
Research Article
Austin J Biotechnol Bioeng. 2015;2(2): 1040.
Drought Stress Tolerance and Enhancement of Banana Plantlets In Vitro
Eglal M. Said¹, Rania A. Mahmoud², Rana Al-Akshar³ and Gehan Safwat³*
1Breeding Research Department for fruit trees, Ornamental Plants and Woody Trees, Horticulture Research Institute, Agricultural Research Center, Egypt
2Deciduous Fruit Research Department, Horticulture Research Institute, Agricultural Research Center, Egypt
2Faculty of Biotechnology, October University for Modern Sciences and Arts, Egypt
*Corresponding author: Gehan Safwat, Faculty of Biotechnology, October University for Modern Sciences and Arts, Egyp.
Received: March 12, 2015; Accepted: May 08, 2015; Published: May 12, 2015
Abstract
Environmental stresses severely restrict the developmental rates of plants; one prominent environmental stress is drought stress. Drought stress remains an ever-growing environmental problem that severely limits crop production worldwide and causes important agricultural losses particularly in arid and semiarid areas. Drought stress targets bananas which are one of the most important crops grown and consumed. Tissue culture is a novel technique that enabled the evaluation of tolerance to environmental stresses because it allowed their manipulation in vitro. The study investigated the adverse effects of drought stress on growth, yield and endogenous phytohormones of the banana (Musa acuminata); and focused on the progress towards the development of stresstolerant lines through tissue culturing based on in vitro selection. Drought stress was induced by applying Polyethylene Glycol (PEG) solutions with elevated strengths of (1, 2 & 3%). Tolerance effect was acquired by pre-treating the plantlets with trehalose using different concentrations (20, 60 & 100 mM), to test which concentration provides the most eminent outcome. Results exposed that trehalose has positive significant effects combating drought stress.
Keywords: Drought stress; Polyethylene glycol; Trehalose; Tissue culturing
Introduction
The banana and plantains (Musa spp.) are belonging to the family Musacea. Banana (Musa acuminata) is grown in wide scale in tropical and subtropical regions. The plantains originated in Malaysia through a complex hybridization process [1]. Bananas are crops of vital importance to hundreds of millions of people in developing countries. Inhabitants of many countries make their living directly or indirectly from Musa crops as a source of food or export earnings [1]. It is one of the most important cash crops in the world and has high acceptability amongst consumers. In addition, it is considered as main source of food that is rich in carbohydrate, minerals, phosphorus, calcium, potassium and vitamin-C. Furthermore, it is a chief producer of tannins, latex and fibers.
Environmental stress severely restricts the distribution and productivity of plants [2]; particularly drought is a major abiotic factor that limits crop productivity [3]. Drought stress remains an evergrowing environmental problem that severely limits crop production worldwide and causes important agricultural losses particularly in arid and semi-arid areas [4]. Water stress enforces a serious threat to banana productivity. The consequences of water deficit include its adverse effects on plant phenology, development, assimilate partitioning, carbon assimilation, growth, and plant reproduction processes. Drought induced osmotic stress triggers a wide range of perturbations ranging from growth and water status disruption to the modification of ion transport and uptake systems [5-7]. Upon water deficit exposure, plants exhibit physiological, biochemical and molecular responses at both cellular and organismal [8,9].
Utilization of tissue culture techniques for quantifying stress tolerance of various crops has been increasing rapidly. Tissue culturing systems are useful for the evaluation of tolerance to environmental stresses because stress conditions can be easily controlled in vitro [10]. Moreover, in vitro cultures provide a uniform population of synchronously developing plant cells without involving regulatory mechanisms that naturally are repaired at the organismal level [11]. Also, In vitro culture techniques minimize environmental variations due to defined nutrient media, controlled conditions and homogeneity of stress application. In addition, the simplicity of such manipulations enables the study of large plant population and stress treatments in a limited space and a short period of time. Simulation of drought stress under in vitro conditions during the regeneration process constitutes a convenient way to study the effects of drought on the morphogenic responses [12]. Tissue culturing is also an efficient procedure to study the effect of abiotic stress on the cell metabolism [3,13-15].
Drought stress-induction is one of the most popular approaches takes use of high molecular weight osmotic agents, such as Polyethylene Glycol (PEG) [16,17]. These agents have no detrimental or toxic effects on the plant [18]; however, they inhibit the plant’s growth by lowering the water potential of the culture medium in a way similar to soil drying, so that cultured explants are unable to take up water [19].
Plants subjected to abiotic stress use various defense mechanisms to cope with the stress. A common strategy is the synthesis and accumulation of osmoprotectants or compatible solutes like proline, glycine betaine, polyamines or trehalose. Tolerance to abiotic stresses can be acquired by pre-treatment with such a protective compound [20]. Trehalose, also known as mycose or tremalose, is a natural alpha-linked disaccharide formed by an a,a-1,1-glucoside bond between two a-glucose units. It can be synthesized by bacteria, fungi, plants, and invertebrate animals. It is widely dispersed in biological systems of fungi, yeast, bacteria, higher and lower plants, as well as invertebrates and insects.
