
Special Article - Fungal Biotechnology: Current and Future Perspective
Austin J Biotechnol Bioeng. 2015; 2(4): 1051.
Mycofumigation for the Biological Control of Post- Harvest Diseases in Fruits and Vegetables: A Review
Gomes AAM¹, Queiroz MV¹ and Pereira OL²*
¹Departamento de Microbiologia, Universidade Federal de Viçosa, Brazil
²Departamento de Fitopatologia, Universidade Federal de Viçosa, Brazil
*Corresponding author: Pereira OL, Departamento de Fitopatologia, Universidade Federal de Viçosa, Av. Peter Henry Rolfs, s/n - Campus Universitário, Viçosa – MG, CEP. 36570-900, Brazil
Received: June 25, 2015; Accepted: August 28, 2015; Published: September 02, 2015
Abstract
There are several causes of post-harvest losses in fruits and vegetables, and microbial infections are responsible for the greatest losses that occur during the transport, storage, and sale of these products. Chemical control is the most used method to control post-harvest diseases in fruits and vegetables by directly applying synthetic fungicides to the product to be consumed. However, the indiscriminate use of fungicides may be associated with serious toxicity problems in humans and environmental imbalance. Mycofumigation, which is the use of volatile antimicrobial organic compounds produced by fungi to inhibit microbial growth, has become a promising alternative for controlling phytopathogenic fungi associated with post-harvest diseases in fruits and vegetables. The technique has some advantages relative to traditional disease control methods, for example, it does not require direct contact between the antagonist and the plant product, the antimicrobial volatiles diffuse easily in closed environments, they do not leave residues on the plant product to be consumed, and most of the antimicrobial volatile mixtures exhibit bioactivity against a wide range of microorganisms, including many phytopathogens associated with post-harvest diseases. This review highlights mycofumigation as a method for controlling post-harvest diseases in fruits and vegetables, emphasizing the effects of volatile compounds on phytopathogenic fungi and their potential to be applied during the transport and storage of fresh fruits and vegetables.
Keywords: Biofumigation; Muscodor; Antimicrobial volatiles
Abbreviation
VOCs: Volatile Organic Compounds
Introduction
As fruits and vegetables are usually tender and juicy, they can become rich and adequate substrates for microbial growth and, consequently, post-harvest infections. These infections are usually responsible for the greatest post-harvest losses observed in horticultural products. For example, in citrus fruit, the Penicillium digitatum (Pers.) Sacc. fungus is responsible for more than 90% of post-harvest production losses [1].
Physical and physiological damage favors microbial infections, and fruits’ and vegetables’ natural resistance to disease decreases with maturation, favoring phytopathogen invasion. These phytopathogens require an entry site to start an infection and may become a serious problem in products stored for long periods of time [2].
Post-harvest decay during the supply chain has been identified as the greatest cause of post-harvest losses in fruits and vegetables, which results in significant economic losses [3]. It is estimated that approximately 20-25% of the fruits and vegetables harvested in developed countries are lost due to action/attack by phytopathogenic microorganisms during post-harvest handling. In developing countries, post-harvest losses are usually higher, especially due to inadequate storage methods and transport difficulties [4].
Fungi are often involved in the decay of fruits and vegetables. This microbial group stands out as important post-harvest diseasecausing agents with the highest frequency and activity, and they are responsible for 80 to 90% of the total losses caused by microbial agents (Figure 1). Many fungal species within the most varied genera have been reported to be associated with post-harvest diseases in fruits and vegetables worldwide: Penicillium Link, Aspergillus P. Micheli, Geotrichum Link, Botrytis P. Micheli, Fusarium Link, Alternaria Nees, Colletotrichum, Dothiorella Sacc, Lasiodiplodia Ellis & Everh, Phomopsis Sacc. & Roum, Cladosporium Link, Phytophthora De Bary, Pythium Nees, Rhizopus Ehrenb, Mucor P. Micheli ex L., Sclerotium Tode, Rhizoctonia D.C. [5-12].
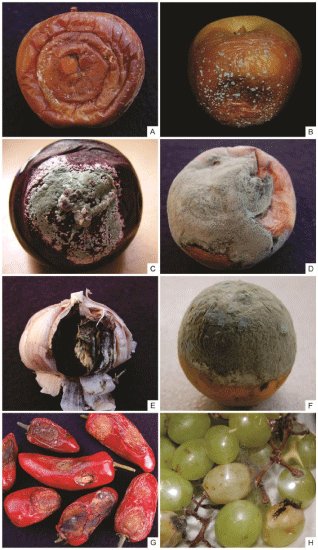
Figure 1: Examples of post-harvest diseases of fruits and vegetables. Bitter
Rot (A) and blue mold (B), postharvest decay of apple caused by the fungus
Colletotrichum spp. and Penicillium expansum respectively; (C) - Decay of
nectarine fruitcaused by P. expansum; (D) - Brown Rot of peach caused by
Monilinia fructicola; (E) - Black Mold caused by Aspergillus niger on garlic;
(F) - Green mold caused by P. digitatum on citrus fruits; (G) - Anthracnose of
pepper fruit caused by Colletotrichum sp.; (H) - Decay of table grapes caused
by Rhizopus stolonifer and Aspergillus niger.
In addition to their potential to cause rot, some fungi that are associated with fruits and vegetables have high potential for mycotoxin production. These secondary metabolites exhibit bioactivity associated with toxic effects in humans, animals, and plants [13]. Several toxins produced by Aspergillus, Penicillium, and Fusarium species and their toxic effects on humans have been reported [14,15].
Practices have been adopted to reduce the incidence of fungi and consequent damage and losses caused by post-harvest diseases in fruits and vegetables, including manipulation of the storage environment and resistance induction. However, the main method used to control post-harvest diseases in fruits and vegetables is by applying fungicides via spraying or even by immersing the horticultural products in fungicide solution [12,16].
