N-Acetyl L-Tryptophan (L-NAT) against radiation induced toxicity in IEC-6 and Neuro2A cells by reducing radiation-mediated oxidative stress, maintaining mitochondrial membrane hyperpolarization, and inhibiting radiation-induced apoptosis [14,15]. In the present study, the underlying mechanism of L-NAT-mediated radioprotection involving substance P/NK-1R expression inhibition and subsequent inflammatory and apoptotic response down-regulation was studied using murine Intestinal Epithelial Cells (IEC-6) and C57BL/6 mice.
Materials and Methods
Ingredients
Phosphate- Buffered Saline (PBS) was procured from Calbiochem, Merck India Pvt. Ltd., Mumbai, India. Dulbecco Modified Eagle Medium with high glucose, fetal bovine serum, NBT/BCIP solution, and N-acetyl L-tryptophan, Radioimmune Precipitation Assay (RIPA) lysis buffer, Bradford reagent, Apaf-1 (MAB3504), and GAPDH (SAB2108668) monoclonal antibodies were purchased from Sigma-Aldrich, St Louis, MO. 3-(4,5-dimethyl-2-yl)-2,5-diphenyl-2II-Tetrazolium bromide (MTT), and Penicillin-Streptomycin solution (5000 U/mL) were procured from Himedia Laboratory Pvt. Ltd., Mumbai, India. All ELISA kits were bought from the Bioassay Technology Laboratory (Shanghai, China). The primary antibodies for NF-kB p65 antibody (PA5-16545), p-AKT (700392), p-ERK1/2 (700012), ERK1/2 (136200), and Alkaline Phosphatase (AP)-tagged goat anti-rabbit or anti-mouse secondary antibody and Fluo-4 AM (F14201) were procured from Thermo Fischer, Invitrogen (MA, USA). All other chemicals used were of analytical grade.
Cell Culture, Drug Treatment, and Irradiation
The IEC-6 cell line originates from normal rat intestinal crypt cells and was acquired from the National Centre for Cell Science (NCCS) in Pune, India. These cells were maintained in a DMEM culture medium containing 4 g/L glucose. The medium was enriched with 10% Fetal Bovine Serum (FBS), 2 mM L-glutamine, and 1.5 g/L sodium bicarbonate (NaHCO3). The culture medium containing antibiotics, particularly 50 IU/mL of penicillin and 50 μg/mL of streptomycin, was combined with IEC-6 cells. The cell-contained media was subsequently incubated in a humidified CO2 incubator with a CO2 concentration of 5% at an ambient temperature of 37°C. The IEC-6 cells that underwent exponential growth were subjected to pre-treatment with L-NAT (0.1μg/mL) for 1 hour before getting exposed to radiation. A radiation treatment was performed utilizing a GC-5000 60Co radiation source with a dose rate of 0.374 kGy/h (6.2 Gy/min) at a temperature of 25 °C. The irradiation doses used were 20 Gy or 5 Gy, which were estimated to be the LD50 dose [14].
Evaluation of Neurokinin-1 Receptor (NK-1R) Involvement in Radioprotection offered by L-NAT using the MTT assay
Exponentially growing cells were divided into the following experimental groups:
Gp 1: Control: Untreated Intestinal IEC-6 Cells
Gp 2: IEC-6 cells irradiated with gamma radiation (20 Gy)
Gp 3: IEC-6 cells treated with L-NAT (0.1 μg/mL)
Gp 4: Irradiated (20 Gy) IEC-6 cells that were pretreated (-1 hr) with L-NAT (0.1μg/mL).
Gp 5: Irradiated (20 Gy) intestinal IEC-6 cells that were pretreated with the NK-1R antagonist CP99994 (20 nM)
Gp 6: Intestinal IEC-6 cells were treated first with L-NAT (0.1μg/mL), then with the NK-1R antagonist CP99994 (20 nM; +30 minutes), and then irradiated with gamma radiation (20 Gy). Corresponding controls with L-NAT and CP99994 treatment but without irradiation were also used.
Gp 7: Intestinal IEC-6 cells were initially treated with CP99994 (20 nM), followed by (+30 minutes) with L-NAT (0.1μg/mL), and then exposed to gamma radiation (20 Gy). Corresponding control with NK-1R antagonist CP99994 and L-NAT treatment but without irradiation was also used.
The IEC-6 cells were subjected to incubation at 37°C for 48 hours with a 5% CO2 concentration after each treatment, as previously outlined. 10 μl of MTT solution with a concentration of 5 mg/mL was added to the 96 wells containing the cell culture, followed by an additional incubation period of 3 hours at 37°C. Following incubation, formazan crystals of purple color in the wells were dissolved with 100 μl of DMSO. The UV/visible microplate spectrophotometer (Biotek, Gene5 software; Powerwave XS2) was employed to analyze the purple-colored solution at a dual wavelength of 570–670 nm. The estimation of metabolic activity was conducted through the implementation of the following formula: [Absorbance (treatment)/Absorbance (no treatment)] × 100.
Evaluation of the Role of the Neurokinin-1 Receptor (NK1R) in the Radioprotection offered by L-NAT using a Clonogenic assay
Exponentially growing IEC-6 cells were divided into six experimental groups, as mentioned above (except Group 3). In brief, a total of 2 × 102 cells were seeded onto 35 mm culture dishes and incubated at 37°C with 5% CO2 for an overnight period to promote adherence. Subsequent to the previously stated treatment, the cells were subjected to a lethal dose (LD50) of 5 Gy of γ-radiation. The cells were then permitted to proliferate and develop colonies for a duration of 10 days at a temperature of 37°C with a CO2 concentration of 5%. After a period of 10 days of incubation, colonies were treated with 70% chilled ethanol and subsequently stained with 2% crystal violet [14].
Determination of Intracellular Calcium level in L-NAT-Pretreated and Irradiated IEC-6 cells
Radiation-mediated calcium release was determined by flow cytometry (BD-Pharmingen, Arya III, USA). Cells were categorized into four experimental groups: i. Control; untreated cells; ii. Irradiated cells (20 Gy); iii. Irradiated cells that were pre-treated with L-NAT (0.1μg/mL); iv. Cells treated with L-NAT (0.1μg/mL) only. Subsequent to each treatment, cells received an incubation of 5 M Fluo-4 AM in Hank’s balanced salt solution (HBSS) for 45 M at 37°C in the dark for 24-48 h. Cells were then washed twice with calcium-free HBSS buffer, and intracellular calcium levels were estimated. The fluorescence intensity of the Fluo-4 AM-bound cells was measured at a wavelength of 494/506 nm (excitation/emission).
Radiation Induced Ubiquitination and Mitochondrial Complex V Functional Activity Estimation using an ELISA assay
Radiation-driven ubiquitination and mitochondrial complex V functional activity were determined by using polyubiquitin B and mitochondrial ATP F (0) complex subunit B1 Elisa kits (BT Laboratory, Shanghai, China). Briefly, followed by all treatments (as mentioned above), proteins were collected and estimated [15]. An equal amount of protein was added to Elisa plate wells pre-coated with polyubiquitin B and mitochondrial ATP F(0) complex subunit B1 antibodies. Following incubation, streptavidin-HRP conjugated secondary antibodies were added to the wells and further incubated at 37°C for 60 min. Following the washing, Substrate A and subsequently Substrate B were added to the wells and again incubated for 10 minutes in the dark at 37°C. The blue color developed and turned yellow upon adding the stop solution. The Optical Density (OD) was measured at a wavelength of 450 nm using an ELISA microplate reader.