Materials and Methods
This study was carried out in the Biotechnology Laboratory of the Horticulture Research Institute of the Agricultural Research Center, Egypt.
In vitro raised shoots resulting from proliferation of Grand Nain banana shoot-tips were used as experimental materials. Before a subculture, explants were transferred to ½ MS medium supplemented with trehalose in different concentrations (0, 20, 60 and 100 mM). After two days of growing on trehalose medium, the stress treatments were applied.
Media specifically formulated to induce drought stress supplemented with modified MS salts and vitamins [21], 3% sucrose, BA at 22 μM and 0.0, 1.0, 2.0 or 3.0% PEG. The pH of the prepared medium was adjusted at 5.7 prior to addition of agar at 5 g/L. The medium was distributed into the culture jars (325 ml.), where each jar contained 30 ml. of the medium. The culture jars were autoclaved at 121oC at 15 lb/inch2 for 20 min. Cultures were allowed to grow for 8 weeks at 27°C of day and night temperature. Light was provided by white fluorescent tubes giving intensity of about 1500 Lux at the explants’ level; then data including number of the proliferated shoots, average shoot length (cm), number of roots, average root length (cm) and wet weight (gm) were recorded.
After the stress treatment, proliferated shoots were re-cultured each at the same concentration of PEG on a free growth regulators modified MS medium and allowed to grow for another 8 weeks under growth room conditions, then tests were carried out and data were recorded. The measured growth parameters included plant height (cm), number of leaves, number of roots, average length of roots (cm), and fresh weight (gm). Samples of shoot and root tissues were subjected to further chemical analysis.
Extraction and determination of trehalose
The trehalose contents of the shoots and roots were determined by using HPLC. The trehalose extraction was carried according to Ferreira et al. [22]. It was carried out by boiling 0.5 gm of ground tissues in 2 ml of ethanol. Ethanol was then evaporated at 60°C and the residue was dissolved in 5 ml of the mobile phase (5 mM H2SO4) of the HPLC. This solution was then centrifuged at 10,000 rpm for 10 min in a micro-centrifuge and filtered. Then the extract was incubated in a water bath for 1 h to hydrolyze the sucrose in the extract, because the sucrose retention time is the same as that of trehalose. Then, sample of this extract was analyzed by the method described by [23] using HPLC (Hewlett Packard, HP 1090 liquid chromatography). Trehalose content was determined by comparing its chromatogram with that of different concentration of commercial trehalose.
Determination of antioxidant properties
Radical scavenging ability using DPPH (2, 2-diphenyl-1- picrylhydrazyl) radical: The antioxidant activity of plant methanol extracts was determined based on the radical scavenging ability in reacting with a stable DPPH free radical according to Blois [24]. Briefly, 0.1 mM of DPPH in methanol was prepared and 1 ml of this solution was added to 3 ml of methanolic plant extract (50- 150 μg/ml) .The mixture was shaken vigorously and allowed to stand at room temperature for 30 min in the dark. Then the absorbance was measured at 517 nm. The radical scavenging activities of BHT and BHA were also determined as positive controls. Lower absorbance of the reaction mixture indicated higher free radical scavenging activity. Purple colored stable free radicals were reduced to the yellow colored diphenyl picryl hydrazine when antioxidant was added. The corresponding blank readings were taken and the capability to scavenge the DPPH radical was calculated using the following equation:
DPPH’ scavenging effect (%) = [(A0 - A1/ A0)] x100
Where: A0 = the absorbance of the control reaction (containing all reagents except the test compounds)
A1= the absorbance in the presence of the tested extracts
Statistical analysis
Statistical analysis was carried out according to Snedecor and Cochran [25] using analysis of variance and the significance was determined using L.S.D values at P = 0.05.
Multiplication stage (1st experimental stage)
Number of proliferated shoots/ explants: Concerning number of shoots the results as illustrated in Figure 1 revealed that trehalose untreated explants which were sub cultured on free PEG medium produced the highest number of shoots (10.3). On the other hand, increasing concentrations of PEG in the culture media led to decreasing number of shoots gradually. The most harmful effects of PEG were noticed in trehalose untreated explants where the number of shoots decreased dramatically with increasing concentrations of PEG in the culture media. At the highest PEG concentration (3%), pretreated explants with higher concentrations of trehalose (60 and 100 mM) significantly increased number of shoots (8.0 and 6.0, respectively) compared with trehalose untreated explants which represented 3.7.
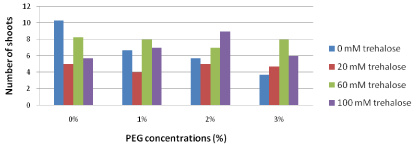
Figure 1: Effect of pretreatment with different concentrations of trehalose
(mM) on number of proliferated shoots/explants of Grand Nain banana sub
cultured on media containing different concentrations of PEG (%).