Studies have indicated the efficiency of several fungicides with different active ingredients in controlling post-harvest decay in fruits and vegetables. Solutions of borax, sodium bicarbonate, and more recently synthetic fungicides such as sodium ortho-phenyl phenate, imazalil, and thiabendazoleare often used for controlling post-harvest decay in fruits and vegetables by immersing the fruit in fungicide solution [17,18]. One classic example is the use of 2,6-dichloro- 4-nitroaniline to control post-harvest decay in peaches, plums, and nectarines [19]. Another very widespread technique involves using benzimidazoles to control post-harvest decay in cherries by application before and after fruit harvest [20].
Although the use of pesticides such as fungicides has positive aspects, the vast majority of products applied are extremely toxic, endangering human health and environmental balance. Several studies have demonstrated the presence and persistence of fungicide residues in fruits and vegetables [21-23]. The application of fungicides together with high temperatures for controlling post-harvest diseases led to increased 2,6-dichloro-4-nitroaniline residue levels in plum and nectarine and increased sodium o-phenylphenate residue levels in citrus fruit [24]. Imazil residue was also detected in citrus fruit after being applied post-harvest, and the residue level was associated with treatment method, where dip-treated fruit exhibited higher quantities of residue than fruit treated with the same fungicide and at the same concentration but by spraying [25].
Intensive pesticide use for disease control has admittedly caused several environmentally related problems, such as contamination of food, soil, water, and animals; toxicity to farmers; resistance of pathogens to certain active ingredients in the pesticides; development of iatrogenic diseases (occurring due to pesticide use); biological imbalance, altering nutrient and organic matter cycling; elimination of beneficial organisms; and reduction of biodiversity, among others [24].
The identification of these problems has increased the demands for residue-free products, making it necessary to search for disease control/management techniques in fruits and vegetables that do not endanger consumers and to reduce the risk of toxicity to farmers and the environmental imbalance generated by using synthetic fungicides.
Mycofumigation for Controlling Post- Harvest Diseases
Studies involving alternative control of plant diseases have increased significantly over the last 20 years, particularly emphasizing biological control as a promising alternative for reducing synthetic fungicide use. The potential of several microorganisms for controlling different disease-causing pathogens in fruits and vegetables has been reported [26-29].
However, the development of commercial products intended for the biocontrol of post-harvest diseases has been limited, most likely due to the long time period necessary to identify, develop, and market the products, in addition to the process’s high financial cost. Several features characterize a microorganism as an antagonist with potential for the development of commercial products, such as: genetic stability; effective at low concentrations; simple nutritional requirement; capacity to survive under adverse environmental conditions; effective against a wide range of phytopathogens in different products; resistant to the chemical products used in the post-harvest environment; compatible with commercial processing procedures; and lack of risk to human health [27].
The vast majority of the studies related to post-harvest biological control involve the use of fungi or bacteria as microbiological control agents. However, the positive effect on disease control/management is often only observed when the biological agent is directly applied to the fruits or vegetables. This effect may occur mainly due to the main antimicrobial action mechanisms triggered by antagonistic microorganisms, namely competition for space and nutrients, and antibiosis [4,29].
However, some questions have been raised regarding the introduction of antagonists to the human diet and concerns for human health and food security [29]. In addition, the fact that most registered biocontrol products, such as Biosave (Pseudomonas syringae Van Hall), Shemer (Metschnikowia fructicola Kurtzman & Droby), BioNext, AspireTM, Leasaffre International (Candida oleophila Kaisha & Iizuka), and Yield Plus [Cryptococcus albidus (Saito) C.E.Skinner], have similar application methods that involve directly applying a cell suspension to horticultural products can generate fear in the population regarding their consumption.
Mycofumigation is a different biological control strategy for post-harvest diseases in fruits and vegetables that can be an effective alternative to directly applying microorganisms to horticultural products. This strategy consists of the use of antimicrobial Volatile Organic Compounds (VOCs) produced by fungi.
The concept of mycofumigation started developing with the description of Muscodor albus Worapong, Strobel & W.M.Hes, an endophytic fungus obtained from Cinnamomum zeylanicum Breyne, and its potential for emitting volatile compounds that inhibit the growth and/or promote the death of many plant pathogenic agents [30,31].
A peculiarity of antimicrobial VOCs is that they can diffuse in the air, reaching difficult-to-access habitats in closed environments [32]. This property makes antimicrobial VOCs emitted by fungi an additional valuable strategy for post-harvest disease biocontrol. For example, without any direct contact between isolates, the M. albus volatiles inhibited growth of a wide range of fungal species, including Aspergillus fumigatus Fresen, A. carbonarius (Bainier) Thom, A. flavus Link, A. niger Tiegh, A. ochraceus Wilh, Penicillium verrucosum Dierckx, P. digitatum (Pers.) Sacc. Fusarium culmorum (Wm.G.Sm.) Sacc. F. graminearum Schwabe, Botrytis cinera Pers, Colletotrichum acutatum J.H.Simmonds, Geotrichum candidum Link, Monilinia fructicola (G.Winter) Honey, and Rhizopus sp., important fungal species associated with post-harvest decay and mycotoxin production [31,33,35].
Diversity of Antimicrobial Volatile Organic Compound-Producing Fungi
After the discovery of M. albus, many antimicrobial VOCproducing fungal species were identified (Table 1). The vast majority of these species were isolated from healthy plant tissue, especially from tropical plants commonly used in alternative medicine, such as Ananas ananassoides (Baker) L. B. Sm., Aegle marmelos (L.) Corr., Cinnamomum spp. And Myroxylon balsamum (L.) Harms [30,36-39].
