
Research Article
Austin J Biotechnol Bioeng. 2024; 11(2): 1136.
Effect of Astaxanthin in Cytoprotection and Maturation in Vitro Differentiation Process to Insulin-Producing Cells
Sanchez GI¹; Flores HFY¹; Bravo MJ¹; Flores VMA¹; Gaona BJ²; Marino MEN¹*
1Department of Medical and Pharmaceutical Biotechnology, Center for Research and Assistance in Technology and Design of the State of Jalisco, Mexico
2Department of medical microbiology, University of Guadalajara, Mexico
*Corresponding author: Marino MEN, 1Department of Medical and Pharmaceutical Biotechnology, Center for Research and Assistance in Technology and Design of the State of Jalisco Av. Normalistas 800, Colinas de La Normal, 44270 Guadalajara, Jalico, Mexico. Tel: (33) 33455200 ext. 1671 Email: emarino@ciatej.mx
Received: October 04, 2024; Accepted: October 25, 2024 Published: November 01, 2024
Abstract
Asx is a fat-soluble xanthophyll carotenoid, one of its most relevant properties is its antioxidant activity. Depending on the dose and environment, it participates in an adequate regulation of ROS/RNS at optimal levels, either directly scavenger or indirectly through the stimulation of “antioxidant” pathways such as NRF2. This regulation and interactions within the signaling pathways are studied here in order to find information that provides data to elucidate the effects that may influence the processes of obtaining IPC and the study of the effect of molecules that increase the efficiency of the cellular response during the trans differentiation process. We found that adding Asx to a cocktail of differentiation molecules to obtain IPC from DPSC increases the efficiency of insulin production, as well as the expression of important markers for the maturity and identity of pancreatic β-type cells (NGN3, PDX1, MAFA). It was also revealed that Asx favors the proper regulation of oxidative stress caused during the process of cellular trans differentiation of DPSC towards IPC, through the direct inactivation of ROS and the increase in the expression of NRF2 as well as the decrease of their principal inhibitor KEAP1, favoring the maturity of the IPC. These results suggest the potential use of Asx to deepen the knowledge of its interaction with other signaling pathways that favor the generation of IPC and other cells sensitive to oxidative stress, and thereby lay the foundations for a possible cell replacement therapy as in DM1.
Keywords: Astaxanthin; Antioxidant; Oxidative stress; Mesenchymal cells; NRF2
Introduction
Diabetes mellitus is a chronic metabolic disorder characterized by a state of chronic hyperglycemia. DM is a disease of worldwide relevance and constant growth [1]. The expenses related to this disease are high for the patient and for the health systems. It is estimated that in 2021, there were 537 million people with DM in the world. In addition, stakeholders in this condition had risen to 966 billion USD [1,2]. Despite great advances in the development of palliative therapies, side effects have been reported in efforts to provide patients with recurrent treatments. In DM type1, there is an altered immune tolerance to specific proteins, determining the destruction of β cells [3]. While in DM2 a state of insulin resistance prevails, triggering several problems such as glucotoxicity among others [3]. This increases oxidative stress, increasing the production of reactive oxygen species and favoring the direct destruction of the β-cell mass [4]. Consequently, small percentages of β cells prevail in both types of DM, 2% in DM1 and 20% in DM2 [5]. The transplant of pancreas or islets from cadavers is one of the most effective therapies, however the low availability of donors, a low percentage of islets per patient, histocompatibility, surgery costs, among other reasons, makes this therapy very complicated [6]. Therefore, more effective and cheaper long-term therapies are required. Derived from the interest in having replacement cells, which has ventured into the field of cell therapy, the use of adults Mesenchymal Stromal Cells (MSC), may have some advantages including the potential to produce differentiated cells obtained from the same patient (autologous) or from a different one (heterologous), thus minimizing the problem of cell or donor supply and possibly reducing the cost of treatment per patient [7]. Further to this, they are not teratogenic, immunogenic, and also have immunomodulatory functions [8-10]. The generation of replacement cells obtained from stromal cells such as MSC continues to be of interest due to their availability from different sources or niches (bone marrow, dental pulp, umbilical cord, adipose tissue, among others); they have been a topic of growing interest both in the academic and health industry fields for about 50 years, for which isolation, culture and expansion protocols have been improved to facilitate cell replacement techniques [11]. The DPCS in particular have an advantage since their extraction can be done from third molars after dental surgeries, in addition to the fact that they do not present ethical conflicts in their use [9,12-14]. Insulin-producing cells can be generated from MSC through targeted differentiation protocols [15,16]. It is now known that the resulting insulin-producing cells can control hyperglycemia to induced diabetes mice, preclinic studies [17-19]. Although the mechanisms have not been fully elucidated, great progress has been made in studying the effect of molecules that potentially increase the efficiency in obtaining IPC. Asx is a fat-soluble xanthophyll carotenoid and it is contained in various microorganisms like Blakeslea trispora or Haematococcus pluvialis. Asx exerts an action on the response to insulin and the metabolism of glucose in a diet with a high amount of fat and fructose mediated by the modulation of signaling pathways such as activation of IRS, PI3K, PKB [20-22]. Other studies refer to the usefulness of Asx as a neuroprotective and enhancer in increasing the proliferation of neuronal progenitors obtained from MSC [23,24]. These studies reported that Asx had a protective effect and induced proliferation of neuronal progenitors at almost twice the rate observed when Asx was not used. It was also discovered to induce the expression of neuronal genes (NGN3, NEUROD1), involved in the formation of the definitive endoderm during embryonic development. Further, these genes participate in the maturation and function of pancreatic cells and insulin secretion., which is why the correlation of the use of Asx and stimulation in the process of transdifferentiation into pancreatic β-type cells became of interest for our study [25-27].
One of the most relevant properties of Asx is its antioxidant activity, and depending on the environment, it participates in an adequate regulation of ROS at optimal levels, either directly as a ROS/ RNS scavenger or indirectly through the stimulation of "antioxidant" pathways such as NRF2 or HO-1, among others [21,28]. It has been reported that adequate regulation of ROS can also favor different cellular processes such as cell differentiation, among which is the organogenesis of insulin -producing β cells [29]. It has been reported that this regulation favored the expression of genes such as SOX9 or the NGN3 [30,31]. It should be noted that it has been necessary to control oxidative stress in the in vitro differentiation processes in which MSC are used, since this allows the cells to specialize more efficiently towards the lineage these are aimed to differentiate to [32,33].
It is believed that the use of antioxidants during protocols for obtaining IPC in vitro may favor its efficiency, probably due to its cytoprotective effect similar to studies in Diabetes models or induction of pancreatic type genes, however, its possible mechanism of action has not been fully elucidated [22,34,35]. As a consequence, in this exploratory study we investigate the implications of the use of Asx, the efficacy of the transdifferentiation process to obtain IPC in vitro, through its participation on oxidative stress, specifically on some ROS/RNS such as H2O2, O2• and O2• (direct antioxidant or scavenging activity) and the expression of antioxidant pathways such as KEAP1/NRF2, which regulates antioxidant enzymes such as SOD, CAT and GPX (indirect antioxidant activity).