L.S.D. at 0.05 for trehalose & PEG treatments is 1.2
L.S.D. at 0.05 for interaction between trehalose x PEG is 2.4
Average shoots length (cm): The average shoot length results are illustrated in Figure 2 where they cleared out that explants pretreated with the highest trehalose concentration (100 mM) and sub cultured on free PEG medium produced the highest average shoot length (3.2). On the other hand, increasing concentrations of PEG in the culture media led to decreasing average shoot length gradually. Generally, the lowest harmful effects of PEG were observed at pretreatments with higher concentrations of trehalose (60 and 100 mM) which significantly increased average shoot length compared with trehalose untreated explants and pretreatment with the lowest trehalose concentration (20 mM) at different concentrations of PEG.
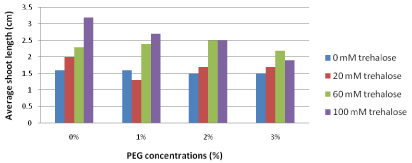
Figure 2: Effect of pretreatment with different concentrations of trehalose
(mM) on average shoot length (cm) of Grand Nain banana sub cultured on
media containing different concentrations of PEG (%).
L.S.D at 0.05 for trehalose & PEG treatment is 0.4
L.S.D at 0.5 for Interaction between trehalose & PEG is 0.9
Number of roots: The effect of pretreatment with different concentrations of trehalose on number of roots of Grand Nain banana sub cultured on media containing different concentrations of PEG are represented in Figure 3. Plantlets sub cultured on PEG free medium revealed insignificant differences between various trehalose pretreatments. On the other hand, adding PEG to the culture media decreased number of roots with different levels. Decreasing number of roots were more aggressive for trehalose untreated explants which represented the lowest average number of roots (12.2) as compared with trehalose pretreated explants at different concentrations (16.6, 17.3 and 20.3 for 20, 60 and 100 mM trehalose, respectively).
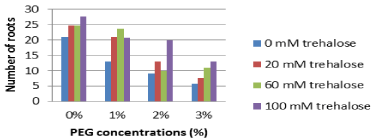
Figure 3: Effect of pretreatment with different concentrations of trehalose
(mM) on number of roots of Grand Nain banana sub cultured on media
containing different concentrations of PEG (%).
L.S.D. at 0.05 for trehalose & PEG treatments is 4.1
L.S.D. at 0.05 for interaction between trehalose x PEG is 8.1
Average root length (cm): Concerning average root length, the results represented in Figure 4 illustrated that explants pretreated with higher concentrations of trehalose (60 and 100 mM) and sub cultured on free PEG media produced the highest average root length (6.8 and 6.3, respectively). On the other hand, increasing concentrations of PEG in the culture media led to decreasing average root length gradually. Plantlets sub cultured on medium containing the highest PEG concentration (3%) revealed insignificant differences between various trehalose pretreatments.
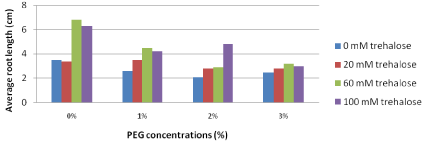
Figure 4: Effect of pretreatment with different concentrations of trehalose
(mM) on average root length (cm) of Grand Nain banana sub cultured on
media containing different concentrations of PEG (%).
L.S.D. at 0.05 for trehalose & PEG treatments is 0.6
L.S.D. at 0.05 for interaction between trehalose x PEG is 1
Fresh weight (gm): Concerning fresh weight, the results in Figure 5 showed that, explants pretreated with higher concentrations of trehalose (60 and 100 mM) and sub cultured on free PEG media produced the highest fresh weight (10.7 and 9.5, respectively). On the other hand, increasing concentrations of PEG in the culture media led to a gradual decrease in fresh weight. Plantlets sub cultured on medium containing the highest PEG concentration (3%) revealed insignificant differences amongst various trehalose pretreatments.
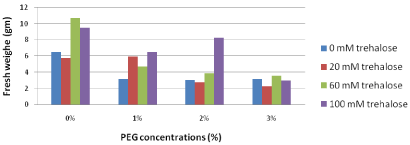
Figure 5: Effect of pretreatment with different concentrations of trehalose
(mM) on fresh weight (gm) of Grand Nain banana subcultured on media
containing different concentrations of PEG (%).
L.S.D. at 0.05 for trehalose & PEG treatments is 1.9
L.S.D. at 0.05 for interaction between trehalose x PEG is 3.8
Elongation stage (2nd experimental stage)
Plant height (cm): The results for plant height illustrated in Figure 6 pointed out that trehalose unpretreated explants were the shortest (9.2 cm) under long term non-stress conditions compared with explants received different concentrations of trehalose prior subject to drought stress (10.3, 10.7 and 11.0 cm for 20, 60 and 100 mM trehalose, respectively). On the other hand, adding PEG to the culture media decreased plant height. Generally ,low harmful PEG effect affected the plant height where it was observed that pretreatments with 20 and 60 mM trehalose produced longest plants under the highest PEG concentration (3%) ; it resulted in 10.7 and 10.0 cm, respectively, compared with trehalose non-pretreated explants and pretreatment with the highest concentration of trehalose (100 mM trehalose) which recorded 9.0 and 9.1, respectively.