Species
Host
Lifestyle
Site isolation
Taxonomic position
References
Filamentous fungi
Muscodor albus
Cinnamomum zeylanicum
Endophytic
Honduras
Ascomycota, Sordariomycetes, Xylariales
[30]
M. kashayum
Aegle marmelos
Endophytic
India
Ascomycota, Sordariomycetes, Xylariales
[37]
M. crispans
Ananas ananassoides
Endophytic
Bolivian
Ascomycota, Sordariomycetes, Xylariales
[36]
M. roseus
Grevillea pteridifolia
Endophytic
Honduras
Ascomycota, Sordariomycetes, Xylariales
[66]
M. oryzae
Oryza rufipogon
Endophytic
Thailand
Ascomycota, Sordariomycetes, Xylariales
[47]
M. musae
Musa acuminate
Endophytic
Thailand
Ascomycota, Sordariomycetes, Xylariales
[47]
M. cinnanomi
C. bejolghota
Endophytic
Thailand
Ascomycota, Sordariomycetes, Xylariales
[39]
M. strobelii
C. zeylanicum
Endophytic
India
Ascomycota, Sordariomycetes, Xylariales
[38]
M. darjeelingensis
C. camphora
Endophytic
India
Ascomycota, Sordariomycetes, Xylariales
[67]
M. tigerii
C. camphora
Endophytic
India
Ascomycota, Sordariomycetes, Xylariales
[68]
M. suthepensis
C. bejolghota
Endophytic
Thailand
Ascomycota, Sordariomycetes, Xylariales
[47]
M. yucatanensis
Bursera simaruba
Endophytic
Mexico
Ascomycota, Sordariomycetes, Xylariales
[69]
M. vitigenus
Paullinia paullinioides
Endophytic
Peru
Ascomycota, Sordariomycetes, Xylariales
[49]
M. equiseti
Equisetum debile
Endophytic
Thailand
Ascomycota, Sordariomycetes, Xylariales
[47]
M. sutura
Prestonia trifidi
Endophytic
Colombia
Ascomycota, Sordariomycetes, Xylariales
[52]
M. fengyangensis
Actinidia chinensis
Endophytic
China
Ascomycota, Sordariomycetes, Xylariales
[48]
Hypoxylon sp.
Persea indica
Endophytic
Canary Islands
Ascomycota, Sordariomycetes, Xylariales
[55]
Nodulisporium sp.
Myroxylon balsamum
Endophytic
Ecuador
Ascomycota, Sordariomycetes, Xylariales
[56]
Nodulisporium sp.
Lagerstroemia loudoni
Endophytic
Thailand
Ascomycota, Sordariomycetes, Xylariales
[57]
Myrothecium inunduatum
Acalypha indica
Endophytic
India
Ascomycota, Sordariomycetes, Hypocreales
[53]
Gliocladium sp.
Eucryphia cordifolia
Endophytic
USA
Ascomycota, Sordariomycetes, Hypocreales
[60]
Trichoderma atroviride
Ascomycota, Sordariomycetes, Hypocreales
[70]
Bionectria ochroleuca
Nothapodytes foetida
Endophytic
India
Ascomycota, Sordariomycetes, Hypocreales
[58]
Phomopsis sp.
Odontoglossum sp.
Endophytic
Ecuador
Ascomycota, Sordariomycetes, Diaporthales
[54]
Phoma sp.
Larrea tridentate
Endophytic
USA
Ascomycota, Dothideomycetes, Pleosporales
[71]
Gloeosporium sp.
Tsuga heterophylla
Endophytic
USA
Ascomycota, Leotiomycetes, Helotiales
[59]
Oxyporus latemarginatus
Capsicum annum
Endophytic
Basidiomycota, Agaricomycetes
[65]
Schizophyllum commune
Saprofit
Chile
Basidiomycota, Agaricomycetes
[72]
Yeast fungi
Aureobasidium pullulans
Saprophytic
Ascomycota, Dothideomycetes, Dothideales
[61,62]
Saccharomyces cerevisiae
Ascomycota, Saccharomycetes, Saccharomycetales
[40,41]
Candida intermedia
Ascomycota, Saccharomycetes, Saccharomycetales
[42]
Wickerhamomyces anomalus
Ascomycota, Saccharomycetes, Saccharomycetales
[40]
Metschnikowia pulcherrima
Ascomycota, Saccharomycetes, Saccharomycetales
[40]
Table 1: Species, host, lifestyle and taxonomic position of filamentous and yeast fungi reported as VOCs producers.
Hitherto, most filamentous fungi related to antimicrobial volatile emission have belonged to Ascomycota, order Xylariales, and other related ascomycetes are found in the classes Sordariomycetes, Dothideomycetes, and Leotiomycete, all of which are endophytic (Table 1). In a more phylogenetically distant group, the basidiomycetes Oxyporus latemarginatus (Durieu & Mont.) Donkand and Schizophyllum commune Fr. are also related to antimicrobial volatile production, and S. commune is noteworthy because, unlike the others, it was isolated from decomposing material, exhibiting a saprophyticlife style in nature.
In addition to filamentous fungi, some yeasts have the potential for emitting the VOCs described. Aureobasidium pullulans (de Bary & Löwenthal) G. Arnaud, Saccharomyces cerevisiae Meyen ex E.C.Hansen, Candida intermedia (Cif. & Ashford) Langeron & Guerra, Wickerhamomyces anomalus (E.C.Hansen) Kurtzman, Robnett & Bas.-Powers, and Metschnikowia pulcherrima Pitt & M.W.Mill. were reported emitting volatile compound mixtures that inhibit the growth of fungi associated with post-harvest decay in fruits and vegetables [40-42].
The identification of fungi associated with antimicrobial VOC production has been conducted through morphology studies and mainly by molecular analyses of the internal transcribed spacer (ITS) region sequences of their DNA. For species of the Muscodor genus, identification and even the proposal of new species have been performed via phylogeny based on ITS region sequencing, accompanied by the volatile compound production profile, as specialized structures in sexual and asexual reproduction have never yet been observed for this genus. This feature is useful for identifying and differentiating fungal species.
Antimicrobial Volatile Organic Compounds (VOCs)
VOCs are solid/liquid carbon-based compounds that easily enter the gas phase via vaporization at 0.01 KPa and temperature close to 20oC, i.e., exhibit high vapor pressure and low water solubility, which allows them to evaporate and diffuse easily through the air [16,43].
