
Research Article
Austin J Biotechnol Bioeng. 2015;2(1): 1034.
Enhanced Production of Novel Glutaminase Free Recombinant L-Asparaginase II of Erwinia Carotovora Subsp. Atroseptica Scri 1043 in Escherichia Coli BL21 (DE3) in a Batch and Fed-Batch Culture
Rachna Goswami1,2, Venkata Dasu Veeranki1* and Krishnamoorthy Hegde1
1Department of Biotechnology, Indian Institute of Technology Guwahati, India
2Department of Bioscience, AP IIIT Nuzvid, Rajiv Gandhi University of Knowledge Technologies (RGUKT), India
*Corresponding author: Venkata Dasu V, Biochemical Engineering Laboratory, Department of Biotechnology, Indian Institute of Technology Guwahati, Guwahati 781039, Assam, India.
Received: November 06, 2014; Accepted: December 03, 2014; Published: January 29, 2015
Abstract
L-asparaginase (E.C.3.5.1.1) is used for treatment of acute lymphoblastic leukemia. It is also used as a processing aid for reducing the formation of acrylamide in starchy foods preparations. In this study, the effects of glucose, controlled pH and Dissolved Oxygen Concentration (DOC) level on cell growth and production of novel glutaminase free recombinant L-asparaginase II of Erwinia carotovora subsp. atroseptica SCRI 1043, expressed in Escherichia coli BL21 (DE3) were investigated in a batch bioreactor using Taguchi experimental design technique. At optimum circumstances of glucose (1.5 g/l), controlled pH (7.0) and DOC (40%), the maximum dry cell weight and production of recombinant L-asparaginase II was found to be 1.84 g/l and 24.57 U/ml, respectively. Fed-batch culture is used frequently to increase expression of heterologous recombinant proteins in Escherichia coli. Therefore, the production of recombinant L-asparaginase II was performed in fed batch culture. In fed-batch fermentation, 7.35 g/l of dry cell weight and 96.78 U/ml of recombinant glutaminase free L-asparaginase II were achieved, corresponding to about four fold increase in dry cell weight and production as compared with the batch culture.
Keyword: Erwinia carotovora subsp. atroseptica SCRI 1043; Recombinant L-asparaginase II; Taguchi’s method; Batch and Fed batch
Introduction
L-asparaginase (L-asparagine amidohydrolase, EC 3.5.1.1) has been widely used for the treatment of acute lymphoblastic leukemia and non-Hodgkin’s lymphoma [1]. It is also used in food industry for acrylamide free food production [2] model enzyme for the development of new drug delivery system [3] and L-asparagine biosensor for leukemia [4]. The antileukemic effect of L-asparaginase is postulated to result from the rapid and complete depletion of the circulating pool of L-asparagine, as most of the cancer cells are dependent on an exogenous source of this amino acid for survival. However, normal cells are able to synthesize L-asparagine and thus are less affected by its rapid depletion due to treatment with this enzyme. The L-asparagine deficiency rapidly impairs the protein synthesis and leads to a delayed inhibition in DNA and RNA synthesis and hence an impairment of cellular functions, resulting in cell death [5,6]. Studies on the molecular structure [4], catalysis [7], clinical aspects [5], genetic determinants involved in regulation [8] and stabilization to enhance biological half-life [9] of L-asparaginase have been reported. Several gram-negative bacteria contain two L-asparaginases, a low affinity cytoplasmic enzyme and a highaffinity periplasmic enzyme. In Escherichia coli and several other bacteria, the synthesis of cytoplasmic asparaginase I is constitutive, while expression of periplasmic asparaginase II is activated during anaerobiosis. It has been suggested that the latter one probably has a special function in anaerobic fumarate respiration by providing aspartate, which is then converted to fumarate. Further, only the type II enzyme has shown substantial antitumor activity [10]. The various side effects of this drug are mainly due to the presence of partial glutaminase activity [11]. Hence, for successful clinical studies, glutaminase free L-asparaginase is highly desirable.
The production of L-asparaginase has been studied in Serratia marcescens [12,13], Erwinia carotovora [14], E. coli [15], Enterobacter aerogenes [16], Pseudomonas aeruginosa [17], and Bacillus subtilis [10] with various carbon and nitrogen sources. The synthesis of L-asparaginase by gram negative bacteria is regulated by environmental and nutritional factors. The results are contradictory in terms of the effect of glucose [12,15] and oxygen [16] on the production of this enzyme. On the other hand, the production of L-asparaginase from wild strain is very limited. Therefore, recombinant L-asparaginases were developed to increase the expression levels [5,8,9,18]. The interest in E. carotovora L-asparaginase II arose from its significantly lower glutaminase activity as compared to that exhibited by E. coli and E. chrysanthemi enzymes [5].