Figure 6: Effect of pretreatment with different concentrations of trehalose
(mM) on growth of Grand Nain banana subcultured on media containing
different concentrations of PEG (%).
Number of leaves: During long term stress conditions, the effects of increasing PEG concentrations in the culture media on number of leaves were insignificant as shown in Figure 7. Explants pretreated with the lowest trehalose concentration (20 mM) prior culture under drought stress conditions produced relatively more leaves than explants pretreated with the highest trehalose concentration (100 mM) especially on the medium contained higher concentration of PEG (3%) 6.0 and 4.0, respectively.
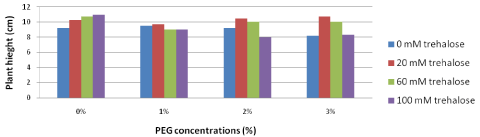
Figure 7: Long term effect of pretreatment with different concentrations of
trehalose (mM) on plant height (cm) of Grand Nain banana recultured on
media containing different concentrations of PEG (%).
L.S.D. at 0.05 for trehalose & PEG treatment is 0.8
L.S.D. at 0.05 for interaction between trehalose x PEG is 1.5
Number of roots: Elevating PEG concentrations in the culture media decreased number of roots gradually as illustrated in Figure 8. Explants pretreated with the highest trehalose concentration (100 mM) before culturing under long term drought stress conditions were the most negatively affected ones. Whereas, explants received the lowest trehalose concentration (20 mM) produced largest number of roots (10.3) in the presence of the highest PEG concentration (3%) in the culture medium.
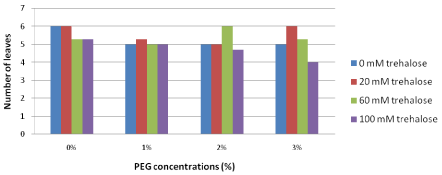
Figure 8: Long term effect of pretreatment with different concentrations of
trehalose (mM) on number of leaves of Grand Nain banana recultured on
media containing different concentrations of PEG (%).
L.S.D. at 0.05 for interaction between trehalose x PEG is 1.4
L.S.D. at 0.05 for trehalose & PEG treatments is 0.7
Average root length (cm): Excessive PEG concentrations in the culture media negatively affected the average root length as shown in Figure 9. Explants pretreated with the low trehalose concentration (20 mM) produced the longest roots length (8.4 cm) in the presence of the highest PEG concentration (3%) in the culture medium.
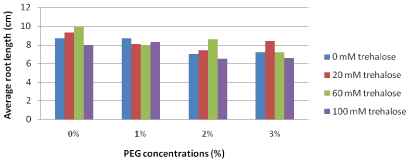
Figure 9: Long term effect of pretreatment with different concentrations
of trehalose (mM) on average root length (cm) of Grand Nain banana recultured
on media containing different concentrations of PEG (%).
L.S.D. at 0.05 for trehalose & PEG treatments is 0.8
L.S.D. at 0.05 for interaction between trehalose x PEG is 1.5
Fresh weight (gm): Data illustrated the long term effect of pretreatment with different concentrations of trehalose on fresh weight (gm) of Grand Nain banana re-cultured on media containing different concentrations of PEG which are represented in Figure 10. The data extracted revealed that the fresh weight decreased in the presence of PEG in the culture media. The pretreated explants with low trehalose concentration (20 mM) were the heaviest (3.1 gm) with the high PEG concentration (3%) in the culture medium.
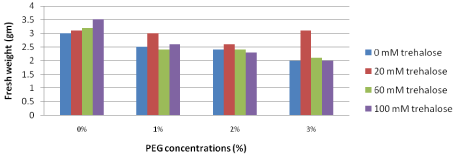
Figure 10: Long term effect of pretreatment with different concentrations of
trehalose (mM) on fresh weight (gm) of Grand Nain banana re-cultured on
media containing different concentrations of PEG (%).
L.S.D. at 0.05 for trehalose & PEG treatments is 0.4
L.S.D. at 0.05 for interaction between trehalose x PEG is 0.9
Trehalose content (mg/100gm): Data presented in Table 1 shows the long term effect of pretreatment with different concentrations of trehalose on root and shoot content of trehalose of Grand Nain banana re-cultured on media containing different concentrations of PEG (Figure 11). Plants accumulated more trehalose under drought stress conditions. On the other hand, shoot tissues contained higher concentrations of trehalose in comparison with the root tissues. Plantlets pretreated with the low trehalose concentration (20 mM) contained highest values of trehalose under both control (0% PEG) or stress conditions (3% PEG) ; which recorded the highest trehalose content in shoot tissues on the medium that contained 3% PEG (328.2 mg/100gm); where it was followed by root tissues on the same medium which contained 304.4 mg/100gm trehalose.