Radiation-Induced Apoptotic Protein Expression Analysis
Following experimental treatments (as mentioned above), IEC-6 cells were harvested using the RIPA method [16]. The protein from the confluent IEC-6 cell monolayer was obtained using the RIPA method, with subsequent gentle agitation at 10-minute intervals for 1 hour at 4°C. The cell supernatants were collected through centrifugation at 13,500 × g for 30 min. The cellular protein fraction was obtained, and the protein content was determined using the Bradford method. The protein samples underwent size fractionation using 12% SDS-polyacrylamide gels. Subsequently, they were electro-transferred to a Nitrocellulose membrane (NC) at 60 V for 1 hour and 30 minutes at a temperature of 4 °C. The protein on the membrane was immobilized using TBS-T (composed of 50 mM Tris-HCl pH 7.4, 200 mM NaCl, 0.5% Tween-20, and 5% BSA) for a duration of 2 h at the normal temperature. Subsequently, the membrane was subjected to incubation with primary antibodies targeting p-ERK1/2 (1:1000), ERK1/2 (1:1000), p-Akt (1:1000), PI3K p85 (1:1000), NF-κB (1:1000), Apaf-1 (1:1000), Cytochrome C (1:1000), Bcl-2 (1:1000), and GAPDH (1:1000) at a temperature of 4°C for an entire night. Subsequently, the membrane underwent a minimum of three washes lasting 5 minutes each with TBS-T. Following this, the membrane was subjected to incubation with either alkaline phosphatase-conjugated anti-mouse or anti-rabbit secondary antibody (diluted at a ratio of 1:3000, Invitrogen, USA) for a duration of 2 h at room temperature. The membranes were subsequently observed utilizing the NBT/BCIP substrate, which is a colorimetric technique. The images were acquired using the HR55000 imaging system (Gene Genome, United States). The quantification of the bands was conducted through densitometric analysis using Image J software v1.44p (NIH) in Bethesda, MD.
Unrevealing the Gastro-Intestinal System Radioprotective Activity of L-NAT in a Mouse Model
Approximately 8–10-week-old C57BL/6 strain (male) mice were issued from the Institutional Experimental Animal Facility after IAEC approval. Mice were randomly divided into the following four experimental groups:
Gp. 1 Control (n = 6) untreated mice
Gp. 2 Irradiated mice (n = 6) received whole body irradiation (9 Gy)
Gp. 3 L-NAT treated mice (n=6; 150mg/kg.by.im)
Gp. 4 Irradiated (9Gy; whole body) mice that were pre-treated with L-NAT (n = 6; 150 mg/kg.b.wt, im)
Mice were irradiated using 60Co irradiator (LDI-2000 gamma chamber; Board of Radiation and Isotope Technology, Government of India, Department of Atomic Energy) at a dose rate of 1.723 Gy/min. All studies were conducted in compliance with the Institutional Animal Ethics Committee's (IAEC) standards and regulations. The GI system radioprotective activity of L-NAT was evaluated in terms of radiation-induced intestinal cellular damage and recovery analysis in L-NAT pre-treated mice using histological and intestinal crypt stem cell marker expression analysis.
Histological Analysis of the Jejunum Section of the Intestine of Irradiated and L-NAT-Pretreated Mice
Following euthanasia, the jejunum sections of the irradiated and L-NAT-pretreated mice were collected at different time intervals. The fat and connective tissues attached to the jejunum section were carefully scraped out. Intestinal tissue was fixed in buffered paraformaldehyde at room temperature for 24 hours. Subsequently, the tissue specimens underwent dehydration using a series of graded ethanol and were then embedded in paraffin blocks. For H&E staining, 5-m-thick sections were prepared. Digital images of the stained sections were captured and subjected to analysis using NIS element software integrated with an automated microscope (Nikon Ti series, Japan).
Immunohistochemical Analysis of the Jejunum Section of the Intestine of Irradiated and L-NAT-Pretreated Mice
The histological sections were deparaffinized with xylene twice, for 10 minutes each. The sections were then immersed in a 1:1 solution of xylene and ethanol. Rehydrate the sections in 100% ethanol for 3 minutes, and then in 70% ethanol for 10 minutes before washing with water. The slides are then immersed in antigen retrieval buffer (10 mM). Sodium citrate, 0.05 percent Tween 20, pH 6.0) for 45 minutes at 60°C. The slides were blocked with 1% BSA for 2 hours at room temperature. After that, the slides were incubated overnight in a moist chamber at 4°C. The primary antibodies for KN-1R (TACR1; ZN003, Thermo Fisher, USA) were prepared in 1% BSA in PBS. The slides are then washed with PBST for 5 minutes each time. After that, the slides are incubated in 0.3% hydrogen peroxide for 15 minutes. In a moist environment, HRP-conjugated secondary antibodies were applied for 1 hour at room temperature, followed by washing with 0.35% PBST three times for 5 minutes each. Slides were incubated in the presence of DAB for 10 minutes, and then washed gently at room temperature. The slides are then counterstained with hematoxylin, dehydrated with a graded ethanol solution, and examined under a bright field microscope.
Expression Analysis of Inflammatory Cytokines, Chemokines Markers using ELISA assays
Tissue lysate was prepared by homogenizing 100 mg of jejunum tissue with RIPA buffer to extract total soluble proteins [17]. To determine the cytokines (TGF-β1, IL-10, IL-6, IL-4), inflammatory marker (NFkB), chemokines (ICAM-1, MCP-1, MIP-1a, MIP-2), and NK-1R and Substance P expressions, commercial ELISA kits procured from Fine Test, Wuhan, China, and ELK Biotek, USA, were used. The individual protein’s expression analysis was carried out by following the instructions and protocols mentioned in the operational manual of the respective kits.
Statistical Analysis
Three replicates of each experiment were conducted, and the data was expressed as means ± SD. To test for statistically significant differences between these groups, Prism 8.0.1 (GraphPad Software, San Diego, CA, USA) was employed and performed a one-way ANOVA for the MTT and clonogenic assay and a two-way ANOVA with Tukey's test for other parameters. It was considered that a P value (=0.05) indicated statistically significant variation between treatment groups.
Results
The NK-1R Antagonistic Effect of CP99994 and L-NAT Provides Protection to IEC-6 cells Against Radiation-Induced cell Death
To investigate whether NK-1R is involved in radioprotection, a cell viability and proliferation (MTT) and clonogenic (CFU) assays were performed. MTT assay revealed that pre-treatment with NK-1R antagonist CP99994 (20 nM) in irradiated (20 Gy) IEC-6 cells significantly improved (82.5±3.3%) cell survival as compared to irradiated cells (49.2±2.8%) that were not pre-treated with CP99994 (20 nM) (Figure 1A). Similarly, irradiated cells (5 Gy) pretreated with CP99994 (20 nM) showed a significant (p<0.05) increase in Colony Forming Unit (CFU) as compared to only irradiated cells that not pretreated with CP99994 (20 nM) (Figure 1B, C). Interestingly, pre-treatment with L-NAT (0.01μg/ml) alone or along with CP99994 (20nM) on the irradiated IEC-6 cells showed a significant improvement in the cell survivability, i.e., 79.2±7.4% and 80.9±5.5%, respectively, as compared to irradiated cells (Figure 1A).