More than 250 VOCs have been identified from fungi, occurring in the form of mixtures of simple hydrocarbons, heterocyclic hydrocarbons, aldehydes, ketones, alcohols, phenols, thioalcohols, thioesters and their derivatives, including benzene and cyclohexanes [32].
VOCs may be derived from primary and secondary metabolic pathways of microorganisms. The microorganism releases VOCs as products of primary metabolism when it decomposes substrates to extract nutrients necessary for its maintenance. In contrast, in secondary metabolism, VOC production is usually related to competition for resources in nutrient-poor environments [44].
The profiles of volatiles produced by a certain species or isolates may vary, depending on the substrate used for growth, incubation duration, nutrient type present, temperature, and other environmental parameters [32,45]. The same M. albus 620 isolate shows variation in volatile profile composition depending on the nutrient concentration in the growth medium, where the number of volatile compounds detected was higher in culture media that exhibited a greater quantity of the carbon source [46].
The VOCs produced by Muscodor species consist mainly of lowmolecular- weight esters, alcohols, and acids, with differences between the compound mixtures produced by different species of the genus. However, the VOC mixture produced by most Muscodor species has antimicrobial bioactivity [47,48].
Muscodor species vary regarding the VOC mixture emitted. Muscodor crispans Mitch, Strobel, Hess, Vargas & Ezra, for example, do not produce naphthalene or azulene derivatives, compounds observed in other species of the genus Muscodor [36]. In contrast, naphthalene predominates in the VOC mixture emitted by M. vitigenus Daisy, Strobel, Ezra & Hess, and the VOC mixture emitted by this fungus does not exhibit antifungal bioactivity, though it has previously demonstrated lethality in insects [49].
Gas chromatography/mass spectrometry analyses of the VOC mixture produced by M. albus reveal the presence of at least 28 different VOCs, representing at least five classes of organic substances, where the esters contributed the highest percentage in the mixture, followed by alcohols, acids, lipids, and ketones [31].
The antimicrobial action spectra of the compounds emitted by certain species or isolates seem to be affected by the compound mixture emitted by each isolate. Several studies have demonstrated that the volatile mixture among Muscodor species varies, and the action spectrum also varies, with some being more efficient in inhibiting the growth of certain fungi than others [31,37-39,47-49].
Antimicrobial Effects of the VOCs Produced by Fungi in Post-Harvest Pathogens in Fruits and Vegetables
Most studies on the antimicrobial effects of volatiles produced by fungi involve Muscodor species (Figure 2), although the biological functions of the toxic compounds produced are still not well elucidated. Most Muscodor spp. isolates and other antimicrobial volatile-producing species are endophytic.VOC emission by these fungi may act as a defense mechanism for the host plant against pathogen attack, helping the antimicrobial VOC-producing endophyte survive by preventing colonization of the host plant by microorganisms that compete for the same ecological niche [31].
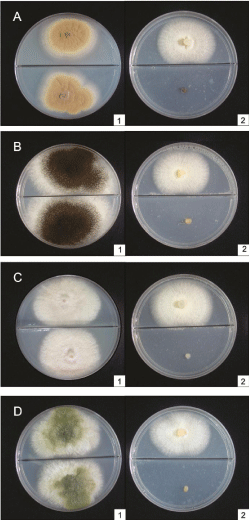
Figure 2: In vitro effect of VOCs produced by Muscodor sp. (2 – upper side
of the plate) inhibiting the mycelial growth of A. ochraceus (A2 – bottom of the
plate); A. niger (B2 – bottom of the plate); F. semitectum (C2 – bottom of the
plate); A. flavus (D2 – bottom of the plate). Control (A1–D1).
Toxicity from exposure to M. albus appears to be associated with combined action of the compounds present in the mixture. Each of the five classes of volatile compounds produced by the fungus (alcohols, esters, ketones, acids, and lipids) had some inhibitory effect against fungi and bacteria when tested alone but did not cause their death. However, they acted synergistically when collectively tested in the mixture, killing a wide range of fungi and bacteria pathogenic to plants and humans [31].
A recent attempt to elucidate the action mechanism of the volatile compounds emitted by M. albus shows DNA damage in Escherichia coli cells when exposed to VOCs emitted by the fungus, which most likely resulted in the interruption of the replication and/or transcription processes; the compounds also caused morphological changes in the cells, generating increased fluidity of the cell membrane [50].
The antimicrobial potential of the compounds emitted by M. albus against diverse microbial groups among fungi, bacteria, and oomycetes has been described in the literature. Growth (in vitro) of B. cinerea, A. fumigatus, Tapesia yallundae Wall work & Spooner, Rhizoctonia solani Kühn, Sclerotinia sclerotiorum (Lib.) de Bary, Candida albicans (C.P.Robin) Berkhout, Pythium ultimumTrow, Verticillium dahliae Kleb, Phytophthora cinnamomi Rands, E. coli, Bacillus subtilis, Staphylococcus aureus, and Micrococcus luteus, representative of diverse groups of fungi, oomycetes and bacteria, was inhibited, and their cells died after exposure to VOCs emitted by M. albus isolates [30,31].
The effects of the VOCs emitted by M. albus 620 were reported (in vitro) against three important fungi frequently associated with post-harvest decay, S. sclerotiorum, B. cinerea and Penicillium expansum Link. The volatiles emitted by the M. albus 620 isolate exhibited significant effects in the germination of B. cinerea and P. expansum spores, preventing the conidia of these fungi to germinate and reducing S. sclerotiorum colony diameter growth. For both treatments, the source of M. albus 620 used was rye grain colonized by the fungus, and higher grain weight (0.25 g to 1.25 g/L) in each treatment corresponded to a stronger observed effect, where 1.25 g/L completely inhibited B. cinerea and P. expansum spore generation and S. sclerotiorum growth [51].