The production of any metabolite varies from shake flask to bioreactor fermentation. This might be possible due to uncontrolled pH, agitation pattern and aeration in shake flask fermentation. The strategy of studying one variable at a time and keeping all others at a predetermined level is very ineffective in many cases and also a time consuming technique [19,20]. Factorial designs are not preferred to optimize variables in bioreactors, as the experiments cannot be carried out in blocks. Additionally, they need more information to design parameter levels [21]. Taguchi’s method has been used in industrial process design, mostly in developmental trials. This method is used to produce adequate process information to set up the screening and optimal conditions of parameters for particular process using a minimum number of experiments possible. The basic principle of this technique serves as screening filters, which evaluate the effects of various process variables and identify those factors which have main effects on the process using less number of experiments [22].
Escherichia coli have been used most widely as a host system for the expression of recombinant proteins, as it was characterized in terms of its physiology, molecular genetics and expression systems [23]. The High Cell Density Culture (HCDC) techniques have been developed using E. coli systems for the production of recombinant proteins with high productivities [24-26].
The selection of nutrient feeding strategy plays a vital role in high cell density cultivations as it affects the metabolic pathway fluxes, and consequently influences the the specific productivity of recombinant proteins, formation of by-products (acetic acid) and cell growth. A significant relation was observed between the post-induction glucose feeding strategy and recombinant protein production [27,28]. The feed rate of glucose would be adjusted to control the formation of acetate in fed-batch cultures. To date, there is no rule established for selecting a particular feeding strategy to achieve maximum productivity for a given recombinant protein. Prior to carbon source, the nitrogen source is also very much important for extending the cell growth and improving protein production [29,30].
In this work, we investigated the influence of initial glucose concentration, controlled pH and Dissolved Oxygen Concentration (%) (DOC) level on the production of glutaminase free recombinant L-asparaginase II of E. carotovora subsp. atroseptica SCRI 1043 in E. coli BL21 (DE3) using Taguchi’s experimental technique in a batch bioreactor. Recombinant L-asparaginase II production was also performed in fed batch culture for enhanced productivity.
Materials and Methods
Chemicals
Isopropyl- ß-d-Thiogalactopyranoside (IPTG) and ampicillin were purchased from Sigma, India. Culture media and their constituents, L-asparagine, ammonium sulfate, were procured from Hi-Media, India. Nessler’s reagent was purchased from Loba Company, India. All chemicals were procured from Sigma unless otherwise stated and were of the highest quality.
Bacterial strains and plasmid
Erwinia carotovora subsp. atroseptica SCRI 1043 was kindly provided by Dr. Paul Birch, Scotland Crop Research Institute (SCRI), Scotland. Escherichia coli BL21 (DE3) and plasmid pET 22b(+) were purchased from Novagen, USA. The gene encoding L-asparaginase II of E. carotovora subsp. atroseptica SCRI 1043 was amplified by PCR, and introduced between BamHI and XhoI restriction sites of pET 22 b(+) in the downstream of the T7 promoter and resultant recombinant construct was transformed in E. coli BL21 (DE3) for recombinant L-asparaginase II expression [20]. The over-expression of cloned gene was controlled by T7 polymerase responsive promoter. The positive clone was confirmed by restriction digestion analysis and colony PCR (data not shown). It was maintained in 20% sterile glycerol at -80oC.
Inoculum development
A loopful of frozen glycerol stock culture (kept at -80°C) was streaked on a LB-agar plate containing ampicillin (100 μg/ml) and incubated at 37°C for 14–16 h. A single colony was isolated and transferred in 20.0 ml of sterile LB medium containing ampicillin (100 μg/ml) in Erlenmeyer flask (100 ml) on a rotary shaker at 37°C and 200 rpm for 6–8 h and this pre-inoculum was transferred at a rate of 2.34 % (v/v) to the inoculum medium (tryptone: 14.50 g/l, yeast extract: 5.30 g/l and NaCl: 4.03 g/l).