Abbreviations: Asx: Astaxanthin; CAT: Catalase; DAFDA: 4-Amino-5-Methylamino-2',7'- Difluorofluorescein Diacetate; DCFDA: 2',7'-Dichlorofluorescein diacetate; DPSC: Dental Pulp Stromal Cells; GAPDH: Glyceraldehyde-3-Phosphate Dehydrogenase; GPX: Glutathione Peroxidase; IPC: Insulin-Producing Cells; KEAP1: Kelch Like ECH Associated Protein 1; MAFA: V-Maf Avian Musculoaponeurotic Fibrosarcoma Oncogene; MSC: Mesenchymal Stromal Cells; NGN3: Neurogenin 3; NRF2: Nuclear Factor Erythroid 2-Related Factor 2; PBS: Phosphate-Buffered Saline; PDX1: Pancreatic and Duodenal Homeobox 1; RNS: Reactive Nitrogen Species; ROS: Reactive Oxygen Species; SOD: Superoxide Dismutase; SOX9: (Sex determining region Y)-box transcription factor 9; SP: Standard Protocol without astaxanthin; SP+Asx: Standard Protocol with Astaxanthin; FBS: Fetal Bovine Serum.
Material and Methods
Isolation and Culture of DPSC
DPSC were obtained from a dental piece following the protocol reported by Hernandez et al. [36], was obtained from a patient (female 18 years old) undergoing dental surgery at the Civil Hospital of Guadalajara, under informed consent. Within a biosafety cabinet, the dental piece was cleaned with a 0.25mg/mL amphotericin B (Sigma cat. A2942) gauze, then it was placed in 8% povidone-iodine, later in PBS 1X pH 7.4 (Sigma) with 100U/10mg/ml of Penicillin/ Streptomycin (Sigma cat. P4333) and finally in Chlorhexidine at 0.12% (Sigma cat. 282227). A mechanical method was used to obtain the cells, cutting the dental organ at the neck, dividing the crown and the root, using a low-speed hand piece (Medidental) and a diamond disc (Mestra). Upon reaching the chamber, the pulp tissue was removed with forceps. The tissue was fractionated with a scalpel and digested by adding 1 mL of 0.005% trypsin in DPBS (ATCC cat. 30- 2101, Sigma cat. D8537) shaking it at 160 rpm and at 37°C for 20 min, vortexing for 1 min, shaking it at 220 rpm for 20 min, and vortexing for 1 min again. The sample was centrifuged at 1500 rpm for 5 min., and the cell pellet was resuspended in 5mL of a-MEM medium (Sigma cat. M4526) added with 10% FBS (Gibco), 2mM L-glutamine (Sigma cat. G7513), 10μM L-ascorbic acid 2-phosphate (Sigma cat. A8960), 1% penicillin-streptomycin 100U/100mg/mL and 0.25mg/ mL of amphotericin B. This cell suspension was placed into 25cm2 culture flasks bottles and observed under an inverted microscope daily until adhered cells were observed. The medium was replaced every 72 hours, approximately between 7 and 15 days the first cells with fibroblastoid morphology were observed. For the maintenance of cell cultures, DMEM medium (Sigma cat. D0822) supplemented with 10% FBS and 200mM L-glutamine were used. When the cells were expanded, they were cryopreserved in vials with 7% DMSO (Supelco cat. 94563). Subsequent cell expansion for use was carried out in 75 cm2 bottles, with DMEM/F12 medium (Sigma cat. D8437) supplemented with 7% FBS. They were grown at a density of 3,000 cells per cm².
Characterization of DPSC - Like MSC
The characterization was carried out from the second cell passage and up to 10 passages. Cells were harvested and counted (approximately 500,000 cells), washed with PBS1X/BSA 0.1% (Roche cat. 10735108001) 2 min at 1200 rpm, fixed with cold 4% paraformaldehyde (PFA) (Sigma cat. P6148), shaken 20 min at 120 rpm, washed again with PBS/BSA 0.1% and labeled with fluorescent conjugate assays CD105-PerCP, CD90-FITC, CD73-APC CD44-PE, Hematopoietic cocktail (CD344, CD11, CD45, HLA-DR)-PE) (BD Biosciences cat. 562245) at 4°C overnight. Then, they were washed with PBS/BSA 0.1% and resuspended in PBS/BSA 0.1%. Unlabeled cells were used as negative control. Cells were analyzed using a flow cytometer (BD Accuri C6™). For Mult differentiation, cells were cultured with differentiation media for adipocytes (Sigma cat. 811D- 250), chondrocytes (Sigma cat. 411D-250) and osteocytes (Sigma Cat. 417D-250) for 28 days in 6-well plates. Oil red staining was used for characterizing adipocytes: culture medium was removed and rinsed twice with PBS 1X, fixed with 4% PFA for 15 min, PFA was removed and PBS 1X added 1 min, PBS 1X was removed and 60% isopropanol was added 15 sec. Isopropanol was removed and Oil Red was added for 20 min and rinsed twice with PBS. Alcian blue staining was used for characterizing chondrocytes: the medium was separated and the cells were fixed with 96% ethanol for 20min, washed with 1X PBS, 1% acetic acid was added for 5min, then washed with 1X PBS and placed the blue solution 1% alcian blue solution for 1h and subsequently washed with 1X PBS. Alizarin red staining was used for characterizing osteocytes: cells were fixed with 4% PFA for 1 h, then washed twice with distilled water and 2% alizarin red was added and allowed to stand for 3-5 min. Finally, cells were washed wash 3 times with distilled water. Images of stained cells were captured using phase contrast microscope Optika, XDS-2FL where the DPSC were used as undifferentiated control.
Influence of Asx on the Production of Insulin During the Procurement of the CPI
Differentiation assays were performed in a stepwise protocol as shown in Figure 1. To perform the DPSC differentiation assays towards insulin-producing cells, passages 8-11 were used. The maintenance medium was removed and added in a first stage bFGF 5 ng/mL, IGF 15 ng/mL, Activin A 100ng/mL, CHIR99021 3μM in DMEM/F12 with 7% FBS for 5 days to induce the formation of progenitor pancreatic cells. The endocrine progenitors were obtained by changing the medium for bFGF 4ng/mL, IGF 50ng/mL, Noggin 100ng/mL, dorsomorphin 1μM and retinoic acid 2μM in DMEM-F12 with 1% FBS, for 6 days. For the last stage and to obtain insulin-producing cells, a medium change was made for DMEM-F12 with 10 μM Forskolin, 3 μM taurine, 10 mM nicotinamide, 4 ng/mL GLP-1, 10 μM dexamethasone and 1% B27 supplement for 6 days. The Asx (Sigma Cat. SML09082) dose used in protocol was 10 ng/mL (16.75 nM) in every stage. DPSC were used as a negative differentiation control, cultivating them for the same time, changing the medium according to each stage of differentiation. At the end of each stage, cell viability was analyzed by MTT assay, the expression of pancreatic mRNA and the NRF2 pathway by RT-qPCR, different intracellular ROS/RNS (H2O2, O2•, NO•); and at the end of differentiation, pancreatic mRNAs, intracellular insulin, and Glucose- Stimulated Insulin Secretion (GSIS) in the supernatant were analyzed.