The volatiles emitted by M. albus were also tested against important toxin-producing fungi. Conidia of Aspergillus carbonarius (Bainier) Thom, A. flavus, A. niger, A. ochraceus, P. verrucosum, F. culmorum, and F. graminearum died or their germination was inhibited (in vitro) when exposed to volatiles produced by M. albus colonizing rye grain at 20oC. When conidia of the same fungi were separately exposed to the compounds most abundant in the compound mixture emitted by M. albus, isobutyric acid and 2-methyl-1-butanol, the same magnitude of effect was not observed [34].
In addition to M. albus, other Muscodor species have also been reported to inhibit the growth of fungi associated with post-harvest decay. VOCs emitted by M. crispans were effective against a wide range of phytopathogens, among which B. cinerea, Colletotrichum lagenarium Caruso & Kuc, Fusarium avenaceum (Fr.) Sacc., F. culmorum, Phytophthora palmivora Butler (Butler), P. ultimum, S. sclerotiorum, G. candidum, A. fumigatus, and Curvularia lunata (Wakker) Boedijn exhibited inhibited colony growth. Additionally, except for the last three, 24-hour exposure to the compound mixture emitted by M. crispans led to cell death [36].
The volatiles emitted by M. strobelii exhibited a broad spectrum of activity against yeasts, bacteria, and filamentous fungi and, among the fungi tested, the VOCs completely inhibited the growth of Penicillium citreonigrum Dierckx, B. cinerea, and Aspergillus japonicus Saitoafter three days of exposure. The mixture of compounds emitted by M. strobelii is different from the mixtures of other species of the genus Muscodor, exhibiting 4-octadecylmorpholine as the most abundant compound, along with tetraoxapropellan and aspidofractinine-3- methanol; the last two compounds are not encountered among the volatiles of the other Muscodor species [38].
Variation in compounds present in the VOC mixture among Muscodor species also occurred in M. sultura, where there is variation in the compound mixture profile compared with other Muscodor species, producing higher abundances of propanoic acid, 2-methyl, and thujopsene. The VOCs emitted by M. sultura exhibited antimicrobial bioactivity against a wide range of fungi, inhibiting the growth of A. fumigatus, B. cinerea, C. lagenarium, Ceratocystis ulmi (Buisman) C. Moreau, Cercospora beticola Sacc., G. candidum, Mycosphaerella fijiensis M. Morelet, P. cinnamomi, P. palmivora, Pythium ultimum, R. solani, S. sclerotiorum, and V. dahliae after two days of exposure, promoting death of their cells. Many of these species are important phytopathogenic fungi associated with postharvest decay in fruits and vegetables [52].
Other Muscodor species, such as M. musae, M. oryzae, M. suthepensis and M. equiseti N. Suwannarach & S. Lumyong, were described together with the antimicrobial potential of VOCs emitted. These VOCs showed antimicrobial activity against several microorganisms, including important post-harvest phytopathogens, such as A. flavus, B. cinerea, Colletotrichum capsici (Syd. & P. Syd.) Butler & Bisby, Colletotrichum gloeosporioides (Penz.) Penz. & Sacc., Colletotrichum musae (Berk.& Curtis) Arx, Penicillium digitatum, and P. expansum, and in most cases, the exposure to the compounds emitted by these Muscodor species inhibited 100% of phytopathogen growth and caused death of their cells [47].
Muscodor species are not the only fungi that have been reported to emitanti microbial volatiles with the potential to inhibit growth and even kill post-harvest phytopathogenic fungi in fruits and vegetables. For Myrothecium inundatum Tode, Phomopsis sp., Hypoxylon sp., Nodulisporium sp., Bionectria ochroleuca (Schwein.) Schroers & Samuels, Schizophyllum commune RF., Gloeosporium sp., and Gliocladium sp., even though these fungi do not exhibit the same effects observed in Muscodor spp. compounds in vitro, the VOCs produced by isolates of these fungi reduced the growth of important fungi associated with post-harvest diseases, such as Aspergillus ochraceus, A. flavus, A. fumigatus, B. cinerea, C. capsici, C. gloeosporioide, C. lagenarium, C. musae, G. candidum, Penicillium digitatum, Penicillium expansum, Phytophthora palmivora, Pythium ultimum, and Sclerotinia sclerotiorum [53-60].
In addition to in vitro assays, some studies have been performed to elucidate the potential of VOCs produced by fungi to control postharvest diseases in fruits and vegetables by mycofumigation of the horticultural product. The VOCs emitted by Aureobasidium pullulans yeast isolates inhibited (in vitro) conidial germination of post-harvest disease-causing phytopathogens in apple. Furthermore, when tested in vivo, the VOCs reduced the incidence of blue mold and bitter rot in apple caused by Penicillium expansum and Colletotrichum acutatum, respectively; however, the greatest effect was observed after directly applying the antagonists to the fruit [61]. In later tests (in vivo), VOCs of the same isolates significantly reduced B. cinerea and P. expansum infection in apple, as observed by the smaller size of damage in the fruit compared with the control treatment; in this assay, the antagonist was inoculated in culture medium deposited at the bottoms of glass boxes containing apples artificially inoculated with the phytopathogens, thus preventing direct contact between the antagonist and the fruit [62].
Other yeasts, such as Candida intermedia, Wickerhamomyces anomalus, and Metschnikowia pulcherrima, have been tested for post-harvest disease control in fruit. Isolates of these yeasts were used to control B. cinera colonization in strawberry and table grape. The VOCs emitted inhibited B. cinera growth in vitro, and the yeasts reduced disease severity when applied in vivo. However, the effect on the inhibition of disease development was more intense after directly applying yeast suspension to the strawberries inoculated with B. cinera [40,42].
The potential of volatiles produced by M. albus to control postharvest diseases in fresh fruit by mycofumigation was also studied. Mycofumigation of apple with M. albus culture controlled blue mold (Penicillium expansum) and gray mold (Botrytis cinerea) in apples inoculated with the phytopathogens, without requiring direct contact between the fruit and the M. albus culture. The same was observed in peaches inoculated with Monilinia fructicola, where fumigation with M. albus culture promoted complete control of brown rot in an assay performed using closed plastic boxes. In organic table grape (‘Thompson Seedless’ and ‘Red Seedless’ varieties), mycofumigation with M. albus culture in plastic boxes reduced the incidence of postharvest decay [35,63,64].