Optimization methodology for enhanced production of recombinant L-asparaginase II of E. carotovora subsp. atroseptica SCRI 1043 in batch bioreactor
In order to minimize the number of experiments and for medium components optimization, shake flask experiments were carried out by exploiting central composite design (data not shown). It is well accounted that production of any metabolite varies from shake flask to bioreactor fermentation. This might be because of uncontrolled pH, agitation pattern and aeration in shake flask fermentation. It is reported that on addition of glucose less than 0.05%, the plasmid stability and protein yields improves [20,31,32]. Therefore, Taguchi’s method was applied to know the parameters, which significantly affect the recombinant L-asparaginase II production in a batch bioreactor. Three parameters (glucose, controlled pH and DOC) in nine experiments were used to evaluate the influence of variables on recombinant L-asparaginase II production according to Taguchi’s orthogonal array. The parameters and their levels employed in Taguchi’s experimental design are mentioned in the Table 1. The glucose concentration and levels of controlled pH and DOC changed according to the experimental plan given in the Table 2. MINITAB® Release 15.1, PA, USA was used in the current study. In each experimental run, the result was recorded as the recombinant L-asparaginase II production (U/ml) and corresponding signal-tonoise (S/N) ratio was calculated by Eq. 1 with an overall objective for estimating the effects of a range of parameters on recombinant L-asparaginase II production, where a large S/N ratio is desired.
Parameters
Constituents
Levels
1
2
3
A
Glucose (g/l)
0.5
1.0
1.5
B
pH
6.5
7.0
7.5
C
DOC (%)
10
25
40
Table 1: Parameters and their levels employed in the Taguchi’s robust design method for recombinant L-asparaginase II production for batch study in bioreactor.
Where, Y is the outcome [enzyme activity (U/ml)] and n is the number of experimental runs. Statistical analysis of the results was performed, in the form of analysis of variance (ANOVA). Validity of the model was verified by conducting the experiment at optimal level of parameters in bioreactor.
All batch fermentations were carried out in a 3.0 L bioreactor (Applikon, Holland) with 1.0 L working volume. The bioreactor was fitted with essential controllers. With 2 M NaOH and/or 2 M HCl, the pH of the medium was adjusted. According to experimental plan by varying airflow and impeller speed, the levels of pH (controlled) and The DOC was maintained. During growth phase, microorganism was grown at 37°C whereas, for production of recombinant L-asparaginase II, temperature was decreased to 30°C (previously optimized temperature for production). When cell density at Ab600nm reached 1.50 to 1.80 (~ after 3 h), production of recombinant protein was induced with 1mM IPTG and further cultivated for 12 h at 30°C. The samples were collected at regular intervals of time and enzyme activity was measured in triplicates and averages of the results were taken as response.
Fed-batch cultivation
To enhance the cell concentration and productivity, the production of recombinant L-asparaginase II of E. carotovora subsp. atroseptica SCRI 1043 in E. coli was performed in Fed-batch culture (3.0 L Bioreactor, Applikon, Holland). Initial batch culture was started with 1.0 L of medium. After the utilization of glucose, fed-batch experiments were performed by feeding the solutions containing glucose (100 g/l), yeast extract (100 g/l) and 100 mg/l ampicillin. Concentration of glucose in the medium was observed at regular interval of time and fed by intermittent pulse feeding in relative to the cell mass. Feeding of glucose with specific feed rate of 0.5 g glucose/g DCW/ h and 0.25 g glucose/g DCW/ h was performed in the growth phase and production phase, correspondingly. The residual glucose was monitored and maintained below 1.0 g/l [20,28,33-35]. During the growth phase, microorganism was grown at 37°C and in production phase, temperature was reduced to 30°C. The biomass concentration in the bioreactor was measured throughout the fermentation, by calculating the optical density (OD) as well as the dry cell weight. The DOC was restricted between 35-40% of saturation by changing agitation (350-1200 rpm) and aeration (2.0- 3.0 vvm). The pH was maintained at 7.0 by adding 2N HCl and/or 2N NaOH. The recombinant protein expression was induced by adding 1mM IPTG at 30°C, when the cell OD at Ab600nm reached ~10.00.
Analytical methods
Assays for L-glutaminase and L-asparaginase activity: L-asparaginase activity was measured in terms of hydrolysis rate of L-asparagine by calculating the quantity of ammonia released in the reaction assay of L-asparaginase. Assay for glutaminase and L-asparaginase was carried out as described earlier [20,36]. The ammonia produced in the reaction was determined on the basis of standard curve obtained with ammonium sulfate as standard. The amount of enzyme that liberates 1 μmol of ammonia per minute at 37°C was defined as one unit of enzyme activity. Specific activity is denoted as units per milligram of protein.
Protein determination: The total protein contents of the samples were determined according to the method described by Lowry et al. [37] using bovine serum albumin (Sigma) as standard.