Real Time Quantitative PCR Analysis
For RNA extraction, Promega RNA Reliaprep extraction Kit (cat. Z6010) was used according to the supplier's instructions. Subsequently, for the RT-qPCR analysis, the Invitrogen RNA ultrasense kit (cat. 11732927) was used according to the supplier's instructions, using GAPDH as internal control. The thermal cycler procedure was used: Lysis and RNA extraction at 37°C for 5 min and 75°C for 5 min; for RT-qPCR 50 °C for 25 min, 95 °C for 5 min, followed by 40 cycles, 95 °C for 15 s, 60°C for 45 s. The primers used were listed in Table 1.
Gene
Forward primer (5´ - 3´)
Reverse primer (5´ - 3´)
NRF2 human
caaaaggagcaagagaaagcc
tctgatttgggaatgtgggc
KEAP1 human
ggagtacatctacatgcattttgg
ttgacccagttgatgcagg
SOD human
tcgagcagaaggaaagtaatgg
ctggatagaggattaaagtgaggac
GPX human
ctgctttccctgctcctg
gctccgtactcgtaaatggtg
CAT human
tctcgttggaaataacacccc
tgcagagactcaggacgtag
GAPDH human
ggtgtgaaccatgagaagtatga
gagtccttccacgataccaaag
PDX1 human
agaatccagacctgcacaac
gccggtacttgtagttggg
NGN3 human
ggcagtctggctttctcag
gggagaagcagaaggaacaag
MAFA human
ctggtgtccatgtcggtg
cacttctcgctctccagaatg
Table 1: Sequences of the primers and probes employed for reverse transcription-quantitative polymerase chain reaction.
Intracellular Insulin Measurement
Once the cells were harvested, they were washed with PBS 1X at 1200 rpm for 5 min, then the cell button was fixed and permeabilized with a 1:1 solution of cold methanol: acetone for 1 min [37], washed with PBS 1X/BSA 1% and incubated with anti- insulin (Alexa Fluor 647 Mouse) at 100 rpm for 1 h in the dark at room temperature. They were washed with PBS1X and read in a cytometer (Accuri C6 BD). DPSC was taken as a negative control.
Assay of Glucose-Stimulated Insulin Secretion (GSIS)
At the end of differentiation step, the cells were washed 3 times with PBS 1X, pre-incubated with Krebs-Ringer buffer (NaCl 129mM, KCl 4.7mM, NaHCO3 5mM, CaCl2 2.5mM, MgSO4 1.2mM, KH2PO4 1.2mM, HEPES 10mM, BSA 0.2%, pH 7.4) without glucose for 90 min. The supernatant was then collected and replaced with Krebs-Ringer buffer supplemented with 2.8 mM D-glucose at 37°C for another 60 min. The supernatant was also collected and exchanged again for Krebs Ringer buffer with 16.7 mM D-glucose. The supernatant was also collected to detect the insulin release by a tertiary laboratory DILABIM®, using electrochemiluminescence (CMIA) assay.
Detection of Intracellular ROS/RNS (H2O2, O2-, NO-)
DPSC were cultured in 12-well plates until they reached 80% confluence. For H2O2 detection, cells were harvested with trypsin and washed with PBS 1X. DCF-DA (2',7'-dichlorofluorescein diacetate) (Sigma) was added at 50 μM in PBS and incubated at 37 °C for 30 minutes protected from light. Subsequently, the sample was read in a flow cytometer (BD Accuri C6™) at 480/530 nm (DCFDA Cellular ROS Detection Assay Kit Abcam). Cells were cultured in dark 96-well plates, 3x105 cells/well. Cells were washed with PBS and incubated at 37°C with 100 μL/well for 45 min. The staining solutions for O2- detection were made with Superoxide Detection Reagent (Abcam ab139476) at 2 μM in culture medium per supplier indications and for NO- with DAF-FM™ (Invitrogen) (4-amino-5-methylamino-2 ',7'-difluororescein) 5 μM in PBS (Invitrogen protocol MP23841). Afterwards, it was washed with PBS 1x and proceeded to read in a fluorometer (Cytation3®) at 550/620 for O2- and at 485/535 nm for NO-. *For O2- reading, cells were not washed with PBS Cell viability (MTT)
Cell viability was detected by the MTT assay (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide). For this analysis, the cells were seeded at a density of 5x103 cells/well in a 96-well plate. After the corresponding treatment, the medium was removed and washed with PBS, then added 100 μL of 0.3 mg/ml MTT for 4 h. Subsequently, 150 μL/well of DMSO: SDS 30% in a ratio of 3:1 was added. It was placed under stirring at 120 rpm for 20 min in dark. The absorbance was read at 570 nm on ELISA spectrophotometer (Xmark Bio-rad).
Statistical Analysis
Graphics were presented as means ± Standard Deviation (SD) from at least 2 independent experiments. Results were analyzed by one-way Analysis of Variance Analysis (ANOVA) after corroborating the normality of the data and Tukey's post hoc test was used, unless otherwise stated. Differences were considered statistically significant at p < 0.05. GraphPad Prism version 8.0 (Graphpad Software Inc., La Jolla, CA) was used for statistical analyses.
Results
Characterization of DPSC-like MSC
Primary DPSC isolated from dental pulp were observed with an inverted microscope showing adherence to plastic and a fibroblastoid morphology (Figure 2A). As shown in Figure 2B, the mesenchymal cell-specific membrane markers CD44, CD73, CD90 and CD105 were found to be expressed above 95%. To investigate the high plasticity of MSC, they were differentiated into osteogenic, adipogenic, and chondrogenic lineages (Figure 2C). By direct observation under the microscope, particular characteristics of each lineage were observed, such as lipid droplets stained red (adipocytes), collagen fibers stained blue (chondrocytes), and deposits of calcium stained red (osteocytes). These results confirm that DPSC characteristics of MSC.
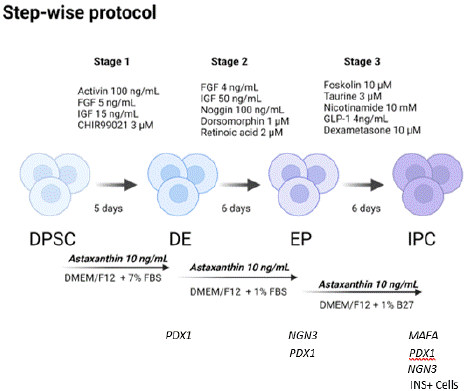
Figure 1: Schematic representation of the stepwise differentiation protocol
used to obtain IPC from DPSC. Protocol showing the specialization of an
MSC towards an IPC, beginning by expressing the definitive endoderm
marker (PDX1), then expressing endocrine progenitor markers (NGN3,
PDX1). MSC, Mesenchymal stem cells; DE, Definitive endoderm; EP,
Endocrine progenitor; IPC, Insulin producing cells. INS+: Insulin positive
cells.