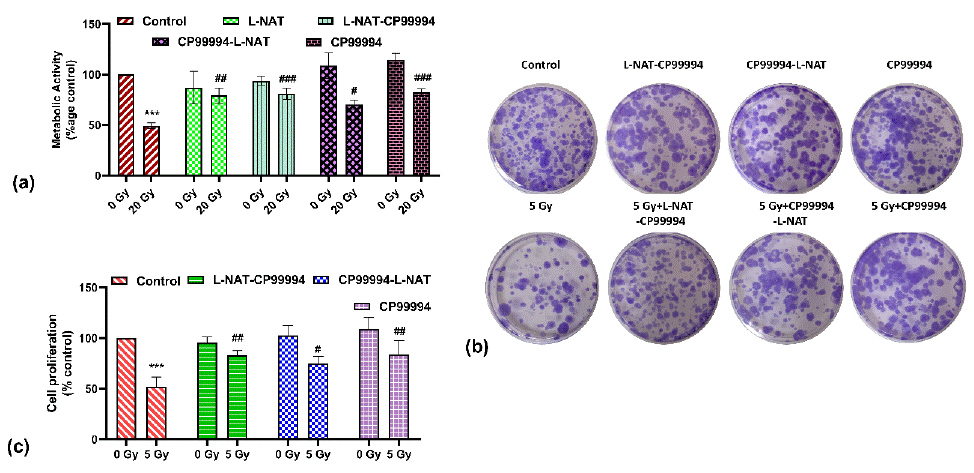
Figure 1: Determination of radioprotective activity of N-acetyl- L-tryptophan (L-NAT) and Neurokinin-1 receptor (NK-1R) antagonist CP99994 in IEC-6 cells. Metabolic survival was estimated using MTT assay (A), while, reproductive cell survival evaluated using clonogenic assay (B and C) using Neurokinin-1 receptor (NK-1R) antagonist CP99994. Results were expressed as mean±SD of three separate experiments. Significance of variance was denoted as: ***p=0.001 Vs Control; and #p=0.05, ##p=0.01, ###p=0.001 Vs Radiation treatment (20 Gy for MTT assay or 5 Gy for CFU assay).
Similar observations were found with the clonogenic assay (Figure 1B, C). These findings strongly suggested that CP99994 and L-NAT inhibits substance P/NK-1 receptor system inhibition, thus may providing significant protection against gamma radiation via modulating subsequent down-stream signaling.
L-NAT Pretreatment Reduces Intracellular Ca2+ Accumulation, Maintains ATP Synthase Activities, and helps in Oxidized Protein Degradation in Irradiated IEC-6 Cells
A significant increase (~2 fold; p<0.05) in intracellular calcium was noticed in irradiated cells at 1–24 h as compared to untreated controls. But a maximum increase in calcium level was noticed with irradiated cells at 48 h as compared to control (Figure 2A, B). Nevertheless, when analyzing the impact of L-NAT pre-treatment on irradiated cells, a noteworthy decrease in intracellular calcium levels was observed (p<0.05) compared to only irradiated cells at 1-48 hours (Figure 2A, B). Furthermore, intracellular calcium level, and ATP F (0) complex subunit B1 activity was found to be decreased significantly (p<0.05) in irradiated cells as compared to untreated control (Figure 2C) at 1-48 hrs. However, L-NAT pre-treatment to irradiated cells was found to enhance ATP synthase F(0) complex subunit B1 activity (Figure 2C) as compared to irradiated cells. These findings were suggesting a link between higher intracellular calcium and lower ATP production in the mitochondria of irradiated IEC-6 cells (Figure 2C) that not pretreated with L-NAT.
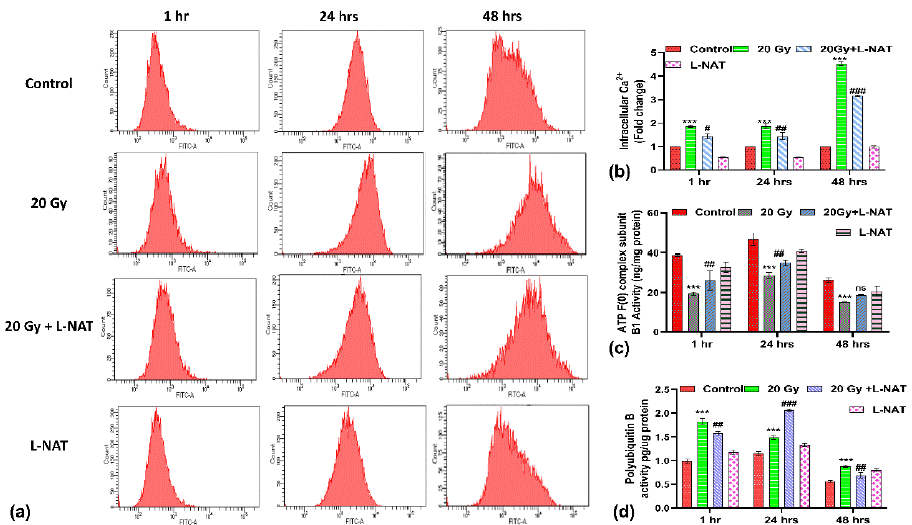
Figure 2: Effect of L-NAT pre-treatment on intracellular Calcium accumulation, protein ubiquitination and mitochondrial ATP F(0) complex functioning in irradiated IEC-6 cells. Flow cytometric analysis (representative histogram) of IEC-6 cells pretreated with L-NAT and gamma radiation (20 Gy) was done using Fluo 4 AM probed (A). Quantification of intracellular calcium was carried out by measuring mean fluorescence intensity (B). Measurement of the mitochondrial complex F(0) proteins (C) and Polyubiquitin B (D) activities was measured in irradiated IEC-6 cells pretreated with L-NAT using ELISA assay. The data were represented as mean ± SD of three independent experiments. Significance of variance was denoted as: ***p =0.001 vs Control; and #p = 0.001, ##p=0.01, ###p=0.001 Vs Radiation treatment (20Gy).
Surprisingly, a noticeable increase in polyubiquitin B activity was reported in irradiated cells pre-treated with L-NAT at 24 h in comparison with irradiated cells (Figure 2D), which was almost subsidized at 48 h. These observations may support the view that L-NAT pre-treatment probably accelerates the degradation of oxidized proteins from the cellular milieu within 24 h of post-radiation exposure.
L-NAT Pre-Treatment of IEC-6 Cells Inhibits Radiation Induced Apoptosis by Modulating apoptosis Regulating protein Expression
To determine the anti-apoptotic activities of L-NAT in irradiated IEC-6 cells, western blot analysis was performed. Observations obtained demonstrated a significant (p<0.05) increase (~ 3-fold) in phosphorylated ERK1/2 (pERK1/2) expression in irradiated cells that were pretreated with L-NAT as compared to only irradiated cells (Figure 3A, B) at 48 h. Though no significant modulation in non-phosphorylated ERK1/2 expression was evident with any treatment group (Figure 3C, D). Similarly, significant (p<0.05) induction (~ 3.5-fold) in phosphorylated AKT (pAKT) expression was observed with L-NAT-pretreated plus irradiated group of cells as compared to only irradiated cells that were not pretreated with L-NAT at 24-48 h (Figure 3E). Furthermore, observations also indicated a significant (p<0.05; ~ 3-fold) increase in NFkB expression with irradiated IEC-6 cells as compared to untreated control cells (Figure 3F) at 24-48 h. In contrast, a significant decrease in NFkB expression was noticed in the irradiated cells that were pre-treated with L-NAT as compared to the irradiated cells that were not pre-treated with L-NAT (Figure 3F). Moreover, remarkable induction in Apaf-1, cytochrome C, and caspase-9 expression, along with a significant reduction in Bcl-2 expression, was observed in irradiated (20 Gy) cells as compared to untreated control (Figure 4) at 24-48 h. Conversely, significant inhibition in Apaf-1 (p<0.01), cytochrome C (p<0.001), and caspase-9 (p<0.01) expressions, along with induction in Bcl-2 (p<0.01) expressions, were observed in the irradiated cells that were pre-treated with L-NAT as compared to only irradiated cells at 24-48 h. Taken altogether, the present findings suggest that radiation induced calcium accumulation in the cells leads to disturbed mitochondrial functions and cell death. However, L-NAT pre-treatment may regulate Ca2+ homeostasis, resulting in down-regulation of inflammation as well as apoptosis-inducing protein expression and thus provide protection against radiation induced cell death in IEC-6 cells.