Estimation of DCW: The cell density was calculated by measuring the culture’s optical density at Ab600nm with a UV-visible spectrometer. Dry cell weight was calculated by centrifuging the sample broth at 8000g for 10 min and drying the washed cells to constant weight at 80°C.
Estimation of glucose: Residual sugar in the samples was determined by the 3,5-Dinitrosalicylic Acid (DNS) method. Glucose was used as standard [38].
Measurement of plasmid stability: Plasmid stability was measured as described earlier by Goswami et al. 2014 [20].
Results and Discussion
The results of the Taguchi’s experiments are presented in the Table 2. Depending upon the combination of the levels of chemical and physical process parameters, recombinant L-asparaginase II production altered in each run significantly, signifying the strong effect of the variables and their levels on the response. Their ranking was performed based on the calculated delta S/N ratio, to evaluate the effect of these variables on recombinant L-asparaginase II production. In general, delta value for a factor, calculated by determining the difference between the maximum and minimum characteristic average S/N ratio of the factor, indicates its relative significance over others on a particular response. For a factor, higher value of delta signifies a larger significant effect than others, while S/N ratio points to effect of factors on a response. Thus delta S/N ratio have used as an important factor for ranking the variables for their effects on the response [40]. In this study, based on the delta S/N ratio obtained for each factor, all parameters were ranked accordingly which has shown that pH had the maximum effect and followed by glucose concentration and DOC (Table 3). To validate these findings on the importance of the individual parameters and their involvement on L-asparaginase production, ANOVA was employed. Table 4 presents the ANOVA of L-asparaginase II production of E. carotovora subsp. atroseptica SCRI 1043 in E. coli). In general, low P value of a term in ANOVA specifies high importance of the term. In this study, all the parameters viz., glucose concentration, pH and DOC were observed to have a significant effect on recombinant L-asparaginase II production. The effect of parameters on L-asparaginase production by ANOVA is in good agreement with those observed previously from the factors ranking based on their delta S/N ratio.
Run No.
Glucose (g/l)
pH
DOC (%)
E. carotovora subsp. atroseptica SCRI 1043
Activity (U/ml)
Sp. Activity (U/mg)
S/N
1
0.5
6.5
10
3.81
27.41
11.61
2
0.5
7.0
25
14.98
49.84
23.51
3
0.5
7.5
40
12.35
42.62
21.83
4
1.0
6.5
25
6.02
41.06
15.59
5
1.0
7.0
40
16.98
57.95
24.59
6
1.0
7.5
10
11.12
35.63
20.92
7
1.5
6.5
40
13.13
49.02
22.36
8
1.5
7.0
10
19.24
52.21
25.68
9
1.5
7.5
25
16.99
51.25
24.60
Table 2: Taguchi’s robust experimental design matrix and recombinant Lasparaginase II production of E. carotovora subsp. atroseptica SCRI 1043 in E. coli in bioreactor.
Level
Glucose
pH
DOC
1
18.99
16.53
19.41
2
20.37
24.60
21.24
3
24.22
22.45
22.93
Delta
5.23
8.07
3.52
Rank
2
1
3
Table 3: Values of average S/N ratio of the process variables at various levels and their ranking on the basis of delta S/N ratio.
Source
DF
Seq SS
Ads SS
Ads MS
F
P
Glucose
2
63.678
63.678
31.8392
120.51
0.008
pH
2
135.455
135.455
67.7275
256.34
0.004
DOC
2
11.477
11.477
5.7387
21.72
0.044
Residual Error
2
0.528
0.528
0.2642
Total
8
211.139
R-Sq = 99.7%, R-Sq(adj) = 99.0%
Table 4: Analysis of variance for production of recombinant L-asparaginase II of E. carotovora subsp. atroseptica SCRI 1043.
Selection of optimal levels of parameters for enhanced recombinant L-asparaginase II production
All selected parameters (glucose, pH and DOC) were tested at different levels; particular levels of the variables caused a significant increase in the mean response as compared to other levels of the variables. For e.g. level 3 of glucose and DOC and level 2 of pH were found to exhibit a noteworthy positive consequence on the response (Figure 1). Therefore, these levels of the variables were selected as optimum for the highest production of recombinant L-asparaginase II in the medium. In the optimized conditions, production of recombinant L-asparaginase II was increased. At the most favorable levels of factors, recombinant L-asparaginase II production was carried out in a batch-operated 3 L bioreactor (Applikon, Holland) with 1.0 L medium. It was observed that the production of recombinant L-asparaginase was very high (24.57 U/ ml) in the bioreactor, as compared to any of those values given in Table 2. The production of recombinant L-asparaginase was found to be 1.40 fold higher under optimal levels of parameters as compared to un-optimized conditions (17.58 U/ml. Due to higher growth of cells in bioreactor, sp. activity of enzyme was lower (61.71 U /mg) than shake flask (64.62 U/mg). Maximum production of L-asparaginase was achieved at 7 h of fermentation in a batch bioreactor and plasmid stability analysis was carried out by comparing the number of CFUs (colony forming units) on amp+ and amp- plates formed after plating the culture, withdrawn at different time-points throughout cultivation. The presence of ampicillin resistance was used as an indicator for the existence of recombinant plasmid in the cells. It was found that 74% of the culture retained the recombinant plasmid at 24 h post-induction, comparable to the shake flask cultivation.