Mycofumigation with Oxyporus latemarginatus isolate culture also reduced development of gray mold caused by B. cinera in apples [65]. In citrus, mycofumigation with Nodulisporium sp. isolate culture controlled green mold decay in Citrus limon caused by Penicillium digitatum and blue mold decay in Citrus aurantifolia and C. reticulata caused by P. expansum [57].
Conclusion
Mycofumigation is a promising alternative for reducing postharvest losses in fruits and vegetables caused by fungi. The method has potential to be applied during the transport and storage of fresh fruits and vegetables, where the presence of antimicrobial VOCs, such as compound mixtures produced by M. albus cultures, may increase the shelf lives of these horticultural products by reducing the incidence of post-harvest diseases. The potential of some fungi to emit VOCs able to inhibit or cause death of important phytopathogenic fungi associated with post-harvest decay, without requiring direct contact with the product to be consumed, together with the wide range of microorganisms sensitive to VOCs from fungal species, makes mycofumigation an interesting method for controlling post-harvest diseases, which, unlike traditional methods, reduces risks to human health and environmental contamination.
Acknowledgement
The authors thank the Conselho Nacional de Desenvolvimento Científico e Tecnológico – CNPq, Coordenação de Aperfeiçoamento de Pessoal de Nível Superior – CAPES and Fundação de Amparo a Pesquisa do Estado de Minas Gerais – FAPEMIG for financial support.
References
- Macarisin D, Cohen L, Eick A, Rafael G, Belausov E, Wisniewski M, et al. Penicillium digitatum Suppresses Production of Hydrogen Peroxide in Host Tissue During Infection of Citrus Fruit. Phytopathology. 2007; 97: 1491-1500.
- Droby S, Chalutz E, Wilson CL, Wisniewski ME. Alternative to the use of synthetic fungicides. Phytoparasitica. 1992; 20: 149-153.
- Prusky D. Reduction of the incidence of postharvest quality losses, and future prospects. Prospects Food Secur. 2011; 3: 463-474.
- Sharma RR, Singh D, Singh R. Biological control of postharvest diseases of fruits and vegetables by microbial antagonists: A review. Biol Control. 2009; 50: 205-221.
- Aloui H, Khwaldia K, Licciardello F, Mazzaglia A, Muratore G, Hamdi M, et al. Efficacy of the combined application of chitosan and Locust Bean Gum with different citrus essential oils to control postharvest spoilage caused by Aspergillus flavus in dates. Int J Food Microbiol. 2014; 170: 21-28.
- Auger J, Pérez I, Esterio M. Diaporthe ambigua Associated with Post-Harvest Fruit Rot of Kiwifruit in Chile. Plant Dis. 2013; 97: 843.
- Bourret TB, Kramer EK, Rogers JD, Glawe DA. Isolation of Geotrichum candidum pathogenic to tomato (Solanum lycopersicum) in Washington State. N Am Fungi. 2013; 8: 1-7.
- Frank CO, Kingsley CA. Role of Fungal Rots in Post-harvest Storage Loses in Some Nigerian Varieties of Dioscorea Species. Br. Microbiol Res J. 2014; 4: 343-350.
- Fischer IH, Lourenço SF, Spósito MB, Amorim L. Characterisation of the fungal population in citrus packing houses. Eur J Plant Pathol. 2009; 123: 449-460.
- Kou LP, GaskinsVL, Luo YG, Jurick II WM. First Report of Fusarium avenaceum Causing Postharvest Decay of ‘Gala’ Apple Fruit in the United States. Plant Dis. 2014; 98: 690.
- Youssef K, Ligorio A, Sanzani SM, Nigro F, Ippolito A. Control of storage diseases of citrus by pre- and postharvest application of salts. Postharvest Biol Tec. 2012; 72: 57-63.
- Snowdon AL. Post-Harvest: Diseases and Disorders of Fruits and vegetables. Manson Publishing. 1991.
- Placinta CM, D’mello JPF, MacDonald AMC. A review of worldwide contamination of cereal grains and animal feed with Fusarium mycotoxins. Anim Feed Sci Tech. 1999; 78: 21-37.
- Kamei K, Watanabe A. Aspergillus mycotoxins and their effect on the host. Med Mycol. 2005; 43 Suppl 1: S95-99.
- Sweeney MJ, Dobson AD. Mycotoxin production by Aspergillus, Fusarium and Penicillium species. Int J Food Microbiol. 1998; 43: 141-158.
- Jamalizadeh M, Etebarian HR, Aminian H, Alizadeh A. A review of mechanisms of action of biological control organisms against post-harvest fruit spoilage. EPPO Bulletin. 2011; 41: 65-71.
- Montesinos-Herrero C, Smilanick JL, Tebbets JS, Walse S, Palou L. Control of citrus postharvest decay by ammonia gas fumigation and its influence on the efficacy of the fungicide imazalil. Postharvest Biol Tec. 2011; 59: 85-93.
- Hao W, Zhong G, Hu M, Luo J, Weng Q, Rizwan-Ul-Haq M. Control of citrus postharvest green and blue mold and sour rot by tea saponin combined with imazalil and prochloraz. Postharvest Biol Tec. 2010; 56: 39-43.
- Wells JM, Harvey JM. Combination heat and 2,6-dichloro-4-nitroaniline treatments for control of Rhizopus and brown rot of peaches, plums, and nectarines. Phytopathology. 1970; 60: 116-120.
- Feliziani E, Santini M, Landi L, Romanazzi G. Pre- and postharvest treatment with alternatives to synthetic fungicides to control postharvest decay of sweet cherry. Postharvest Biol Tec. 2013; 78: 133-138.