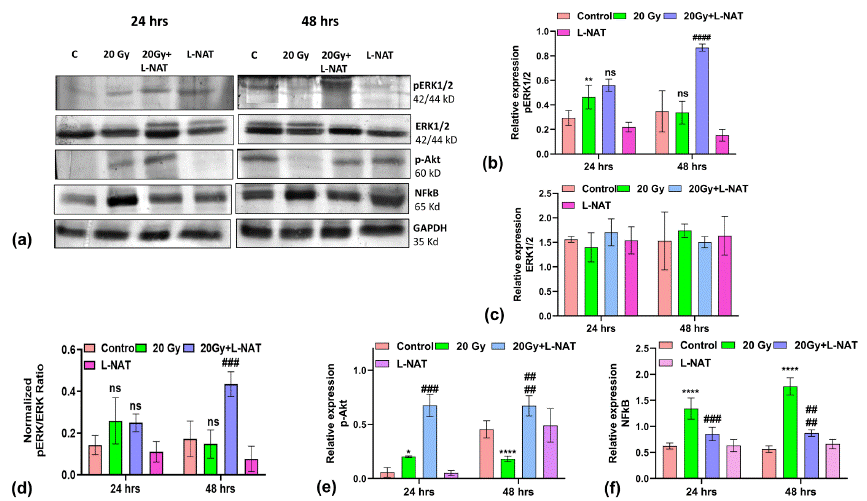
Figure 3: Pro-survival and inflammatory proteins expression analysis in IEC-6 cells upon L-NAT and gamma radiation treatments. Western blot analysis and relative expression quantification of proteins involved in proliferative i.e. pERK1/2 (A,B), ERK1/2 (A,C), ratio of pERK/ERK (A,D), p-Akt (A,E) and inflammatory i.e. NFκB (A,F) pathways in irradiated (20 Gy) IEC-6 cells that pretreated with L-NAT. Measurement of proteins expressions were carried out by Image J software v1.44p (NIH, Bethesda, MD). Data were represented as mean±SD of three independent experiments. Significance of variance was denoted as: *p=0.05, **p=0.01, ***p=0.001 Vs Control; and ###p=0.01, ####p=0.001 Vs Radiation treatment (20Gy)/ NS: Non Significant.
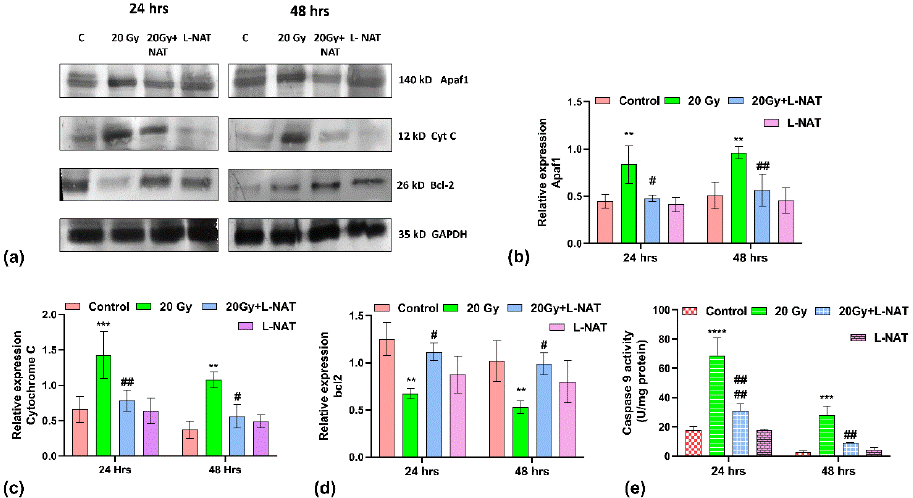
Figure 4: Apoptosis regulating proteins expression analysis in IEC-6 cells treated with L-NAT and gamma radiation. Western blot analysis and relative quantification of proteins involved in radiation induced apoptosis i.e. Apaf-1 (A,B), Cytochrome C (A,C), and pro-survival protein Bcl2 (A,D) proteins was performed with irradiated and L-NAT pre-treated IEC-6 cells. While, Caspase-9 activities (E) of IEC-6 cells was analysed using ELISA assay. Data were expressed as mean±SD of three experiments. Significance of variance was denoted as: **p=0.01, ***p=0.001, ****p=0.0001 Vs Control; and #p=0.05, ##p=0.01, ####p=0.0001 Vs Radiation treatment (20 Gy).
Histological Analysis
Histological observations were evident of rapid structural damage, including villi shortening, loss of epithelial cell lining, severe crypt damage, and cell necrosis to the small intestine within three days that was found to accelerate in subsequent 5 and 7 days in irradiated mice as compared to untreated control mice (Figure 5). By day 5th, the epithelial cell lining of the villi was completely lost, and the villi became shortened, wider, and sparser, while unhealthy shortened crypts were still observable in the irradiated mice. In contrast, the damage to the intestinal architecture was found to be less severe, as the crypt lining significantly restored and the crypt-villus axis of the jejunal epithelial cells eventually recovered in irradiated mice that pre-administered L-NAT (Figure 5) within 5-6 days.
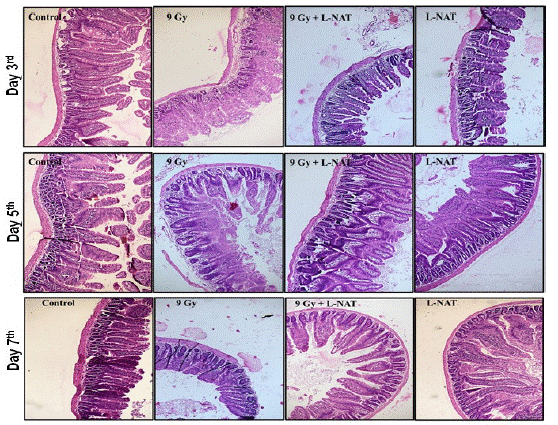
Figure 5: Histological analysis of small intestine (jejunum) of whole-body gamma irradiated (9 Gy) mice pre-treated with L-NAT. Histological analysis of C56BL/6 small intestine (jejunum) prior administration of L-NAT to Whole-body irradiation (9 Gy). (A) Day 3, (B) Day 5 and (C) Day 7. Representative images from three mice per group; 100 x magnification.