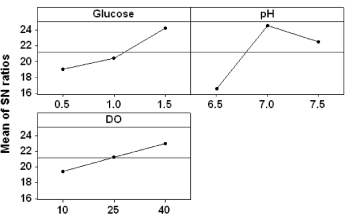
Figure 1: Main effects plot of the process variables.
To the best of our knowledge, we could not find any report on optimization of recombinant L-asparaginase II production by Taguchi’s method for recombinant L-asparaginase II production of E. carotovora subsp. atroseptica SCRI 1043 fin E. coli BL21 (DE3). However, there are many reports available for optimization of production conditions for recombinant and non-recombinant proteins by utilizing Taguchi’s method. Goswami et al. [20] found similar kind of result after optimization of parameters by Taguchi’s method. They have reported 1.34 fold higher production of recombinant L-asparaginase II (23.88 U/ml) of P. carotovorum MTCC 1428 in E. coli after optimization of parameters by Taguchi’s method in batch culture. Dasu et al. [22] has optimized the griseofulvin production from Penicillium griseofulvum MTCC 1898 in a batch bioreactor. Niccolai et al. [41] has optimize the production of recombinant Helicobacter pylori neutrophila activating (NAP) protein from E. coli in bioreactor by Taguchi’s method and an overall 2.91-fold increase in recombinant NAP production was achieved. Hao et al. [42] have also exploited Taguchi’s method to screen the significant medium components for optimization of the medium for cytochrome P450 2C9 production from E. coli DH5á. Ghane et al. [43] have produced the 28% higher production of interferon beta from E. coli using Taguchi’s methodology. Recently Daverey et al. [40] has optimized the sophorolipid (SL) production by the yeast Candida bombicola in a batch bioreactor using Taguchi’s method.
Enhanced production of recombinant L-asparaginase II by fed-batch culture
Fed-batch culture was performed II in a 3 L bioreactor to achieve high production of recombinant L-asparaginase. The glucose was fed at a rate of 0.5 g glucose/g DCW/ h in growth phase and 0.25 g glucose/g DCW/ h in the production phase. As the concentration of glucose dropped below 1.0 g/l, the feeding was started. In fed-batch mode of fermentation, four fold higher production of recombinant L-asparaginase II (96.78 U/ml) of E. carotovora subsp. atroseptica SCRI 1043 in E. coli was achieved as compared to the batch culture and the maximum biomass and plasmid stability was found to be 7.35 g/l and 62%, respectively. The production of recombinant L-asparaginase II was enhanced to a maximum and then started to decline, within 8 h of post-induction. It might be due to considerable plasmid loss was observed after 8 h of post induction (Figure 2). Goswami et al. [20] have also reported recombinant L-asparaginase II production of Pectobacterium carotovorum MTCC 1428 in E. coli. They have achieved maximum 7.32 g/l dry cell weight biomass and 95.85 U/ml of recombinant L-asparaginase II, respectively. It was about four fold higher as compared to batch bioreactor. Khushoo et al. [44] have also produced recombinant L-asparaginase of E. coli in fed batch culture and highest volumetric yield of 8.7×105 units/l was achieved. Zhang et al. [35] have reported improved production of anticancer drug TATm-survivin (T34A) in E. coli using this feeding strategy. Zhang et al. [35] studied three feeding rates of glucose supply (1.06 g/g DCW/ h, 0.64 g/g DCW/ h and 0.25 g/g DCW/ h) and observed the opposite relationship between the glucose feeding rate and the production level and observed that low feeding rate of 0.25 g/g DCW/h gave the highest production of T34A. Giridhar and Srivastava [34] have attained higher production of L-sorbose from Acetobacter suboxydans by pulse feeding strategy. In addition, higher production of Phytolacca insularis protein in fed-batch culture of recombinant E. coli was achieved by step by step increasing feeding, according to the biomass [45].