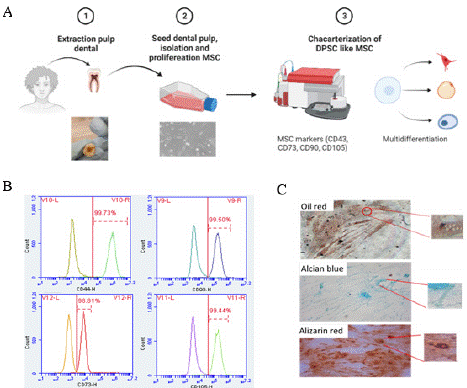
Figure 2: Characterization of primary human Dental Pulp Stem Cells
(DPSC). A. Scheme of extraction, isolation (10X) and characterization of
DPSC. B. Representative flow cytometry analysis of MSC markers indicated
that isolated cells were CD44 (99.73%), CD73 (98.81%), CD90 (99.50%) and
CD105 (99.44%). C. Cellular multidifferentiation: adipogenic cells confirmed
with oil red, chondrogenic cells confirmed with alcian blue and osteogenic
cells confirmed with alizarin red (20X).
Cell Viability of DPSC is not Decreased by Asx.
As shown in Fig. 3, different concentrations (1-1000 ng/mL) of Asx were used on DPSC in vitro for 48h, confirming that none of the doses was cytotoxic according to ISO-109935, since there was no decrease in viability of less than 80% [38]. Instead, we observed a slight increase in cell proliferation capability for all doses of Asx at least during 48h. With this result we verified that the dose of 10ng/mL (to be used in the differentiation protocol) is not cytotoxic.
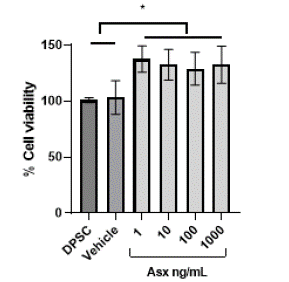
Figure 3: The effect of Asx on the DPSC cells MTT assay. Astaxanthin did
not affect DPSC viability at 48h MTT assay with different concentration of
Asx. n=4. *p <0.05.
During the differentiation process (17 days), there was no significant effect on cell viability compared to the treatment without Asx (Figure 4A), proving to be non-cytotoxic in cultures. Although there was a decrease in cell viability in relation to the control of undifferentiated cells (Figure 4A Stage 3), it has been reported that in vitro cultures of several days present a decreased viability due to the existence in conditions other than the organic (oxidative stress, nutrient availability, etc.), and mainly combined in this type of tests to the process where the cell is forced to leave its multipotent state and go through a process of specialization [39].
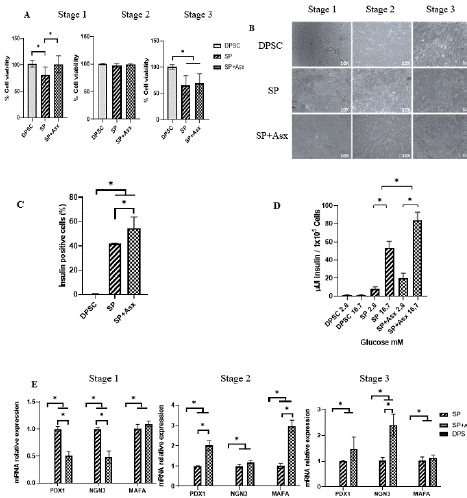
Figure 4: Characterization and functionality of insulin-producing cells
during transdifferentiation. A. Morphology observed by inverted microscope
during differentiation (10X). B. Cell viability during the transdifferentiation
of each treatment (Comparison by stage respect the control DPSC n=6).
C. Intracellular insulin cells by cytometry (Comparison respect the control
DPSC n=2). D. Secreted insulin by electrochemiluminescence (n=3) at final
stage differentiation. E. Pancreatic markers mRNA genes in differentiated
cells (Relative expression respect GAPDH) (*DPSC don´t detect basal
expression) (n=4). DPSC: Dental Pulp Stem Cells; SP: Standard protocol;
SP+Asx: Standard protocol+Astaxanthin, * p=0.05.
Asx Promotes the Generation of Insulin-Producing Cells
During the differentiation process, some cell aggregates were formed in the second stage, however, they did not have structures as organized as an islet, probably due to the lack of stimulation for the formation of other hormone-producing cells (alpha, delta, epsilon, PP) and/or to greater cell communication in a specific cell matrix in a 3D-like environment or biomaterials that help to simulate it (Figure 4B). Regarding the viability by stages, an MTT assay was performed where it can be observed that there was a decrease in cell viability in the differentiation processes in the last stage with or without Asx (Figure 4B), similar to that observed in the cell confluence. For a better understanding of the effect of Asx on the maturation of insulinproducing cells, the cells obtained at the end of differentiation step were characterized. After staged differentiation induction, RT-qPCR analyses showed that pancreatic markers PDX1 (definitive endoderm), NGN3 (endocrine precursor) and MAFA (β-type cell) were elevated from the second stage of differentiation compared to treatment without Asx (Figure 4E); pancreatic-type mRNAs were not found in DPSC. The analyses of intracellular insulin and of insulin released before a glucose stimulus (Figure 4C, D) showed that adding Asx during the differentiation process increased both intracellular insulin (without Asx 22.73 ± 1.74% vs with Asx 37.91 ± 2.04, p=0.05) and the insulin secreted to the medium almost doubled compared to the protocol without Asx, confirming its functionality to secrete insulin in vitro. GSIS (Glucose-stimulated insulin secretion) was tested in the differentiated cells to determine their state of maturity (Figure 4D), showing that at low glucose (2.8 mM) concentrations the release of insulin is lower (without Asx 8.12 ± 2.20 μUI/105 vs with Asx 21.66 ± 5.85 μUI/105 cells p=0.05) while increasing glucose (16.7mM) triggers its release, being higher in cells that were exposed to the Asx (without Asx 53.65 ± 7.11 μUI/105 cells vs with Asx 83.79±9.20 μUI/105 cells p=0.05).
Asx Reduces Oxidative Stress During the Differentiation Process by Decreasing ROS/RNS
ROS are naturally generated in different cellular processes; however, they need to be properly regulated due to the damage they can cause if they remain elevated for a long time. It is known that some cells such as MSC need ROS stimuli to maintain their pluripotency and high state of proliferation, and similar to embryonic cells, by specializing as β cells they tend to decrease and present different patterns of ROS levels. For β cell embryogenesis, it has been shown that a transient increase in ROS is needed during the second phase (endocrine progenitor) and a decrease in the stage of specialization to insulin-producing cells [29]. As shown in Fig. 5A, ROS levels (specifically H2O2) showed a transitory increase in the second stage of differentiation followed by a decrease in the third stage. It should be noted that when using Asx we can observe a significant decrease of H2O2 in stages 1 and 2 mainly. When analyzing other ROS and RNS such as superoxide (O2•) and nitric oxide (NO•), to find out if Asx had a differential effect, it was found that there was no difference in levels when Asx was used or not in the differentiation process (Figure 5B and 5C). However, contrary to H2O2, the NO• level decreased in the first and in the second stage of treatment with Asx, although it remained similar in the third stage (Figure 5C).