Attenuation of Radiation-Induced Up-Regulation of Intestinal NK-1R, Substance P, and NF-κB Expression by L-NAT Pre-treatment
To validate the role of L-NAT pre-treatment in NK-1R-mediated inflammatory signaling modulation in irradiated (9 Gy) mice, NK-1R, substance P, and downstream inflammatory mark er NFkB expression were analyzed. Strong induction in NK-1R (~1-3 fold), substance P (~3-4 fold), and NF-κB (~2.0-3.5 fold) expressions was observed with irradiated mice that were not pre-treated with L-NAT (150 mg/kg.b. wt.im.) (Figure 6a,b,c) as compared to untreated control mice at 1–7 days post-treatment time. However, as a function of L-NAT pre-treatment (150 mg/kg. wt.im. -2h) in irradiated (9 Gy) mice, a significant (p<0.05) reduction in NK-1R, substance P, and NF-κB expressions was observed (Figure 6a, b, c) at 1–7 days. These findings suggested that L-NAT pre-treatment in irradiated mice may down-regulate NK-1 receptor, its agonist substance P, and NFkB expressions and thus inhibits radiation-induced inflammation in the GI system of irradiated mice. Immunohistochemical analysis, also revealed a significant increase in the NK-1R expression in the intestinal epithelial cells (enterocytes) of irradiated mice. However, NK-1R expression was found to be minimized on L-NAT pretreatment to irradiated mice day 7 post-radiation period (Figure 6d, e). These findings suggested that L-NAT pre-treatment in irradiated mice may down-regulate NK-1 receptor, its agonist substance P, and NFkB expressions and thus negatively control radiation-induced inflammation in the GI system of irradiated mice.
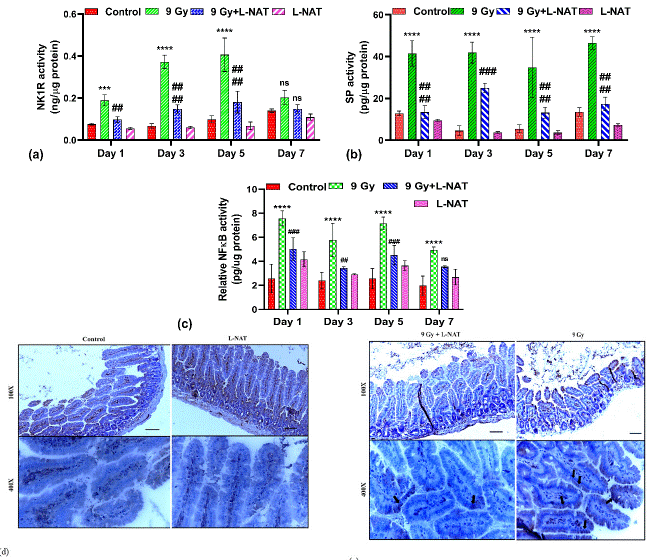
Figure 6: Modulation in Neurokinin-1 receptor and its agonist substance P along with down-stream inflammatory marker NFkB expressions by L-NAT pre-treatment to whole body irradiated (9 Gy) mice jejunum. Significantly high induction in NK-1R (A, E), substance P (B), and NFkB (C) expressions noticed in jejunum of irradiated mice that were found reduced significantly upon L-NAT pre-treatment. Immunohistology revealed radiation induced higher level jejunum tissue expression (localization) of NK-1 receptor (D, E). Data were expressed as mean±SD of three independent experiments. Significance of variance was indicated as: ***p=0.001, ****p=0.0001 Vs Control; ##p=0.01, ###p=0.001 Vs Radiation treatment (9 Gy).
Influence of L-NAT Pre-Treatment on Radiation-Induced Proinflammatory Cytokines and Chemokines Expression in the Nouse Jejunum
To determine the effects of L-NAT pretreatment on proinflammatory cytokines expression in the intestine (jejunum) of irradiated mice, an Elisa assay was performed. Results of the study indicated a significantly high level of pro-inflammatory cytokines IL-6 and IL-4 in irradiated mouse intestines at all the tested time intervals, i.e., 1–7 days, as compared to untreated control group of mice (Figure 7a, D). However, maximum IL-6 expressions (~3.0 folds) were observed 5 days after radiation exposure as compared to control (Figure 7A). Moreover, as a function of L-NAT pretreatment, a significant reduction in IL-6 and IL-4 was observed in irradiated mice at almost all the tested time intervals. On the contrary, a significant increase in the expression of anti-inflammatory cytokines, i.e., IL-10 (on the 3rd and 5th days post-irradiation) and TGF-β1 (at the 1-3rd day post-irradiation), was observed with the irradiated mice that were pretreated with L-NAT as compared to only the irradiated mice that were not pretreated with L-NAT (Figure 7B,C). More interestingly, significantly high levels of RANTES, MCP-1, ICAM-1, MIP-1a, and MIP-2 chemokine proteins were reported in irradiated mice as compared to untreated control at almost all the tested time intervals (1–7 days). However, significant inhibition in RANTES, MCP-1, ICAM-1, and MIP-2 chemokines expression was evident in the irradiated mice that were pre-treated with L-NAT as compared to the irradiated mice that were not pretreated with L-NAT (Figure 8A,B,C,D) at all tested time intervals (1–7 days). Therefore, the present observations strongly suggest that L-NAT pretreatment in irradiated mice significantly suppresses radiation-induced inflammatory responses that contributes to radioprotection.
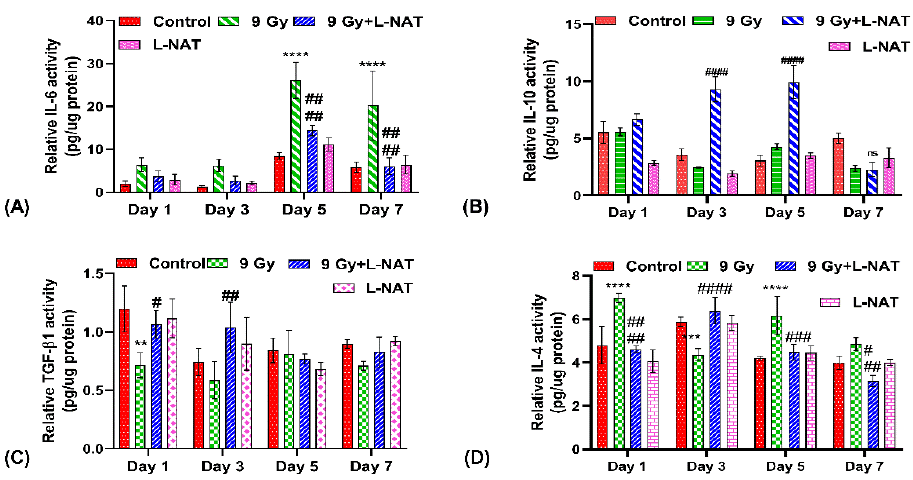
Figure 7: Effect of L-NAT pretreatment on radiation induce pro/anti-inflammatory cytokines IL-6 (A), IL-10 (B), TGF-β1 (C) and IL-4 (D) expression in jejunum of whole body irradiated (9 Gy) mice. Data were represented as mean±SD of three independent experiments. Significance of variance was indicated as: **p=0.01, ***p=0.001, ****p=0.0001 Vs Control; and #p=0.05, ##p=0.01, ###p=0.001, ####p=0.0001 Vs Radiation treatment (9 Gy).