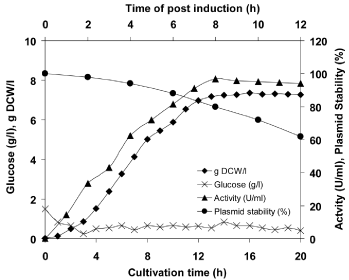
Figure 2: Production of recombinant L-asparaginase II of E. carotovora
subsp. atroseptica SCRI 1043 in a fed-batch culture.
For the mass production of recombinant protein, fed-batch techniques are often employed to achieve a high volumetric yield. Fed-batch cultures have been successfully used to increase the yields of recombinant protein for e.g. recombinant streptokinase from E. coli [39], recombinant phytase from E. coli [46], recombinant 6-deoxyerythronolide B from E. coli [47], the ergosterol production by Saccharomyces cerevisiae [48], recombinant human interferon-γ [49], pectin lyase and pectate lyase from Debaryomyces nepalensis [49], PHB from Methylobacterium sp. ZP24 [51], recombinant human soluble catechol-O-methyltransferase (hSCOMT) from E. coli [52], Microbial production of acetoacetate by recombinant E. coli [53], monoamine oxidase by recombinant E. coli [54] and recombinant potato carboxypeptidase inhibitor from E. coli [55].
Conclusion
Production of recombinant L-asparaginase II of E. carotovora subsp. atroseptica SCRI 1043 in E. coli was performed in batch and fed batch mode of fermentation in bioreactor. Taguchi’s methodology was found to be effective for optimization of fermentation conditions for higher production of recombinant L-asparaginase II. After optimization of fermentation condition 1.40 folds higher production was obtained as compared to un-optimized condition in shake flask. In fed batch mode of fermentation 5.51 folds higher production was achieved than un-optimized condition in shake flask.
Acknowledgement
The authors thankfully acknowledge Department of Biotechnology, Indian institute of Technology Guwahati and Department of Biotechnology (DBT), Government of India for providing instrumentation facility and financial help, respectively.
References
- Narta UK, Kanwar SS, Azmi W. Pharmacological and clinical evaluation of L-asparaginase in the treatment of leukemia. Crit Rev Oncol Hematol. 2007; 61: 208-221.
- Hendriksen HV, Kornbrust BA, Østergaard PR, Stringer MA. Evaluating the potential for enzymatic acrylamide mitigation in a range of food products using an asparaginase from Aspergillus oryzae. J Agric Food Chem. 2009; 57: 4168-4176.
- Teodor E, Litescu SC, Lazar V, Somoghi R. Hydrogel-magnetic nanoparticles with immobilized L-asparaginase for biomedical applications. J Mater Sci Mater Med. 2009; 20: 1307-1314.
- Verma N, Kumar K, Kaur G, Anand S. L-asparaginase: a promising chemotherapeutic agent. Crit Rev Biotechnol. 2007; 27: 45-62.
- Kotzia GA, Labrou NE. Cloning, expression and characterisation of Erwinia carotovora L-asparaginase. J Biotechnol. 2005; 119: 309-323.
- Douer D. Is asparaginase a critical component in the treatment of acute lymphoblastic leukemia? Best Pract Res Clin Haematol. 2008; 21: 647-658.
- Dunlop PC, Roon RJ, Even HL. Utilization of D-asparagine by Saccharomyces cerevisiae. J Bacteriol. 1976; 125: 999-1004.
- Khushoo A, Pal Y, Singh BN, Mukherjee KJ. Extracellular expression and single step purification of recombinant Escherichia coli L-asparaginase II. Protein Expr Purif. 2004; 38: 29-36.
- Kotzia GA, Labrou NE. L-Asparaginase from Erwinia Chrysanthemi 3937: cloning, expression and characterization. J Biotechnol. 2007; 127: 657-669.
- Fisher SH, Wray LV Jr. Bacillus subtilis 168 contains two differentially regulated genes encoding L-asparaginase. J Bacteriol. 2002; 184: 2148-2154.
- Müller HJ, Boos J. Use of L-asparaginase in childhood ALL. Crit Rev Oncol Hematol. 1998; 28: 97-113.
- Khan AA, Pal SP, Raghavan SR, Bhattacharyya PK. Studies on Serratia marcescens L-asparaginase. Biochem Biophys Res Commun. 1970; 41: 525-533.
- Heinemann B, Howard AJ. Production of tumor-inhibitory L-asparaginase by submerged growth of Serratia marcescens. Appl Microbiol. 1969; 18: 550-554.