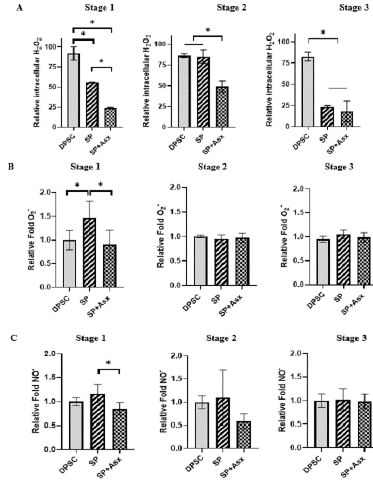
Figure 5: Direct antioxidant activity over intracellular ROS/RNS, during transdifferentiation. A. Levels of intracelular H2O2 by cytometry (DCFH-DA) (n=3). B. Levels of intracelular O2- by fluorescence (Orange® ABcam) (n=6). C. Levels of intracelular NOby fluorescence (DAF-DA) (n=6). DPSC, Dental Pulp Stem Cell control without treatment; SP, Standar protocol; SP+Asx, Standar protocol+Astaxanthin. *: p=0.05.
Asx Favoring the Expression of mRNA of Antioxidant Enzymes through KEAP1/NRF2
Asx, in addition to directly stabilizing reactive molecules by facilitating the transport of unstable electrons to more stable molecules, can participate in antioxidants pathways that are regulated through the action of “antioxidant” enzymes, which favors the transformation of reactive molecules into more stable and less harmful ones for the cell [21,28,40,41]. These enzymes are mainly stimulated by the KEAP1/NRF2 pathway, either by an increase in reactive species or by some molecules, in this case Asx. Cells exposed to Asx showed, at the transcriptional level, an effect of upregulation of NRF2 and downregulation of KEAP1 (p=0.05), its main inhibitor (Figure 6), which acts through binding to NRF2 and its subsequent degradation, preventing it from translocating to the nucleus and favoring the expression of antioxidant enzymes. As it can be seen (Figure 6), during differentiation at the transcriptional level, mRNA encoding for the enzymes CAT, SOD and GPX were also increased (p=0.05) compared to the protocol without Asx, indicating that Asx favors the expression of this signaling pathway and favors a more adequate control of ROS during differentiation of MSC.
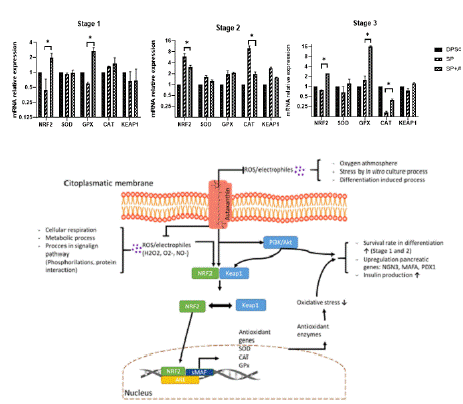
Figure 6: Schematic representation of the signaling pathways proposed
to be the targets of Asx action for regulation of oxidative stress and other
specific β-cell genes transcription.
Discussion
In recent years, cell therapy aimed at offering treatment alternatives for DM, mainly DM1, has faced various challenges, among them the selection of the type of cells, efficiency and optimization of protocols for adequate differentiation into functional pancreatictype cells [16,42-44]. DPSC (and their metabolites or products) have emerged as candidates of special interest in cell therapies, used for the treatment of DM, as well as their application in other diseases. This is due to its various characteristics such as its anti-inflammatory activity, antiapoptotic, regenerative and cytoprotective effects in general [45- 49]. Furthermore, DPSC have some advantages over other pluripotent cells; its ease of obtaining, its genetic stability by not forming teratomas, expression of pancreatic-type transcripts such as SOX17, NGN3, PDX1, PAX4, NEUROD1 and even INS. Even though these elements might have gone undetected in this study, various factors could account for it, such as variations in size, the distinctiveness of the sample, asynchrony in cell growth, and even harvest timing (pending verification in future studies by the research team). Moreover, their remarkable plasticity and capacity for proliferation further complicate detection. [14,35].
In addition, "Antioxidants" have the capability of generating cytoprotection both in vivo and in vitro, coupled with their interaction with signaling pathways that favors the functionality of cells [50-52]. In the family of carotenoids, antioxidant molecules of natural origin, Asx has stood out for providing antioxidant direct antioxidant protection, as a ROS scavenger, and indirectly through the modulation of antioxidant pathways such as NRF2/KEAP1 [21]. This antioxidant property is especially relevant in the processes for obtaining pancreatic cells, therefore in this study it was proven that there is an adequate modulation of ROS in vitro during a differentiation process towards insulin-producing cells, which favors the production of insulin, similar to what it is observed in processes of pancreatic organogenesis [29]. It should be noted that during the stages of pancreatic progenitor and pancreatic β cell formation, a decrease in ROS has been observed since there are relevant genes and processes susceptible to being negatively affected such as INS, MAFA and the insulin sensing process. As for the second stage where endocrine progenitors are generated, there is an increase in controlled oxidative stress, to favor the expression of some genes such as SOX9 or NGN3 [29,53].
In this study DPSC were obtained from a dental piece following the protocol reported by Hernandez et al. [36], which was obtained from a patient (female 18 years old) undergoing dental surgery at the Civil Hospital of Guadalajara, under informed consent. The DPSC were isolated and characterized based on basic criteria (morphology, phenotype, and plasticity) validated according to the ISCT to be MSC.
Asx, in addition to increasing insulin production, can generate cytoprotection by exerting a direct antioxidant effect mainly on H2O2 molecules and an indirect action by promoting adequate signaling of the NRF2 enzymatic antioxidant pathway. These ROS generated in differentiation processes in culture can alter signaling pathways, both for adequate differentiation and generate cellular oxidative damage if they are not adequately regulated, the latter especially in "long" in vitro processes. As reported by some authors such as Mas-Bargues, it is known that when stem cells are cultured at a level of oxygen that is not the same as that offered by the niche microenvironment from which they have been obtained, they suffer a series of alterations. That is, when working with cells in vitro, their manipulation causes changes in ROS generating oxidative stress, other alterations that have been reported are in their proliferation, self-renewal and differentiation potential. When using Asx in culture, we showed a decrease in ROS levels, for these reasons we consider that Asx, added to the differentiation process, helps to offer more optimal levels during the cultivation and differentiation processes [29,54]. We were able to verify that DPSC, like other multipotent cells, by themselves have a state of permanent oxidative stress (Figure 3A), which favors the activation of signaling pathways to maintain their pluripotency [55]. Likewise, the expression of pancreatic-type markers such as INS, PDX1, NGN3 was confirmed in these cells, however, the β cell maturity marker, MAFA, was not expressed (Figure 4E). The expression of these markers is believed to be due to the fact that embryonic cells that are directed to form both endocrine and dental cells share certain early stages in their embryonic development [14].