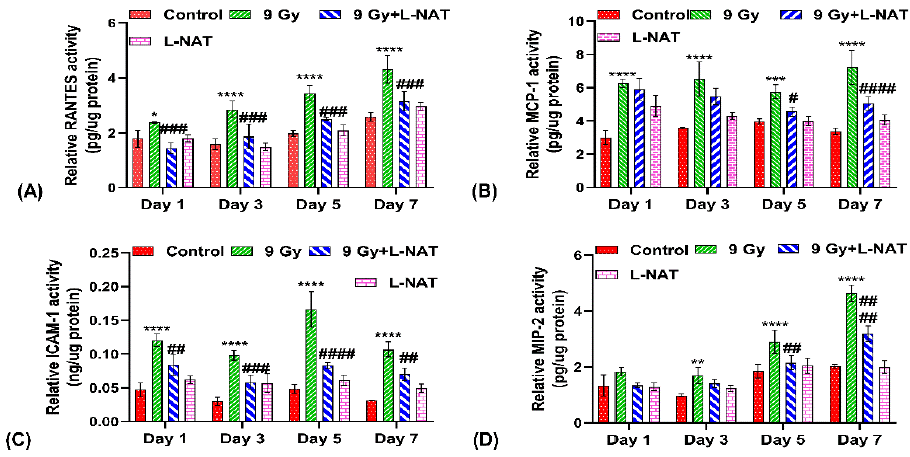
Figure 8: Influence of L-NAT pretreatment on radiation induce pro-inflammatory chemokines RANTES (A), MCP-1 (B), ICAM-1 (C), and MIP-2 (D) expression in jejunum of whole body irradiated (9 Gy) mice. Data were expressed as mean±SD of three repeated experiments. Significance of variation was indicated as: *p=0.05, **p=0.01, ***p=0.001, ****p=0.0001 Vs Control; and #p=0.05, ##p=0.01, ###p=0.001, ####p=0.0001 Vs Radiation treatment (9 Gy).
Discussion
Overexposure to gamma radiation induces emesis in mammals, including humans [18]. Radiation-induced emesis involved neuro-intestinal junction cross-talk in the form of substance P/Neurokinin-1 receptor interaction. Radiation and other cytotoxic therapy including chemotherapy induces substance P release from the ends of enteric neurons that activates NK-1 receptor in intestinal epithelial mucosal cells within the gastro-neuronal junction and initiates emetic and subsequent inflammatory response [19-23].
The present study demonstrated significant protection against lethal gamma radiation in irradiated IEC-6 cells that were pre-treated either with the NK-1R antagonist CP99994 or L-NAT or with a combination of both. Furthermore, no significant difference in cell survival was observed in IEC-6 cells upon either the L-NAT-CP99994 or CP99994-L-NAT pre-treatment regime used before irradiation (Figure 1), suggesting a common molecular/pharmaceutical target (i.e. NK-1R) for the L-NAT and CP99994 mechanisms of action. These findings supported the “Neurokinin-1 receptor antagonism-based mechanism of radioprotection. The positive role of NK-1 receptor antagonists, including N-acetyl-L-tryptophan, in radioprotection, oxidative stress mitigation, neuro-inflammation inhibition, and hepatoprotection has already been reported; [14,15,24-32], providing a strong support to the present investigation. Activation of NK-1R due to radiation exposure has been known to cause pain, nausea, and vomiting, as well as inflammation in the nervous and gastrointestinal systems [20,21,33]. In the present study also, a significant increase in NK-1 receptor and its agonist substance P, along with NFkB expressions, was evident in irradiated (9 Gy, whole body) mice jejunum (Figure 6). However, expressions of all these proteins were found to be significantly inhibited (Figure 6) in irradiated mice that were pre-treated with L-NAT (150mg/kg.b.wt.im). These findings clearly suggest that L-NAT pre-treatment suppresses radiation induced substance P expression and thus its release from enteric neurons within the gastro-neuron junction, leading to significant inhibition in radiation-induced inflammation. Recent molecular docking and MD simulation studies demonstrated possible interactions of L-NAT with NK-1R [34] further supporting the present hypothesis of the involvement of the “NK-1R antagonistic mechanism” in L-NAT mediated radioprotection. However, on the contrary, in vitro, a ligand-receptor binding assay-based study did not support L-NAT/NK-1R physical interaction [35]. Therefore, considering the recent findings [35], the present study suggests that L-NAT mediated inhibition of substance P release from enteric neurons may impair substance P/NK-1R interaction instead of direct physical involvement in the interaction (Figure 6). However, to further confirm the L-NAT/NK-1R physical interaction, if any, we proposed Surface Plasmon Resonance (SPR) spectroscopic study.
A significant increase in intracellular calcium concentration was observed with irradiated cells within 1 h that reached the highest level (~4 fold; Figure 2A, B) at 48 h. Furthermore, ATP levels were found to be decreased in irradiated cells at 48 h (Figure 2C). These observations suggest that radiation induced calcium accumulation in the cells seems to be inversely proportional to ATP generation and subsequent cell death [36,37]. On the contrary, L-NAT pre-treatment of irradiated IEC-6 cells was found to reduce calcium accumulation in cells, and thus improved ATP generation resulted in better cell survival as compared to only irradiated cells (Figure 2A, B). Recent reports from our groups provided strong support for the present findings [14,15]. Mitochondrial calcium disturbs mitochondrial membrane potential, which leads to membrane hyperpolarization, cytochrome C release, and apoptosis [15,36,38]. In full agreement with previous reports, significant inhibition in Cytochrome C, Apaf-1, caspase-9, and NFkB, along with a significant increase in Bcl-2, pERK1/2, and p-AKT expressions, was observed in irradiated IEC-6 cells that were pre-treated with L-NAT (Figures 3 & 4). These findings suggesting that L-NAT pretreatment of irradiated cells significantly controlled intracellular calcium homeostasis, resulted in preserved mitochondrial functions that enhanced cell survival by inhibiting inflammation and apoptosis. Similar findings reported in previous studies closely supporting the current investigation [1,24,31,33,39-42].
The regulatory role of cytokines is crucial in the development of an inflammatory response, intestinal tissue damage, and recovery after radiation exposure [43]. L-NAT pre-treatment in irradiated mice showed significant up-regulation in IL-10 and TGF-β1 along with down-regulation of IL-6 and IL-4 expression as compared to the irradiated control (Figure 7). These observations suggested that L-NAT augments an anti-inflammatory response (Figure 6A, B). NK-1R and substance P expression inhibition are known to initiate anti-inflammatory response [44] further providing gain support to the present study. Further, activation of the anti-inflammatory response in terms of chemokine expression modulation (Figure 8) by L-NAT pretreatment to irradiated mice may facilitate fast tissue recovery and repair in the intestinal epithelium of irradiated mice [45-49]. Intestinal epithelial cell renewal, homeostasis, and tissue repair are totally dependent on highly radiosensitive intestinal crypt stem cell proliferation and differentiation. L-NAT pretreatment to irradiated mice was found to protect crypt stem cells against radiation-induced cell death, as evident by Lgr5, Bmi-1, Msi-1, and Dclk-1 stem cell marker expression (data not shown). Stem cell preservation and regeneration have been found to be the best therapeutic strategies for the replenishment of damaged intestinal epithelial cells to combat radiation toxicity [50], which comprehensively supported by the present study.
Conclusions
Conclusively, the present study offers a new insight into the possible involvement of the NK-1R/substance P system in gamma radiation-induced toxicity in mice models. L-NAT pretreatment was found to be a contributing factor in inhibition of NK-1R/substance P expression resulted in amelioration of radiation induced inflammation, and apoptosis, possibly by maintaining intercellular calcium homeostasis, mitochondrial function preservation, inflammatory cytokine expression inhibition, and finally intestinal crypt stem cell protection against radiation-induced toxicity (Figure 9).