Tissue
Trehalose (mM)
PEG (%)
0
20
60
100
Root
0
214.5
270.5
191.5
197.6
3
254.3
304.4
225.1
195.9
Shoot
0
248.0
286.4
223.9
238.1
3
283.2
328.2
224.0
215.4
L.S.D. at 0.05 for trehalose treatments is 8.9
L.S.D. at 0.05 for PEG treatments & tissue types is 6.3
L.S.D. at 0.05 for interaction between trehalose x PEG x tissue is 17.8
Table 1: Long term effect of pretreatment with different concentrations of trehalose (mM) on root and shoot content of trehalose (mg/100gm) of Grand Nain banana re-cultured on media containing different concentrations of PEG (%).
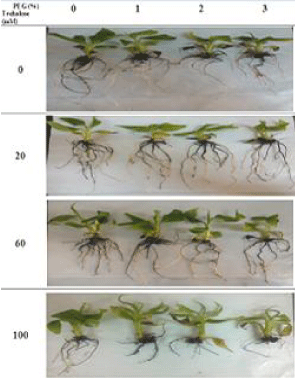
Figure 11: Long term effect of pretreatment with different concentrations
of trehalose (mM) on growth of Grand Nain banana recultured on media
containing different concentrations of PEG (%). The figure illustrated the
results of long term exposure to drought stress, where lower concentration
of trehalose (20mM) enhanced growth of banana plantlets under drought
conditions.
DPPH’ scavenging effects (%): Data presented in Table 2 shows the long term effect of pretreatment with different concentrations of trehalose on DPPH’ scavenging effects (%) of root and shoot extracts of Grand Nain banana re-cultured on media containing different concentrations of PEG. The scavenging effects of extracts on DPPH’ radicals decreased under drought stress with insignificant differences between root and shoot extracts. The shoot extracts with the less considerable decrease of the scavenging effects on DPPH’ radicals as an affect of drought stress was obtained in explants pretreated with 20 mM trehalose to record the highest value (49.3%) in comparison to other trehalose pretreatments which decreased dramatically to reach 43.2, 40.5 and 33.5 within 0, 60 and 100 mM trehalose pretreatments, respectively.
Tissue
Trehalose (mM)
PEG (%)
0
20
60
100
Root
0
51.5
46.4
47.4
46.2
3
47.8
43.9
42.7
44.6
Shoot
0
56.1
55.9
52.9
44.5
3
43.2
49.3
40.5
33.5
L.S.D. at 0.05 for trehalose treatments is 2.8
L.S.D. at 0.05 for PEG treatments & tissue types is 2.0
L.S.D. at 0.05 for interaction between trehalose x PEG x tissue is 5.5
Table 2: Long term effect of pretreatment with different concentrations of trehalose (mM) on DPPH’ scavenging effects (%) of root and shoot extracts of Grand Nain banana recultured on media containing different concentrations of PEG (%).
Discussion
Trehalose is a non-reducing disaccharide (1,1 a-D glucopyranosyl a-Dglucopyranoside) and occurs in a broad range of organisms [26,27]. In yeast, bacteria and certain fungi, it is an important storage carbohydrate and stress protector [27,28]. In higher plants, trehalose is synthesized using a pathway that is common to most organisms [29]. This pathway consists of two steps. First, Trehalose 6-Phosphate (T6P) is synthesized from glucose-6-phosphate and Uridine Diphosphate (UDP)-glucose. This step is catalyzed by Trehalose 6-Phosphate Synthase (TPS). The free sugar trehalose is generated by the action of Trehalose-6-Phosphate Phosphatase (TPP) [30]. Trehalose directly interacts with nucleic acids, facilitates melting of double stranded DNA and stabilizes single-stranded nucleic acids [31]. In the current study, the growth parameters markedly decreased due to PEG induced drought stress. Results showed the enhancement of Banana plantlets growth when high drought stress is preceded by a treatment with trehalose. For short term exposure to drought stress, the higher trehalose pretreatment concentrations (60 and 100 mM) markedly succeeded in combating drought stress.
There is much debate about the function of trehalose in higher plants. Although the involvement of trehalose metabolism in stress tolerance is indisputable [29], some research supports the hypothesis that trehalose is a putative signaling molecule in higher plants while others support the hypothesis that trehalose functions as a direct stress protectant. There is evidence for both hypotheses [20]. The preliminary obtained results are partially agreeing with Stolker [20] who revealed that the protective effect of trehalose seems to be primarily caused by its function as a protector against the damage to lipids and membranes by physical interaction. Relatively high levels of trehalose are necessary to significantly increase the survival after a case in point of drought stress. If trehalose would have a signaling function, one would expect to see the effect of trehalose at the lower trehalose concentrations.