- Howard JB, Carpenter FH. L-asparaginase from Erwinia carotovora. Substrate specificity and enzymatic properties. J Biol Chem. 1972; 247: 1020-1030.
- Barnes WR, Dorn GL, Vela GR. Effect of culture conditions on synthesis of L-asparaginase by Escherichia coli A-1. Appl Environ Microbiol. 1977; 33: 257-261.
- Mukherjee J, Majumdar S, Scheper T. Studies on nutritional and oxygen requirements for production of L-asparaginase by Enterobacter aerogenes. Appl Microbiol Biotechnol. 2000; 53: 180-184.
- Abdel-Fattah YR, Olama ZA. L-Asparaginase production by Pseudomonas aeruginosa in solid-state culture: evaluation and optimization of culture conditions using factorial designs. Process Biochem. 2002; 38: 115–122.
- Cappelletti D, Chiarelli LR, Pasquetto MV, Stivala S, Valentini G, Scotti C. Helicobacter pyloril-asparaginase: a promising chemotherapeutic agent. Biochem Biophys Res Commun. 2008; 377: 1222-1226.
- Stowe RA, Mayer RP. Bioprocess optimization - A challenge. Ind Eng Chem 1996; 56: 36-40.
- Goswami R, Hegde K, Dasu VV. Batch, fed batch production and characterization of glutaminase free L-asparaginase II of Pectobacterium carotovorum MTCC 1428 in Escherichia coli. Adv Microb. 2014; 4: 667-680.
- Panda T, Babu PSR, Kumari JA, Rao DS, Theodore K, Rao JK, et al. Bioprocess optimization - A challenge. J Microbiol Biotechnol. 1997; 7: 367-372.
- Dasu VV, Panda T, Chidambaram M. Determination of major influencing parameters for improved griseofulvin production in a batch bioreactor by Taguchi’s Method. Process Biochem. 2003; 38: 877-880.
- Makrides SC. Strategies for achieving high-level expression of genes in Escherichia coli. Microbiol Rev. 1996; 60: 512-538.
- Gerigk M, Bujnicki R, Ganpo-Nkwenkwa E, Bongaerts J, Sprenger G, Takors R. Process control for enhanced L-phenylalanine production using different recombinant Escherichia coli strains. Biotechnol Bioeng. 2002; 80: 746-754.
- Jeong KJ, Choi JH, Yoo WM, Keum KC, Yoo NC, Lee SY, et al. Constitutive production of human leptin by fed-batch culture of recombinant rpoS- Escherichia coli. Protein Expr Purif. 2004; 36: 150-156.
- Mijts BN, Schmidt-Dannert C. Engineering of secondary metabolite pathways. Curr Opin Biotechnol. 2003; 14: 597-602.
- Wong HH, Kim YC, Lee SY, Chang HN. Effect of post-induction nutrient feeding strategies on the production of bioadhesive protein in Escherichia coli. Biotechnol Bioeng. 1998; 60: 271-276.
- Ramalingam S, Gautam P, Mukherjee KJ, Jayaraman G. Effects of postinduction feed strategies on secretory production of recombinant streptokinase in Escherichia coli. Biochem Eng J. 2007; 33: 34–41.
- Chen HC, Hwang CF, Mou DG. High-density Escherichia coli cultivation process for hyperexpression of recombinant porcine growth hormone. Enzyme Microb Technol. 1992; 14: 321-326.
- Tsai LB, Mann M, Morris F, Rotgers C, Fenton D. The effect of organic nitrogen and glucose on the production of recombinant human insulin-like growth factor in high cell density Escherichia coli fermentations. J Ind Microbiol. 1987; 2: 181–187.
- Zhang Y, Taiming L, Liu J. Low temperature and glucose enhanced T7 RNA polymerase-based plasmid stability for increasing expression of glucagon-like peptide-2 in Escherichia coli. Protein Expr Purif. 2003; 29: 132-139.
- Studier FW. Protein production by auto-induction in high density shaking cultures. Protein Expr Purif. 2005; 41: 207-234.
- Nayak DP, Vyas VV. Improved stability and expression of a recombinant shuttle plasmid in Escherichia coli during fed batch cultivation. World J Microbiol Biotechnol. 1999; 15: 65-71.
- Giridhar R, Srivastava AK. Fed-Batch sorbose fermentation using pulse and multiple feeding strategies for productivity improvement. Biotechnol Bioprocess Eng. 2000; 5: 340-344.