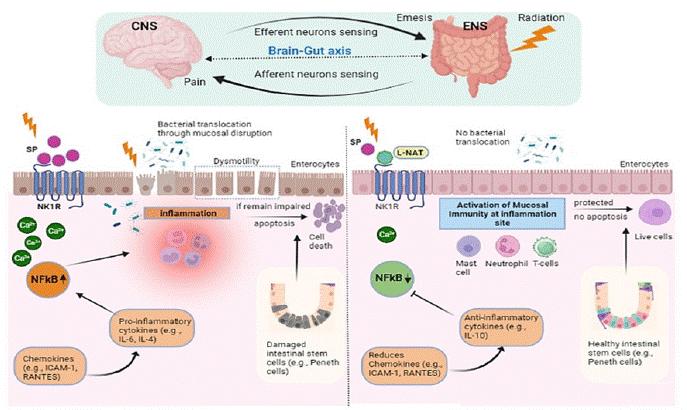
Figure 9: Schematic representation of the molecular mechanism of radioprotection offered by N-acetyl-L-tryptophan to gastro-intestinal system of irradiated mice.
Author Statements
Acknowledgement
The authors express their gratitude to Director INMAS for providing administrative support that facilitated the completion of this work. The authors would like to express their gratitude to Mrs. Namita Kalra for her valuable technical support. We express our gratitude to the Defense Research and Development Organization for their generous funding of the present study, specifically through the Rakshak Project TD-15/INM-313. Mrs. Pratibha Kumari expresses gratitude to the Department of Biotechnology, Government of India, for granting a research fellowship that facilitated the execution of the study.
Conflict of Interest Statements
The authors have stated that there are no competing or conflicting interests.
Author’s Contributions
Each author made contributions to the conception of the study and the design of the experiments. The necessary steps for material preparation, data collection, and analysis were conducted. Dr. Raj Kumar, in his role as the corresponding author of the study, made significant contributions. These contributions included conceptualizing the study, supervising the research process, analyzing the data, and proofreading the manuscript. Pratibha Kumari was responsible for designing and conducting experiments, as well as collecting and analyzing data using statistical analysis techniques. Shravan K. Singh and Ravi Kumar contributed by reviewing and editing the work.
References
- Lu L, Li W, Chen L, Su Q, Wang Y, Guo Z, et al. Radiation-induced intestinal damage: latest molecular and clinical developments. Future oncology. 2019; 15: 4105-18.
- MacVittie TJ, Farese AM. Defining the concomitant multiple organ injury within the ARS and DEARE in an animal model research platform. Health Physics. 2020; 119: 519-526.
- Gehart H, Clevers H. Tales from the crypt: new insights into intestinal stem cells. Nature reviews Gastroenterology & hepatology. 2019; 16: 19-34.
- Mayer EA. Gut feelings: the emerging biology of gut–brain communication. Nature Reviews Neuroscience. 2011; 12: 453-66.
- Birder LA, Kullmann FA. Role of neurogenic inflammation in local communication in the visceral mucosa. Seminars in immunopathology. Springer Berlin Heidelberg. 2018; 40: 261-279.
- Koon HW, Pothoulakis C. Immunomodulatory properties of substance P: the gastrointestinal system as a model. Annals of the New York Academy of Sciences. 2006; 1088: 23-40.
- Patel M, Subas SV, Ghani MR, Busa V, Dardeir A, Marudhai S, et al. Role of Substance P in the Pathophysiology of Inflammatory Bowel Disease and its Correlation with the Degree of Inflammation. Cureus. 2020; 12: e11027.
- McDaniel DK, Eden K, Ringel VM, Allen IC. Emerging roles for noncanonical NF-κB signaling in the modulation of inflammatory bowel disease pathobiology. Inflammatory bowel diseases. 2016; 22: 2265-79.
- Steinhoff MS, von Mentzer B, Geppetti P, Pothoulakis C, Bunnett NW. Tachykinins and their receptors: contributions to physiological control and the mechanisms of disease. Physiological reviews. 2014; 94: 265-301.
- Wang L, Jiang J, Chen Y, Jia Q, Chu Q. The roles of CC chemokines in response to radiation. Radiation Oncology. 2022; 17: 63.
- Kim CK, Yang VW, Bialkowska AB. The role of intestinal stem cells in epithelial regeneration following radiation-induced gut injury. Current stem cell reports. 2017; 3: 320-32.
- Zhong D, Zhang D, Chen W, He J, Ren C, Zhang X, et al. Orally deliverable strategy based on microalgal biomass for intestinal disease treatment. Science Advances. 2021; 7: eabi9265.
- Moussa L, Usunier B, Demarquay C, Benderitter M, Tamarat R, Sémont A, et al. Bowel radiation injury: complexity of the pathophysiology and promises of cell and tissue engineering. Cell Transplantation. 2016; 25: 1723-46.
- Kumari P, Kumar R, Singh D, Kumar R. N-acetyl-L-tryptophan (NAT) provides protection to intestinal epithelial cells (IEC-6) against radiation-induced apoptosis via modulation of oxidative stress and mitochondrial membrane integrity. Molecular Biology Reports. 2023; 15: 1-7.
- Kumar R, Kumari P, Pandey S, Singh SK, Kumar R. Amelioration of Radiation-Induced Cell Death in Neuro2a Cells by Neutralizing Oxidative Stress and Reducing Mitochondrial Dysfunction Using N-Acetyl-L-Tryptophan. Oxidative Medicine and Cellular Longevity. 2022.
- Xu G, Ma T, Zhou C, Zhao F, Peng K, Li B. β-Carotene attenuates apoptosis and autophagy via PI3K/AKT/mTOR signaling pathway in necrotizing enterocolitis model cells IEC-6. Evidence-Based Complementary and Alternative Medicine. 2022; 2022: 2502263.
- Dutta A, Dahiya A. Quercetin 3-O rutinoside prevents gastrointestinal injury through regulation of apoptosis in 7.5 Gy total body irradiated mice. Phytomedicine. 2023; 112: 154692.
- Singh VK, Romaine PLP, Seed TM. Medical countermeasures for radiation exposure and related injuries: characterization of medicines, FDA-approval status and inclusion into the strategic national stockpile. Health physics. 2015; 108: 607.
- Satheeshkumar PS, Mohan MP. Tachykinin peptide, substance P, and its receptor NK-1R play an important role in alimentary tract mucosal inflammation during cytotoxic therapy. Digestive diseases and sciences. 2014; 59: 2864-73.
- Höckerfelt U, Franzén L, Kjörell U, Forsgren S. Parallel increase in substance P and VIP in rat duodenum in response to irradiation. Peptides. 2000; 21: 271-81.
- Höckerfelt U, Franzén L, Forsgren S. Substance P (NK1) receptor in relation to substance P innervation in rat duodenum after irradiation. Regulatory peptides. 2001; 98: 115-26.
- Muñoz M, Coveñas R, Esteban F, Redondo M. The substance P/NK-1 receptor system: NK-1 receptor antagonists as anti-cancer drugs. Journal of biosciences. 2015; 40: 441-63.
- Vijayan M, Joseph S, James E, Dutta D. A review on radiation induced nausea and vomiting: “Current management strategies and prominence of radio sensitizers”. Journal of Oncology Pharmacy Practice. 2021; 27: 1061-72.
- Sirianni AC, Jiang J, Zeng J, Mao LL, Zhou S, Sugarbaker P, et al. N-acetyl-l-tryptophan, but not N-acetyl-d-tryptophan, rescues neuronal cell death in models of amyotrophic lateral sclerosis. Journal of Neurochemistry. 2015; 134: 956-68.