For long term exposure to the highest PEG concentration, a positive correlation between enhancement growth, trehalose content and DPPH’ scavenging effects (%) was observed in trehalose pretreated shoots at the lowest concentration (20 mM). This observation can be explained by the findings of Stolker [20] who suggested that, the protective action of trehalose against drought stress might be through its preservative action on membranes by scavenging of Reactive Oxygen Species (ROS).
When plants are exposed to abiotic stress, this triggers many common responses like cellular dehydration and an increase in Reactive Oxygen Species (ROS) [32-34]. Oxidative stress is closely associated to these unstable but very reactive radicals [35]. Examples of cellular ROS are the Superoxide Radical (O2 -•), Hydrogen Peroxide (H2O2), singlet oxygen (¹O2) and the hydroxyl radical (HO•). These ROS cause oxidative damage to multiple cellular components like proteins, DNA, RNA and lipids [36]. One of the effects of lipid peroxidation is an increase in the leakage of electrolytes since membrane permeability to electrolytes increases [37]. All types of abiotic stresses, for example high salinity, chilling, freezing and drought stress, induce oxidative stress in plant cells [38-40]. ROS are produced continuously, even under non-stress conditions, and are scavenged by antioxidant defense systems. Under stress conditions, the balance between the production of ROS and the antioxidant defense system is disturbed [41]. Since all types of abiotic stresses result in the rapid accumulation of ROS in plant cells, this might indicate that the oxidative stress defense responses are an essential component in cross-protection. Earlier, ROS were seen as toxic byproducts of aerobic metabolism but in recent years is has become evident that they also play an important signaling role in controlling the response to abiotic stress and programmed cell death [37]. At low concentrations, ROS induce defense genes while at high concentrations cell death is initiated [42]. Although the role of ROS in the defense against abiotic stress is well accepted, it is not known what its targets and molecular functions are in the biochemical and transcriptional changes [37].
Trehalose is found to be a direct and indirect scavenger of ROS [20]. To verify if the protective function of trehalose is because of a reduction in level of ROS, explants were treated with trehalose before being subjected to drought stress. After the long term drought treatment, antioxidant activity determined using free radical scavenging activity by DPPH method. The proton radical scavenging action is known as an important mechanism of antioxidants. The model of scavenging the stable DPPH’ radical is a widely used method to evaluate antioxidant activity in a relatively short time compared with other methods. The effect of antioxidants on DPPH’ radical scavenging was thought to result from their hydrogen donating ability [43]. DPPH is a stable free radical and accepts an electron or hydrogen radical to become a stable diamagnetic molecule [44]. The decrease in absorbance of the DPPH’ radical caused by antioxidants, because of the reaction between antioxidant molecules and the radical, progresses, which results in the scavenging of the radical by hydrogen donation. It is visually noticeable as a discoloration from purple to yellow. Hence, DPPH is usually used as a substrate to evaluate the antioxidative activity of natural antioxidants [45]. The demonstrated modified spectrophotometric method makes use of the 2,2-diphenyl-1-picrylhydrazyl (DPPH) radical and its specific absorbance properties. The absorbance decreases when the radical is reduced by antioxidants [46].
For long term exposure to drought stress, lower concentration of trehalose (20 mM) worked as a signal that stimulated biosynthesis of trehalose which reflects on the enhancement of growth parameters and the decrease of the harmful effect of drought stress. This observation partially disagrees with the findings of Stolker [20] who mentioned that, the protective effect of trehalose seems to be primarily caused by its direct function as a protector against the damage of lipids and membranes. Relatively high levels of trehalose are necessary to significantly increase the tolerance to drought and high salinity stress. It seems that using higher trehalose concentration for combating drought stress needs to be consequent whereas lower trehalose concentration is more effective on long term where it stimulates the biosynthesis of trehalose.
Stolker [20] mentioned that, it was hypothesized that the protective function of trehalose is related to ROS scavenging activity. Overall there is a trend for more staining with increasing trehalose concentration or a higher level of superoxide radicals with increasing trehalose concentration. This result does not correspond to observations in other research that has shown that trehalose is as active as a scavenger of ROS and promotes activity of other antioxidant enzymes [47,48]. The function of trehalose as a direct and indirect scavenger of ROS is only shown indirectly. Trehalose pre-treatment results in reduced electrolyte leakage after drought stress. This suggests that the protective function of trehalose can indeed be through the scavenging of ROS. ROS cause lipid peroxidation in membrane that led to increased permeability of the membrane to electrolytes. No conclusions can be drawn from the direct visualization of ROS by staining of oxygen radicals after a stress treatment [20]. On the other hand, our data showed the effective free radical scavenging activity by DPPH method in detection of such relation.
The increased tolerance to drought and high salinity stress after trehalose treatment could be the result of shared steps in the stress response pathways of these stresses [20]. For future research, gene expression analysis could be used subsequently to see if genes are differentially expressed after a trehalose pre-treatment. Such an experiment could result in a better understanding of the exact function of trehalose in the signal transduction pathway of the different abiotic stresses. Understanding the stress response of plants to different types of abiotic stress is the first step in obtaining stress tolerant plants [20].In this research it was conclusive that pre-treating the plantlets with low concentration of trehalose is more affective in combating drought stress.