- Zhang H, Zheng Y, Liu Q,Tao X, Zheng W, Ma X, Wei D. Development of a fed-batch process for the production of anticancer drug TATm-survivin(T34A) in Escherichia coli. Biochem. Eng. J. 2009; 43: 163-169.
- Kumar S, Pakshirajan K, Venkata Dasu V. Development of medium for enhanced production of glutaminase-free L-asparaginase from Pectobacterium carotovorum MTCC 1428. Appl Microbiol Biotechnol. 2009; 84: 477-486.
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951; 193: 265-275.
- Miller GL. Use of DNS reagent for determination of reducing sugar. Anal Chem. 1959; 31: 426–428.
- Goyal D, Sahni G, Sahoo DK. Enhanced production of recombinant streptokinase in Escherichia coli using fed-batch culture. Bioresour Technol. 2009; 100: 4468-4474.
- Daverey A, Pakshirajan K. Kinetics of growth and enhanced sophorolipids production by Candida bombicola using a low-cost fermentative medium. Appl Biochem Biotechnol. 2010; 160: 2090-2101.
- Niccolai A, Fontani S, Kapat A, Olivieri R. Maximization of recombinant Helicobacter pylori neutrophil activating protein production in Escherichia coli: improvement of a chemically defined medium using response surface methodology. FEMS Microbiol Lett. 2003; 221: 257-262.
- Hao D, Pan Z, Sheng Y, Ling Y. Optimization of recombinant cytochrome P450 2C9 protein production in Escherichia coli DH5a by statistically-based experimental design. World J Microbiol Biotechnol. 2006; 22: 1169-1176.
- Ghane M, Khodabandeh YM. Over expression of biologically active Interferon beta using synthetic gene in E. coli. J Sciences Islam Repub Iran. 2008; 19: 203-209.
- Khushoo A, Pal Y, Mukherjee KJ. Optimization of extracellular production of recombinant asparaginase in Escherichia coli in shake-flask and bioreactor. Appl Microbiol Biotechnol. 2005; 68: 189-197.
- Kweon DH, Han NS, Park KM, Seo JH. Overproduction of Phytolacca insularis protein in batch and fed-batch culture of recombinant Escherichia coli. Process Biochem. 2001; 36: 537–542.
- Kleist S, Miksch G, Hitzmann B, Arndt M, Friehs K, Flaschel E. Optimization of the extracellular production of a bacterial phytase with Escherichia coli by using different fed-batch fermentation strategies. Appl Microbiol Biotechnol. 2003; 61: 456-462.
- Lau J, Tran C, Licari P, Galazzo J. Development of a high cell-density fed-batch bioprocess for the heterologous production of 6-deoxyerythronolide B in Escherichia coli. J Biotechnol. 2004; 110: 95-103.
- Shang F, Wen S, Wang X, Tan T. Effect of nitrogen limitation on the ergosterol production by fed-batch culture of Saccharomyces cerevisiae. J Biotechnol. 2006; 122: 285-292.
- Babaeipour V, Shojaosadati S, Robatjazi S, Khalilzadeh R, Maghsoudi N. Over-production of human interferon-g by HCDC of recombinant Escherichia coli. Process Biochem. 2007; 42: 112-117.
- Gummadi SN, Kumar DS. Batch and fed batch production of pectin lyase and pectate lyase by novel strain Debaryomyces nepalensis in bioreactor. Bioresour Technol. 2008; 99: 874-881.
- Nath A, Dixit M, Bandiya A, Chavda S, Desai AJ. Enhanced PHB production and scale up studies using cheese whey in fed batch culture of Methylobacterium sp. ZP24. Bioresour Technol. 2008; 99: 5749-5755.
- Passrinha LA, Bonifacio MJ, Queiroz JA. Application of a fed-batch bioprocess for the heterologous production of hSCOMT in Escherichia coli. J Microbiol Biotechnol. 2009; 19: 972-981.
- Hu D, Shi Z, Wu Q, Chen GQ. Microbial production of acetoacetate by recombinant Escherichia coli. Bioresour Technol. 2010; 101: 8477-8480.
- Voulgarisa I, Arnoldb SA, Speightb R, Harveya LM, McNeil B. Effects of dissolved oxygen availability and culture biomass at induction upon the intracellular expression of monoamine oxidase by recombinant E. coli in fed batch bioprocesses. Process Biochem. 2011; 46: 721-729.
- Puertas J, Ruiz J, Vega MRDL, Lorenzo J, Caminal G, González G. Influence of specific growth rate over the secretary expression of recombinant potato carboxypeptidase inhibitor in fed-batch cultures of Escherichia coli. Process Biochem. 2010; 45: 1334-1341.