- Fernandes J, Mudgal J, Rao CM, Arora D, Mallik SB, Pai KSR, et al. Tachykinin receptor antagonist, N-Acetyl-L-tryptophan alleviates spatial memory deficits in Alzheimer’s disease model in rats. Journal of the Neurological Sciences. 2017; 381: 766.
- Thornton E, Hassall MM, Corrigan F, Vink R. The NK1 receptor antagonist N-acetyl-L-tryptophan reduces dyskinesia in a hemi-parkinsonian rodent model. Parkinsonism & related disorders. 2014; 20: 508-13.
- Fernandes J, Mudgal J, Rao CM, Arora D, Basu Mallik S, Pai KSR, et al. N-acetyl-L-tryptophan, a substance-P receptor antagonist attenuates aluminum-induced spatial memory deficit in rats. Toxicology Mechanisms and Methods. 2018; 28: 328-34.
- Ma T, Cheng H, Li T, Chen Y, Cai T, Bai J, et al. N-Acetyl-l-tryptophan inhibits CCl4-induced hepatic fibrogenesis via regulating TGF-β1/SMAD and Hippo/YAP1 signal. Bioorganic Chemistry. 2022; 126: 105899.
- Pan Y, Yu S, Wang J, Li W, Li H, Bai C, et al. N-acetyl-L-tryptophan attenuates hepatic ischemia-reperfusion injury via regulating TLR4/NLRP3 signaling pathway in rats. Peer J. 2021; 9: e11909.
- Li W, Fotinos A, Wu Q, Chen Y, Zhu Y, Baranov S, et al. N-acetyl-L-tryptophan delays disease onset and extends survival in an amyotrophic lateral sclerosis transgenic mouse model. Neurobiology of disease. 2015; 80: 93-103.
- Li H, Pan Y, Wu H, Yu S, Wang J, Zheng J, et al. Inhibition of excessive mitophagy by N-acetyl-L-tryptophan confers hepatoprotection against Ischemia-Reperfusion injury in rats. PeerJ. 2020; 8: e8665.
- Wang J, Yu S, Li H, Jiang H, Xiao P, Pan Y, et al. Protective role of N-acetyl-L-tryptophan against hepatic ischemia-reperfusion injury by the TLR4/NF-κB signaling pathway. InAIP Conference Proceedings. AIP Publishing LLC. 2020; 2058: 1.
- Wang J, Zheng H, Kulkarni A, Ou X, Hauer-Jensen M. Regulation of early and delayed radiation responses in rat small intestine by capsaicin-sensitive nerves. International Journal of Radiation Oncology* Biology* Physics. 2006; 64: 1528-36.
- Satarker S, Maity S, Mudgal J, Nampoothiri M. In silico screening of neurokinin receptor antagonists as a therapeutic strategy for neuroinflammation in Alzheimer’s disease. Molecular Diversity. 2022; 26: 443-66.
- Matalinska J, Lipinski PFJ. Correcting a widespread error: Neuroprotectant N-acetyl-L-tryptophan does not bind to the neurokinin-1 receptor. Molecular and Cellular Neuroscience. 2022; 120: 103728.
- Zhai K, Liskova A, Kubatka P, Büsselberg D. Calcium entry through TRPV1: a potential target for the regulation of proliferation and apoptosis in cancerous and healthy cells. International journal of molecular sciences. 2020; 21: 4177.
- Qin H, Zhang H, Zhang S, Zhu S, Wang H. Protective effect of sirt1 against radiation-induced damage. Radiation Research. 2021; 196: 647-57.
- Szabo I, Zoratti M, Biasutto L. Targeting mitochondrial ion channels for cancer therapy. Redox Biology. 2021; 42: 101846.
- Agarwal P, Singh D, Raisuddin S, Kumar R. Amelioration of ochratoxin-A induced cytotoxicity by prophylactic treatment of N-Acetyl-L-Tryptophan in human embryonic kidney cells. Toxicology. 2020; 429: 152324.
- Liang Z, Yuan Z, Guo J, Wu J, Yi J, Deng J, et al. Ganoderma lucidum polysaccharides prevent palmitic acid-evoked apoptosis and autophagy in intestinal porcine epithelial cell line via restoration of mitochondrial function and regulation of MAPK and AMPK/Akt/mTOR signaling pathway. International Journal of Molecular Sciences. 2019; 20: 478.
- Kiang JG, Smith JT, Cannon G, Anderson MN, Ho C, Zhai M, et al. Ghrelin, a novel therapy, corrects cytokine and NF-κB-AKT-MAPK network and mitigates intestinal injury induced by com bined radiation and skin-wound trauma. Cell & Bioscience. 2020; 10: 1-7.
- Bonnaud S, Niaudet C, Legoux F, Corre I, Delpon G, Saulquin X, et al. Sphingosine-1-phosphate activates the AKT pathway to protect small intestines from radiation-induced endothelial apoptosis. Cancer Res. 2010; 70: 9905-15.
- François A, Milliat F, Guipaud O, Benderitter M. Inflammation and immunity in radiation damage to the gut mucosa. BioMed research international. 2013; 2013: 123241.
- Beinborn M, Blum A, Hang L, Setiawan T, Schroeder JC, Stoyanoff K, et al. TGF-β regulates T-cell neurokinin-1 receptor internalization and function. Proceedings of the National Academy of Sciences. 2010; 107: 4293-8.
- Guo J, Liu Z, Zhang D, Chen Y, Qin H, Liu T, et al. TLR4 agonist monophosphoryl lipid A alleviated radiation-induced intestinal injury. Journal of Immunology Research. 2019.
- Linard C, Marquette C, Mathieu J, Pennequin A, Clarençon D, Mathé D. Acute induction of inflammatory cytokine expression after γ-irradiation in the rat: effect of an NF-κB inhibitor. International Journal of Radiation Oncology* Biology* Physics. 2004; 58: 427-34.
- Kong L, Liu J, Wang J, Luo Q, Zhang H, Liu B, et al. Icariin inhibits TNF-a/IFN-γ induced inflammatory response via inhibition of the substance P and p38-MAPK signaling pathway in human keratinocytes. International immunopharmacology. 2015; 29: 401-7.
- Kulka M, Sheen CH, Tancowny BP, Grammer LC, Schleimer RP. Neuropeptides activate human mast cell degranulation and chemokine production. Immunology. 2008; 123: 398-410.
- Kosaka H, Gainsbury M, Sheldon H, Cassidy MR, Stucchi A, Becker J. A Neurokinin-1 Receptor (NK1R) antagonist (NK-1RA) that reduces postoperative adhesions reduces the adhesion related chemokines CXCL1 (KC) and CXCL2 (MIP-2) and their receptor, CXCR2. Journal of the American College of Surgeons. 2011; 213: S18-9.
- Gong W, Guo M, Han Z, Wang Y, Yang P, Xu C, et al. Mesenchymal stem cells stimulate intestinal stem cells to repair radiation-induced intestinal injury. Cell death & disease. 2016; 7: e2387.
Citation: Kumari P, Kumar R, Gaurav N, Kumar V, Singh SK, et al. N-acetyl-L Tryptophan Provides Protection Against Gamma Radiation-Induced Inflammation and Apoptosis in the Irradiated IEC-6 Cells and Mouse Intestine via Neurokinin-1 Receptor/Substance P Expression Inhibition. Austin J Biotechnol Bioeng. 2024; 11(2): 1134.