Acknowledgement
A debt of gratitude is owned to the Biotechnology Laboratory of the Horticulture Research Institute of the Agricultural Research Center for their contribution to the development and implementation of this study. In addition, this study would not have been possible without the diligent efforts and expertise of the assisting professors, research assistants and staff who were nothing but supportive.
References
- Novak FJ. Musa (bananas and plantains). Hammerschlag FA, Litz RE, editors. In: Biotechnology of perennial fruit crops. CAB International, Wallingford, UK. 1992.
- Debnath M. Responses of Bacopa monnieri to salinity and drought stress in vitro. Journal of Medicinal Plants Research. 2008; 2: 347- 351.
- Misra AN, Biswal AK, Misra M. Physiological, biochemical and molecular aspects of water stress in plants, and their biotechnological applications. Proceedings of the National Academy of Sciences. 2002; 72: 115- 134.
- Boyer JS. Plant productivity and environment. Science. 1982; 218: 443- 448.
- Santos-Diaz MS, Ochoa-Alejo N. PEG-tolerant cell clones of chili pepper: growth, osmotic potentials and solute accumulation. Plant Cell, Tissue and Organ Culture. 1994; 37: 1- 8.
- Lutts S, Kinet JM, Bouharmont J. NaCl-induced senescence in leaves of rice (Oryza sativa L.) cultivars differing in salinity resistance. Annals of Botany. 1996; 78: 389- 398.
- Bajji M, Lutts S, Kinet JM. Physiological changes after exposureto and recovery from polyethylene glycol-induced water deficit in callus culture issued from durum wheat (Triticum durum) cultivars differing in drought resistance. Journal of plant physiology. 2000; 156: 75- 83.
- Greenway H, Munns R. Mechanism of salt tolerance in nonhalophytes. Annual Review of Plant Physiology. 1980; 33: 149-190.
- Hasegawa PM, Bressan RA, Zhu JK, Bohnert HJ. Plant cellular and molecular responses to high salinity. Annu Rev Plant Physiol Plant Mol Biol. 2000; 51: 463- 499.
- Errabii T, Gandonou CB, Essalmani H, Abrini J, Idaomar M, Skali-Senhaji N. Growth, proline and ion accumulation in sugarcane callus cultures under drought-induced osmotic stress and its subsequent relief. African Journal of Biotechnology, 2006; 5: 1488- 1493.
- Tal M. Selection for stress tolerance. Evans DA, Sharp WR, Ammirato PV, Yamada Y, editors. In: Hand Book of Plant Cell Culture. Volume 1: Techniques for propagation and Breeding. McMillan, London. 1983.
- Sakthivelu G, Akitha Devi MK, Giridhar P, Rajasekaran T, Ravishankar GA, Nedev T, et al. Drought-induced alterations in growth, osmotic potential and in vitro regeneration of soybean cultivars. Gen Appl Plant Physiology. 2008; 34: 103- 112.
- Das N, Misra M, Misra AN. Sodium chloride salt stress induced metabolic changes in callus cultures of pearl millet (Pennisetum americanum L. Leeke): free solute accumulation. Journal of Plant Physiology. 1990; 137: 244-246.
- Das N, Misra M, Misra AN. Sodium chloride salt stress induced metabolic changes in pearl millet callus: oxidases. Proceedings of the National Academy of Sciences, Allahabad, India. 1992; 62: 263- 268.
- Misra AN, Misra M, Das N. Plant responses to salinity: Metabolic changes and the use of plant tissue culture - a perspective. Prakash R, Choubey SM, editors. In: Environmental Concern and Tissue Injury, Part-I. Jagmandir Books, New Delhi. 1990; 77- 84.
- Turkan I, Bor M, Zdemir F, Koca H. Differential responses of lipid peroxidation and antioxidants in the leaves of drought-tolerant P. acutifolius and drought-sensitive P. vulgaris subjected to polyethylene glycol mediated water stress. Plant Science. 2005; 168: 223-231.
- Landjeva S, Neumann K, Lohwasser U, Borner. Molecular mapping of genomic regions associated with wheat seedling growth under osmotic stress. Biologia Plantarum. 2008; 52: 259-266.
- Ober ES, Sharp RE. Electrophysiological responses of maize roots to low water potentials: Relationship to growth and ABA accumulation. Journal of Experimental Botany. 2003; 54: 813- 824.
- Matheka JM, Magni E, Rasha AO, Machuka J. In vitro selection and characterization of drought tolerant somaclones of tropical maize (Zea mays L.). Biotechnology. 2008; 7: 641- 650.
- Stolker R. Combating Abiotic Stress Using Trehalose. Thesis Report, Wageningen University & Research Centre. 2010; 42.
- Murashige T, Skoog F. A revised medium for rapid growth and bioassays with tobacco cultures. Physiologia Plantarum. 1962; 15: 473-497.