Similar to other authors that used antioxidants, the cells showed sensibility to release insulin when faced with a glucose stimulus, similar to what would be expected in a functional beta cell, it should be noted that Asx, have already been integrated into cell maturation processes [35,56-58]. Regarding the stabilizing activity of Asx against ROS/RNS, H2O2 could be identified as the main modulated intracellular ROS, highlighting an increase in stage 2, similar to that reported in a natural maturation process of β cells [29]. Regarding the indirect antioxidant activity, that is, on the KEAP1/NRF2 intracellular pathway, trends of different modulation patterns were observed when using Asx. In some differentiation processes, an oxidative stress stimulus is necessary for them to come out of their undifferentiated state; however, the activation of their regulation is also essential, as in this case through NRF2 [59]. In the second stage of differentiation, we observed an increase in ROS specifically for H2O2, whereas when adding Asx the increase is not as marked, allowing it to be what is necessary for a better differentiation, as reported to carry out the generation of endocrine progenitors, where this oxidative stress stimulus favors some markers such as SOX9 and NGN3 [29]. It should be noted that the CAT transcript, the enzyme responsible for transforming H2O2 into less reactive molecules such as H2O + O2, increases even with possible NRF2 inhibition, which could indicate a different pathway to NRF2; this increase was also reported in the organogenesis of an endocrine progenitor [60].
Finally, we can observe in the third stage a decrease in the expression of the NRF2 transcripts with respect to the CAT transcript, as already reported in functional beta cells where they can have up to 90% less of this enzyme compared to other cells, making it susceptible to damage from oxidative stress once the GSIS (glucose sensing insulin release) system begins to function properly, which generates many ROS that must be properly regulated [29,61]. It is of special interest that by adding Asx we can observe an increase in the expression of the NRF2 transcript and especially of GPx, an enzyme that is also responsible for transforming H2O2 into more stable molecules such as H2O, thus allowing, at least in vitro, maintain antioxidant protection through the expression of this enzyme, although in the organism a β cell they have relatively low levels of these enzymes probably due to greater control of their ROS by adjacent cells and tissues. Based on our findings, we demonstrated that Asx favors cell viability in the differentiation process in the first and second stages, also exerting an effect on the maturation of the insulin-producing cell through the expression of some pancreatic marker transcripts and the production of insulin.
Although it has been reported that Asx favors cell viability, but not in small concentrations such as the one used in the present study (10ng/mL), from the third differentiation stage onwards, however, it seems to exert an effect on other processes such as cell maturation. The insulin-producing cell through the expression of some pancreatic marker transcripts and insulin production, in addition to its effect on oxidative stress. The model of action of Asx during the differentiation process suggested by the previous results and some mechanisms already reported are shown in Figure 7 [21,27] . The foregoing, derived from the characteristics of its structure that facilitates internalization in the cell membrane, which favors its intra and extracellular effect [40].
Among the perspectives for the research team would be the use of Asx at different stages and concentrations during the differentiation protocol; challenge against other antioxidants and oxidants during the same protocol; as well as experimenting in 3D cultures or adding molecules that promote better cell-cell communication (extracellular matrix simulating biomaterials), since it has been reported that cell survival and maturation are limited in 2D culture conditions; and finally experiment with a possible coculture with other islet cells to promote communication similar to their pancreatic organogenesis [62,63].
Conclusion
The results of this work suggest that the in vitro protocol for differentiation towards insulin-producing cells used modulates similar to a natural organogenesis process of the pancreatic beta cell, an increase in the production and release of insulin under a glucose stimulus (GSIS), in addition to relevant pancreatic fate mRNAs such as NGN3 and MAFA. This molecule proved to have cytoprotective effects in differentiation processes in vitro, participating in the modulation of ROS especially H2O2, favoring the expression of NRF2 and its consequent antioxidant enzymes, generating a suitable microenvironment to promote differentiation in insulin-producing cells.
In the present study we show for the first time that the use of Asx throughout the differentiation process towards insulin-producing cells increases the efficiency of the protocol through the alleviation of oxidative stress during the process of trans differentiation. Therefore, it can be deduced that the use of Asx could promotes better cell differentiation through its antioxidant effect.
Highlights
• The Antioxidant Astaxanthin (Asx) improved the performance in this differentiation method from Dental pulp stromal cells (DPSC) to Insulin producing cells (IPC), evidenced by the functionality in insulin production, under glucose stimuli.
• Asx positively influences the maturity of insulin-producing cells, through the expression of pancreatic markers such as PDX1, NGN3 and MAFA.
• The production of reactive oxygen and nitrogen species (ROS/RNS) is decreased during the transdifferentiation process when adding Asx.
• In the KEAP1/NRF2 signaling pathway, there is an increase in the expression of transcripts of antioxidant enzymes CAT, SOD and GPX when Asx is added during the transdifferentiation process.
Author Statements
Author Contributions
Conceptualization, Sanchez G, Marino M and Flores H.; Methodology, Sanchez G, Flores H., Marino M and Gaona B; Investigation, Sanchez G, Flores H. and Marino M; Data Curation, Sanchez G and Bravo M; Writing – Original Draft Preparation, Sanchez G, Flores H and Marino M; Writing – Review & Editing, Sanchez G, Flores H., Marino M, Bravo M, Flores V and Gaona B; Images, Sanchez G; Supervision, Marino M., Flores H., Bravo M, Flores V and Gaona B.
Conflicts of Interest
The authors declare no potential conflicts of interest.
Funding
This work was carried out thanks to the support of CIATEJ A.C. and CONAHCYT through the student scholarship 2018-000068- 02NACF-22846.
Ethics Approval
The registration of the ethics committee for research with human samples was approved by COFEPRIS with number 14CEI14039048; by OPD Hospital Civil de Guadalajara with the number 084/15 HJCM/2015.
Data Availability Statement
The data used to support the findings of this study are available from the corresponding author upon request.
Acknowledgments
The authors thank all the other members, doctors, and partners, in their laboratory for their insight and technical support and the Center of Investigation and assistance in technology and design of state of Jalisco for infrastructure facilities.
References
- Sun H, Saeedi P, Karuranga S, Pinkepank M, Ogurtsova K, Duncan BB, et al. IDF Diabetes Atlas: Global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res Clin Pract. 2021; 183: 109119.
- Goyal R, Jialal I. Diabetes Mellitus Type 2 - StatPearls - NCBI Bookshelf - BAB II [Internet]. Diabetes Mellitus Type 2. 2021.
- Vieira A, Druelle N, Avolio F, Napolitano T, Navarro-Sanz S, Silvano S, et al. β-Cell Replacement Strategies: The Increasing Need for a β-Cell Dogma. Front Genet. 2017; 8: 75.
- Maiese K, Morhan SD, Chong ZZ. Oxidative Stress Biology and Cell Injury During Type 1 and Type 2 Diabetes Mellitus. Curr Neurovasc Res. 2007; 4: 63–71.