- Torres CM, Picó Y, Mañes J. Determination of pesticide residues in fruit and vegetables. J Chromatogr A. 1996; 754: 301-331.
- Lima G, Castoria R, De Curtis F, Raiola A, Ritieni A, De Cicco V. Integrated control of blue mould using new fungicides and biocontrol yeasts lowers levels of fungicide residues and patulin contamination in apples. Postharvest Biol Tec. 2011; 60: 164-172.
- López-Fernández O, Rial-Otero R, González-Barreiro C, Simal-Gándara J. Surveillance of fungicidal dithiocarbamate residues in fruits and vegetables. Food Chem. 2012; 134: 366-374.
- Schirra M, D'Aquino S, Cabras P, Angioni A. Control of postharvest diseases of fruit by heat and fungicides: efficacy, residue levels, and residue persistence. A review. J Agric Food Chem. 2011; 59: 8531-8542.
- Brown GE, Dezman DJ. Uptake of Imazalil by Citrus Fruit after Postharvest Application and the Effect of Residue Distribution on Sporulation of Penicillium digitatum. Plant Dis. 1990; 97: 1491-1500.
- Fravel DR. Commercialization and implementation of biocontrol. Annu Rev Phytopathol. 2005; 43: 337-359.
- Droby S, Wisniewski M, Macarisin D, Wilson C. Twenty years of postharvest biocontrol research: Is it time for a new paradigm? Postharvest Biol Tec. 2009; 52: 137-145.
- Cao J, Zhang H, Yang Q, Ren R. Efficacy of Pichia caribbica in controlling blue mold rot and patulin degradation in apples. Int J Food Microbiol. 2013; 162: 167-173.
- Talibi I, Boubaker H, Boudyach EH, Aoumar AAB. Alternative methods for the control of postharvest citrus diseases. J Appl Microbiol. 2014; 117: 1-17.
- Worapong J, Strobel GA, Ford EJ, Li JY, Baird G, HESS WM. Muscodoralbus anam. nov. an endophyte from Cinnamomum zeylanicum. Mycotaxon. 2001; 79: 67-79.
- Strobel GA, Dirkse E, Sears J, Markworth C. Volatile antimicrobials from Muscodor albus, a novel endophytic fungus. Microbiology. 2001; 147: 2943-2950.
- Morath SU, Hung R, Bennett JW. Fungal volatile organic compounds: A review with emphasis on their biotechnological potential. Fungal Biol Reviews. 2012; 26: 73-83.
- Strobel G. Muscodor species – endophytes with biological promise. Phytochemistry Reviews. 2011; 10: 165-172.
- Braun G, Vailati M, Prange R, Bevis E. Muscodor albus volatiles control toxigenic fungi under Controlled Atmosphere (CA) storage conditions. Int J Mol Sci. 2012; 13: 15848-15858.
- Mercier J, Jiménez JI. Control of fungal decay of apples and peaches by the biofumigant fungus Muscodor albus. Postharvest Biol. Tec. 2004; 31: 1-8.
- Mitchell AM, Strobel GA, Hess WM, Vargas PN, Ezra D. Muscodor crispans, a novel endophyte from Ananas ananassoides in the Bolivian Amazon. Fungal Divers. 2008; 31: 37-43.
- Meshram V, Kapoor N, Saxena S. Muscodor kashayum sp. nov. - a new volatile anti-microbial producing endophytic fungus. Mycology. 2013; 4: 196-204.
- Meshram V, Kapoor N, Saxena S. Muscodor kashayum sp. nov. - a new volatile anti-microbial producing endophytic fungus. Mycology. 2013; 4: 196-204.
- From South India. Mycotaxon. 2014; 128: 93-104.
- Suwannarach N, Bussaban B, Hyde KD, Lumyong S. Muscodor cinnamomi, a new endophytic species from Cinnamomum bejolghota. Mycotaxon. 2010; 114: 15-23.
- Parafati L, Vitale A, Restuccia C, Cirvilleri G. Biocontrol ability and action mechanism of food-isolated yeast strains against Botrytis cinerea causing post-harvest bunch rot of table grape. Food Microbiol. 2015; 47: 85-92.
- Batista-Fialho M, Moraes MHD, Tremocoldi AR, Pascholati SF. Potential of antimicrobial volatile organic compounds to control Sclerotinia sclerotiorum in bean seeds. Pesq Agropec Bras. 2011; 46: 137-142.
- Huang R, Li GQ, Zhang J, Yang L, Che HJ, Jiang DH, et al. Control of postharvest Botrytis fruit rot of strawberry by volatile organic compounds of Candida intermedia. Phytopathology. 2011; 101: 859-869.
- Pagans E, Font X, Sánchez A. Emission of volatile organic compounds from composting of different solid wastes: abatement by biofiltration. J Hazard Mater. 2006; 131: 179-186.
- Korpi A, Järnberg J, Pasanen AL. Microbial volatile organic compounds. Crit Rev Toxicol. 2009; 39: 139-193.
- Schotsmans WC, Braun G, DeLong JM, Prange RK. Temperature and controlled atmosphere effects on efficacy of Muscodor albus as a biofumigant. Biol Control. 2008; 44:101-110.
- Ezra D, Strobel GA. Effect of substrate on the bioactivity of volatile antimicrobials produced by Muscodor albus. Plant Sci. 2003; 165: 1229-1238.
- Suwannarach N, Kumla J, Bussaban B, Hyde KD, Matsui K, Lumyong L. Molecular and morphological evidence support four new species in the genus Muscodor from northern Thailand. Ann Microbiol. 2013; 63: 1341-1351.
- Zhang CL, Wang GP, Mao LJ, Komon-Zelazowska M, Yuan ZL, Lin FC, et al. Muscodor fengyangensis sp. nov. from southeast China: morphology, physiology and production of volatile compounds. Fungal Biol. 2010; 114: 797-808.