- Ferreira JC, Paschoalin VMF, PAnek AD, Trugo LC. Comparison of three different methods for trehalose determination in yeast extracts. Food Chem. 1997; 60: 251-254.
- Čižmárilk J, Hroboňová K, Lehotay J. Determination of monosaccharides and disaccharides in honey by ion-exchange high performance chromatography. Acta Facultatis Pharmaceuticae niversitatis Comenianae. 2004; 51: 73-78.
- Blois MS. Antioxidant determinations by the use of a stable free radical. Nature. 2002; 26: 1199-1200.
- Snedecor GM, Cochran WG. Statistical Methods. 8th edn. Iowa State University Press. 1989.
- Miranda JA, Avonce N, Suárez R, Thevelein JM, Van Dijck P, Iturriaga G. A bifunctional TPS–TPP enzyme from yeast confers tolerance to multiple and extreme abiotic-stress conditions in transgenic Arabidopsis. Planta. 2007; 226: 1411-1421.
- Elbein AD, Pan YT, Pastuszak I, Carroll D. New insights on trehalose: a multifunctional molecule. Glycobiology. 2003; 13: 17R-27R.
- Wingler A. The function of trehalose biosynthesis in plants. Phytochemistry. 2002; 60: 437-440.
- Iordachescu M, Imai R. Trehalose biosynthesis in response to abiotic stresses. J Integr Plant Biol. 2008; 50: 1223-1229.
- Bae H, Herman E, Bailey B, Bae HJ, Sicher R. Exogenous trehalose alters Arabidopsis transcripts involved in cell wall modification, abiotic stress, nitrogen metabolism, and plant defense. Physiologia Plantarum. 2005; 125: 114- 126.
- Bezrukavnikov S, Mashaghi A, van Wijk RJ, Gu C, Yang LJ, Gao YQ, et al. Trehalose facilitates DNA melting: a single-molecule optical tweezers study. Soft Matter. 2014; 10: 7269-7277.
- Ehling-Schulz M, Scherer S. UV protection in cyanobacteria. European Journal of Phycology. 1999; 34: 329-338.
- Rijstenbil JW. Assessment of oxidative stress in the planktonic diatom Thalassiosira pseudonana in response to UVA and UVB radiation. Journal of Plankton Research. 2002; 24: 1277–1288.
- Arora A, Sairam RK, Srivastave GC. Oxidative stress and antioxidative systems in plants. Current Science. 2002; 82: 1227–1238.
- Fang YZ, Yang S, Wu G. Free radicals, antioxidants, and nutrition. Nutrition. 2002; 18: 872–879.
- Jacob RA, Burri BJ. Oxidative damage and defense. Am J Clin Nutr. 1996; 63: 985–990.
- Garg N, Marchanda G. ROS generation in plants: Boon or bane? Plant Biosyst. 2009; 143: 81-96.
- Chen THH, Murata N. Glycinebetaine: an effective protectant against abiotic stress in plants. Trends Plant Sci. 2008; 13: 499-505.
- Groppa MD, Benavides MP. Polyamines and abiotic stress: recent advances. Amino Acids. 2008; 34: 35-45.
- Pastori GM, Foyer CH. Common components, networks, and pathways of crosstolerance to stress. The central role of ‘redox’ and abscisic acid-mediated controls. Plant Physiology. 2002; 129: 460-468.
- De Klerk GJM, Pumisutapon P. Protection of in-vitro grown Arabidopsis seedlings against abiotic stresses. Plant Cell, Tissue and Organ Culture. 2008; 95: 149-154.
- Bhattacharjee S. Reactive oxygen species and oxidative burst: roles in stress, senescence and signal transduction in plants. Current Science. 2005; 89: 1113-1121.
- Shimada K, Fujikawa K, Yahara K, Nakamura T. Antioxidative properties of xanthone on the auto oxidation of soybean in cylcodextrin emulsion. Journal of Agricultural and Food Chemistry. 1992; 40: 945-948.
- Soares JR, Dinis TCP, Cunha AP, Almida LM. Antioxidant activities of some extracts of Thymus zygis. Free Radic Res. 1997; 26: 469-478.
- Mohamed AA, Khalil AA, El-Beltagi HES. Antioxidant and antimicrobial properties of kaff maryam(Anastatica hierochuntica) and doum palm (Hyphaene thebaica). Grasas Y Aceites. 2010; 61: 67-75.
- Marxen K, Vanselow KH, Lippemeier S, Hintze R, Ruser A, Hansen UP. Determination of DPPH Radical Oxidation Caused by Methanolic Extracts of Some Microalgal Species by Linear Regression Analysis of Spectrophotometric Measurements. Sensors. 2007; 7: 2080-2095.
- Luo Y, Li WM, Wang W. Trehalose: protector of antioxidant enzymes or reactive oxygen species scavenger under heat stress? Environmental and Experimental Botany. 2008; 63: 378-384.
- Zhu JK. Plant salt tolerance. Trends in Plant Science. 2001; 6: 66-71.