- Olvera-Granados CP, Leo-Amador GE, Hernández-Montiel HL. Páncreas y células beta: mecanismos de diferenciación, morfogénesis y especificación celular endocrina. ¿Regeneración? Bol Méd Hosp Infant México. 2008; 65: 306–24.
- Rodeman KB, Hatipoglu B. Beta-cell therapies for type 1 diabetes: Transplants and bionics. Cleve Clin J Med. 2018; 85: 931–7.
- Wartchow KM, Rodrigues L, Suardi LZ, Federhen BC, Selistre NG, Gonçalves CA, et al. Short-Term Protocols to Obtain Insulin-Producing Cells from Rat Adipose Tissue: Signaling Pathways and In Vivo Effect. Int J Mol Sci. 2019; 20: 2458.
- Arrighi N. Stem Cells at the Core of Cell Therapy. Stem Cells. 2018;73–100.
- Zakrzewski W, Dobrzynski M, Szymonowicz M, Rybak Z. Stem cells: Past, present, and future. Stem Cell Res Ther. 2019; 10: 1–22.
- Viswanathan S, Shi Y, Galipeau J, Krampera M, Leblanc K, Martin I, et al. Mesenchymal stem versus stromal cells: International Society for Cell & Gene Therapy (ISCT®) Mesenchymal Stromal Cell committee position statement on nomenclature. Cytotherapy. 2019; 21: 1019–24.
- Mastrolia I, Foppiani EM, Murgia A, Candini O, Samarelli AV, Grisendi G, et al. Challenges in Clinical Development of Mesenchymal Stromal/Stem Cells: Concise Review. Stem Cells Transl Med. 2019; 8: 1135–48.
- Baniebrahimi G, Khanmohammadi R, Mir F. Teeth-derived stem cells: A source for cell therapy. J Cell Physiol. 2019; 234: 2426–35.
- Anitua E, Troya M, Zalduendo M. Progress in the use of dental pulp stem cells in regenerative medicine. Cytotherapy. 2018; 20: 479–98.
- Xu B, Fan D, Zhao Y, Li J, Wang Z, Wang J, et al. Three-Dimensional Culture Promotes the Differentiation of Human Dental Pulp Mesenchymal Stem Cells Into Insulin-Producing Cells for Improving the Diabetes Therapy. Front Pharmacol. 2020; 10.
- Wallner K, Pedroza RG, Awotwe I, Piret JM, Senior PA, Shapiro AMJ, et al. Stem cells and beta cell replacement therapy: A prospective health technology assessment study. BMC Endocr Disord. 2018; 18: 1–12.
- Manaph NPA, Sivanathan KN, Nitschke J, Zhou XF, Coates PT, Drogemuller CJ. An overview on small molecule-induced differentiation of mesenchymal stem cells into beta cells for diabetic therapy. Stem Cell Res Ther. 2019; 10.
- Ghoneim MA, Refaie AF, Elbassiouny BL, Gabr MM, Zakaria MM. From Mesenchymal Stromal/Stem Cells to Insulin-Producing Cells: Progress and Challenges. Stem Cell Rev Rep. 2020; 16: 1156–72.
- Jayasinghe M, Prathiraja O, Perera PB, Jena R, Silva MS, Weerawarna PSH, et al. The Role of Mesenchymal Stem Cells in the Treatment of Type 1 Diabetes. Cureus. 2022; 14: e27337.
- Cho J, D’Antuono M, Glicksman M, Wang J, Jonklaas J. A review of clinical trials: mesenchymal stem cell transplant therapy in type 1 and type 2 diabetes mellitus. Am J Stem Cells. 2018; 7: 82–93.
- Ambati RR, Phang SM, Ravi S, Aswathanarayana RG. Astaxanthin: Sources, extraction, stability, biological activities and its commercial applications - A review. Mar Drugs. 2014; 12: 128–52.
- Fakhri S, Abbaszadeh F, Dargahi L, Jorjani M. Astaxanthin: A mechanistic review on its biological activities and health benefits. Pharmacol Res. 2018; 136: 1–20.
- Al-Bulish MSM, Xue C, Waly MI, Xu J, Wang Y, Tang QJ. The Defensive Role of Antioxidants Astaxanthin against Oxidative Damage in Diabetic Rats Injected with Streptozotocin. J Food Nutr Res. 2017; 5: 191–6.
- Grimmig B, Kim SH, Nash K, Bickford PC, Shytle RD. Neuroprotective mechanisms of astaxanthin: a potential therapeutic role in preserving cognitive function in age and neurodegeneration. GeroScience. 2017; 39: 19–32.
- Kim JH, Nam SW, Kim BW, Choi W, Lee JH, Kim WJ, et al. Astaxanthin improves stem cell potency via an increase in the proliferation of neural progenitor cells. Int J Mol Sci. 2010; 11: 5109–19.
- Kim JH, Nam SW, Kim BW, Kim WJ, Choi YH. Astaxanthin improves the proliferative capacity as well as the osteogenic and adipogenic differentiation potential in neural stem cells. Food Chem Toxicol. 2010; 48: 1741–5.
- Soto J, Ding X, Wang A, Li S. Neural crest-like stem cells for tissue regeneration. Stem Cells Transl Med. 2021; 10: 681–93.
- Landon R, Gueguen V, Petite H, Letourneur D, Pavon-Djavid G, Anagnostou F. Impact of Astaxanthin on Diabetes Pathogenesis and Chronic Complications. Mar Drugs. 2020; 18: 357.
- Mohammadi S, Barzegari A, Dehnad A, Barar J, Omidi Y. Astaxanthin protects mesenchymal stem cells from oxidative stress by direct scavenging of free radicals and modulation of cell signaling. Chem Biol Interact. 2021; 333: 109324.
- Wang J, Wang H. Oxidative Stress in Pancreatic Beta Cell Regeneration. Oxid Med Cell Longev. 2017; 2017: e1930261.
- Alfar EA, Kirova Di, Konantz J, Birke S, Mansfeld J, Ninov N. Distinct Levels of Reactive Oxygen Species Coordinate Metabolic Activity with Beta-cell Mass Plasticity. Sci Rep. 2017; 7: 1–12.
- Lenzen S. The pancreatic beta cell: an intricate relation between anatomical structure, the signalling mechanism of glucose-induced insulin secretion, the low antioxidative defence, the high vulnerability and sensitivity to diabetic stress. ChemTexts. 2021; 7: 1–6.
- Denu RA, Hematti P. Optimization of oxidative stress for mesenchymal stromal/stem cell engraftment, function and longevity. Free Radic Biol Med. 2021; 167: 193–200.
- Denu RA, Hematti P. Effects of Oxidative Stress on Mesenchymal Stem Cell Biology. Oxid Med Cell Longev. 2016; 2016.
- Swisa A, Glaser B, Dor Y. Metabolic stress and compromised identity of pancreatic beta cells. Front Genet. 2017; 8: 1–11.