- Daisy B, Strobel G, Ezra D, Castillo U, Baird G, Hess WM. Muscodor vitigenus anam. sp. nov., an endophyte from Paullinia paullinioides. Mycotaxon. 2002; 84: 39-50.
- Alpha CJ, Campos M, Jacobs-Wagner C, Strobel SA. Mycofumigation by the volatile organic compound-producing Fungus Muscodor albus induces bacterial cell death through DNA damage. Appl Environ Microbiol. 2015; 81: 1147-1156.
- Ramin AL, Braun PG, Prange RK, DeLong JM. In vitro Effects of Muscodor albus and Three Volatile Components on Growth of Selected Postharvest Microorganisms. HortScience. 2005; 40: 2109-2114.
- Kudalkar P, Strobel G, Riyaz-Ul-Hassan S, Geary B, Sears J. Muscodor sutura, a novel endophytic fungus with volatile antibiotic activities. Mycoscience.2012; 53: 319-325.
- Banerjee D, Strobel GA, Booth E, Geary B, Sears J ,Spakowicz D, et al. An endophytic Myrothecium inundatum producing volatile organic compounds. Mycosphere. 2010; 1: 229-240.
- Singh SK, Strobel GA, Knighton B, Geary B, Sears J, Ezra D. An endophytic Phomopsis sp. possessing bioactivity and fuel potential with its volatile organic compounds. Microb Ecol. 2011; 61: 729-739.
- Tomsheck AR, Strobel GA, Booth E, Geary B, Spakowicz D, Knighton B, et al. Hypoxylon sp., an endophyte of Persea indica, producing 1,8-cineole and other bioactive volatiles with fuel potential. Microb Ecol. 2010; 60: 903-914.
- Mends MT, Yu E, Strobel GA, Riyaz-Ul-Hassan S, Eric Booth E, Geary B, et al. An Endophytic Nodulisporium sp. Producing Volatile Organic Compounds Having Bioactivity and Fuel Potential. J Pet Environ Biotechnol. 2012; 3: 1-7.
- Suwannarach N, Kumla J, Bussaban B, Nuangmek W, Matsui K, Lumyong S. Biofumigation with the endophytic fungus Nodulisporium spp. CMU-UPE34 to control postharvest decay of citrus fruit. Crop Protection. 2013; 45: 63-70.
- Samaga PV, Rai VR, Rai KML. Bionectria ochroleuca NOTL33—an endophytic fungus from Nothapodytesfoetida producing antimicrobial and free radical scavenging metabolites. Ann Microbiol. 2014; 64: 275-285.
- Schaible GA, Strobel GA, Mends MT, Geary B, Sears J. Characterization of an Endophytic Gloeosporium sp. and Its Novel Bioactivity with "Synergistans". Microb Ecol. 2015; 70: 41-50.
- Stinson M, Ezra D, Hess WM, Sears J, Strobel G. An endophytic Gliocladium sp. of Eucryphia cordifolia producing selective volatile antimicrobial compounds. Plant Sci. 2003; 165: 913-922.
- Mari M, Martini C, Spadoni A, Rouissi W, Bertolini P. Biocontrol of apple postharvest decay by Aureobasidium pullulans. Postharvest Biol Tec. 2012; 73: 56-62.
- Di Francesco A, Ugolini L, Lazzeri L, Mari M. Production of volatile organic compounds by Aureobasidium pullulans as a potential mechanism of action against postharvest fruit pathogens. Biol Control. 2015; 81: 8-14.
- Mercier J, Jiménez-Santamaría JI, Tamez-Guerra P. Development of the Volatile-Producing Fungus Muscodor albus Worapong, Strobel, and Hess as a Novel Antimicrobial Biofumigant. Rev Mex Fitopatol. 2007; 25: 173-179.
- Mercier J, Lego SF, Smilanick JL. In-package use of Muscodor albus volatile-generating sachets and modified atmosphere liners for decay control in organic table grapes under commercial conditions. Fruits. 2010; 65: 31-38.
- Lee SO, Kim HY, Choi GJ, Lee HB, Jang KS, Choi YH, et al. Mycofumigation with Oxyporus latemarginatus EF069 for control of postharvest apple decay and Rhizoctonia root rot on moth orchid. J Appl Microbiol. 2009; 106: 1213-1219.
- Worapong J, Strobel G, Daisy B, Castillo UF, Baird G, Hess WM. Muscodor roseus anam. sp. nov., an endophyte from Grevillea pteridifolia. Mycotaxon. 2002; 81: 463-475.
- Saxena S, Meshram V, Kapoor N. Muscodor darjeelingensis, a new endophytic fungus of Cinnamomum camphora collected from northeastern Himalayas. Sydowia. 2014; 66: 55-67.
- Saxena S, Meshram V, Kapoor N. Muscodor tigerii sp. nov.-Volatile antibiotic producing endophytic fungus from the Northeastern Himalayas. Ann Microbiol. 2014; 65: 47-57.
- Gonzalez MC, Anaya AL, Glenn AE, Macías-Rubalcava ML, Hernandez-Bautista BE, Hanlin RT. Muscodor yucatanensis, a new endophytic ascomycete from Mexican chakah, Bursera simaruba. Mycotaxon. 2009; 110: 363-372.
- Stoppacher N, Kluger B, Zeilinger S, Krska R, Schuhmacher R. Identification and profiling of volatile metabolites of the biocontrol fungus Trichoderma atroviride by HS-SPME-GC-MS. J Microbiol Methods. 2010; 81: 187-193.
- Strobel G, Singh SK, Riyaz-Ul-Hassan S, Mitchell AM, Geary B, Sears J. An endophytic/pathogenic Phoma sp. from creosote bush producing biologically active volatile compounds having fuel potential. FEMS Microbiol Lett. 2011; 320: 87-94.
- Schalchli H, Hormazabal E, Becerra J, Birkett M, Alvear M, Vidal M, et al. Antifungal activity of volatile metabolites emitted by mycelial cultures of saprophytic fungi. Chem Ecol. 2011; 27: 503-513.