- Villa García Torres, Laura Susana. Efecto de la astaxantina en la diferenciación de células troncales de pulpa dental y medula ósea hacia células β pancreáticas productoras de insulina. [Guadalajara, Jalisco]: CENTRO DE INVESTIGACIóN Y ASISTENCIA EN TECNOLOGíA Y DISEÑO DEL ESTADO DE JALISCO AC. 2018.
- Hernandez FYF, Bedoy HMR, Torres LSVG, Pelayo GLG, Santibáñez LPE. Aislamiento, cultivo y caracterización de células estromales mesenquimales de pulpa dental provenientes de población mexicana: perspectivas en el desarrollo de terapia celular. Aisl Cultivo Caracterización Células Estromales Mesenquimales Pulpa Dent Provenientes Poblac Mex Perspect En El Desarro Ter Cel. 1–416.
- Jamur MC, Oliver C. Permeabilization of Cell Membranes. In: Oliver C, Jamur MC, editors. Immunocytochemical Methods and Protocols. Totowa, NJ: Humana Press. 2010: 63–6.
- Biological evaluation of medical devices – Part 5: Tests for in vitro cytotoxicity.
- Natural Compounds as a Strategy to Optimize “In Vitro” Expansion of Stem Cells | Rejuvenation Research. 2023.
- Yamashita E. Let astaxanthin be thy medicine. Pharma Nutrition. 2015; 3: 115–22.
- Merhan O. The Biochemistry and Antioxidant Properties of Carotenoids. In: Cvetkovic DJ, Nikolic GS, editors. Carotenoids. 2017.
- Lukomska B, Stanaszek L, Zuba-Surma E, Legosz P, Sarzynska S, Drela K. Challenges and Controversies in Human Mesenchymal Stem Cell Therapy. Stem Cells Int. 2019; 2019.
- Saeedi P, Halabian R, Fooladi AAI. A revealing review of mesenchymal stem cells therapy, clinical perspectives and Modification strategies. Stem Cell Investig. 2019; 6: 34.
- Memon B, Abdelalim EM. Stem Cell Therapy for Diabetes: Beta Cells versus Pancreatic Progenitors. Cells. 2020; 9: 283.
- Vizoso FJ, Eiro N, Cid S, Schneider J, Perez-Fernandez R. Mesenchymal Stem Cell Secretome: Toward Cell-Free Therapeutic Strategies in Regenerative Medicine. Int J Mol Sci. 2017; 18: 1852.
- Vizoso FJ, Eiro N, Costa L, Esparza P, Landin M, Diaz-Rodriguez P, et al. Mesenchymal Stem Cells in Homeostasis and Systemic Diseases: Hypothesis, Evidences, and Therapeutic Opportunities. Int J Mol Sci. 2019; 20: 3738.
- Makino E, Nakamura N, Miyabe M, Ito M, Kanada S, Hata M, et al. Conditioned media from dental pulp stem cells improved diabetic polyneuropathy through anti-inflammatory, neuroprotective and angiogenic actions: Cell-free regenerative medicine for diabetic polyneuropathy. J Diabetes Investig. 2019; 10: 1199–208.
- Anitua E, Troya M, Zalduendo M. Progress in the use of dental pulp stem cells in regenerative medicine. Cytotherapy. 2018; 20: 479–98.
- Gugliandolo A, Mazzon E. Dental Mesenchymal Stem Cell Secretome: An Intriguing Approach for Neuroprotection and Neuroregeneration. Int J Mol Sci. 2022; 23: 456.
- Sun LY, Pang CY, Li DK, Liao CH, Huang WC, Wu CC, et al. Antioxidants cause rapid expansion of human adipose-derived mesenchymal stem cells via CDK and CDK inhibitor regulation. J Biomed Sci. 2013; 20: 1–11.
- Panahi M, Rahimi B, Rahimi G, Low TY, Saraygord-Afshari N, Alizadeh E. Cytoprotective effects of antioxidant supplementation on mesenchymal stem cell therapy. J Cell Physiol. 2020; 235: 6462–95.
- Liao N, Shi Y, Zhang C, Zheng Y, Wang Y, Zhao B, et al. Antioxidants inhibit cell senescence and preserve stemness of adipose tissue-derived stem cells by reducing ROS generation during long-term in vitro expansion. Stem Cell Res Ther. 2019; 10: 306.
- Leenders F, Groen N, de Graaf N, Engelse MA, Rabelink TJ, de Koning EJP, et al. Oxidative Stress Leads to β-Cell Dysfunction Through Loss of β-Cell Identity. Front Immunol. 2021; 12: 690379.
- Mas-Bargues C, Sanz-Ros J, Román-Domínguez A, Inglés M, Gimeno- Mallench L, El Alami M, et al. Relevance of Oxygen Concentration in Stem Cell Culture for Regenerative Medicine. Int J Mol Sci. 2019; 20: 1195.
- de la Maza DS, Moratilla A, Aparicio V, Lorca C, Alcaina Y, Martín D, et al. Metabolic Reprogramming, Autophagy, and Reactive Oxygen Species Are Necessary for Primordial Germ Cell Reprogramming into Pluripotency. Oxid Med Cell Longev. 2017; 2017: e4745252.
- Hamid ZA, Sellappah P, Bharatham H, Mathialagan RD, Kannan VD. Role of N-acetyl cysteine on bone marrow-derived hematopoietic stem/progenitor cell cryopreservation: Outcome on the oxidative stress-mediated cryodamage and repopulation Capacity into hematopoietic lineages. J Appl Pharm Sci. 2020; 10: 022–31.
- Berniakovich I, Laricchia-Robbio L, Belmonte JCI. N-acetylcysteine protects induced pluripotent stem cells from in vitro stress: impact on differentiation outcome. Int J Dev Biol. 2012; 56: 729–35.
- Horikawa A, Mizuno K, Tsuda K, Yamamoto T, Michiue T. A simple method of hiPSCs differentiation into insulin-producing cells is improved with vitamin C and RepSox. PLOS ONE. 2021; 16: e0254373.
- Kaitsuka T, Hakim F. Response of Pluripotent Stem Cells to Environmental Stress and Its Application for Directed Differentiation. Biology. 2021; 10: 84.
- Liang J, Wu SY, Zhang D, Wang L, Leung KK, Leung PS. NADPH Oxidase- Dependent Reactive Oxygen Species Stimulate β-Cell Regeneration Through Differentiation of Endocrine Progenitors in Murine Pancreas. Antioxid Redox Signal. 2016; 24: 419–33.
- Pi J, Zhang Q, Fu J, Woods CG, Hou Y, Corkey BE, et al. ROS signaling, oxidative stress and Nrf2 in pancreatic beta-cell function. Toxicol Appl Pharmacol. 2010; 244: 77–83.
- Jiang FX, Harrison LC. Extracellular Signals and Pancreatic β-cell Development: A Brief Review. Mol Med. 2002; 8: 763–70.
- Townsend SE, Gannon M. Extracellular Matrix–Associated Factors Play Critical Roles in Regulating Pancreatic β-Cell Proliferation and Survival. Endocrinology. 2019; 160: 1885–94.