
Editorial
Austin J Biotechnol Bioeng. 2015; 2(4): 1052.
Chemoattractants, Scaffolds and Endogenous Stem Cells: Adorable Partners of In Situ Tissue Regeneration
Khushboo Gulati¹ and Krishna Mohan Poluri1,2*
¹Department of Biotechnology, Indian Institute of Technology Roorkee, India
²Center of Nanotechnology, Indian Institute of Technology Roorkee, India
*Corresponding author: Krishna Mohan Poluri, Department of Biotechnology, Indian Institute of Technology Roorkee (IIT-Roorkee), Roorkee – 247667, Uttarakhand, India
Received: September 05, 2015; Accepted: September 14, 2015; Published: September 15, 2015
Editorial
Tissue engineering is a rapidly flourishing cutting-edge biomedical research field promising the regeneration of damaged/ injured tissues and organs through combination of cells, engineered biomolecules and scaffolds along with well-defined biochemical and physiological approaches. Regeneration of the injured tissue can be achieved through two approaches (a) ex vivo or (b) in situ. The former approach involves creation of replacement tissues under ex vivo conditions and then subsequent in vivo transplantation. This is heavily used for the transplantation of new tissues like heart, liver skeletal muscle, bone and functional limb structures etc. However, due to altered signaling responses, immune rejections and reduced homing capacity exhibited by the parental system after implantation, ex vivo approach suffered several limitations [1-3]. The latter approach, often recognized as endogenous regeneration, is based on repairing capacity of endogenous stem cells (Mesenchymal Stem Cells – MSCs, Neuronal Stem Cells – NSCs, smooth muscle progenitor cells, fibroblast progenitors etc) under the influence of the natural/engineered exogenous signaling molecules (Growth factors, Chemokines, Glycosaminoglycans – GAGs, collagen etc), stem cells such as MSCs essentially proliferates and differentiates to have a neotissue maturation in a specific manner to replenish the old damaged tissue by a new functional tissue (Figure 1) [4-6].
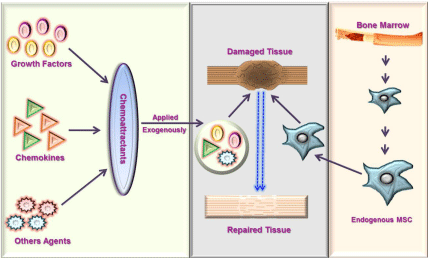
Figure 1: Schematic showing the migration and recruitment of endogenous
MSCs by the exogenous chemoattractants enveloped in an engineered
scaffold at the site of injury for tissue regeneration/repair.
MSCs, also called as mesenchymal stromal cells, are the key cell source of tissue repair and regeneration. Recruitment of MSCs in adult tissues occurs by homing through the vascular network and by interstitial migration within tissue [7]. MSCs are major habitants of bone marrow (bMSC), although their presence was confirmed in other sites such as adipose tissue (aMSC), peripheral blood, cord blood, liver, and fetal tissues and supposed to exist in almost all tissues [8,9]. MSCs are heterogeneous population of stem/progenitor cells and possess multi-lineage potentials to differentiate into mesodermal and non-mesodermal cell lineages, including osteocytes, adipocytes, chondrocytes, myocytes, cardiomyocytes, fibroblasts, myofibroblasts, epithelial cells, and neurons [10]. Clinical investigations established that MSCs can influence various pathophysiological processes, such as injuries, immune and inflammatory responses [11]. In an effort to repair the damaged tissue, it is essential to efficiently recruit sufficient number of MSCs at the target site. The recruitment is a complex multistep process and involves a signal from the injury site causing the release of MSCs from their storage niche into circulation (mobilization), which will essentially migrate towards the target tissue (homing) in order to proliferate and differentiate into matured cells [12]. Such a migration process is based on the principle of chemotaxis, in which chemoattractants forms gradient to guide MSCs [6]. MSC recruitment is a very crucial step for tissue regeneration as it is essential to recruit sufficient number of cells in a best plausible time frame in order to have an accelerated recovery, and in general, combinations of several chemoattractants safeguard this process expeditiously [13]. Indeed this is highly dependent on an uninterrupted production of these endogenous chemoattractants for sustained period at the site of injury.
“Chemoattractants” are the signaling molecules that induce the cell migration and traditionally comprising the families of growth factors, bone morphogenetic proteins, cytokines, chemokines, their coupled receptors and few other extracellular entities [14]. They play a major role in wound repair. Among several chemoattractants, growth factors and chemokines takes a lion’s share in guiding MSCs. (a) Growth factors are the group of proteins, that simulate growth of tissues. A variety of growth factor families are involved during the migration process, they include PDGF (Platelet Derived Growth Factor), Insulin like Growth Factors (IGF-1, IGF-2, IGFBP-3, IGFBP-5), Bone Morphogenetic Proteins (BMP) (BMP-2, BMP-4, BMP-7, TGF-β1, TGF-β3) and Transforming Growth Factors (TGF-β), Epidermal Growth Factor/Receptor (EGF; EGFR), Fibroblast Growth Factors (FGF2), vascular endothelial growth factor (VEGF) etc [6]. (b) Chemokines, classically known as the “chemotactic cytokines” that regulate migration of cells in number of biological processes that includes tissue homeostasis, and inflammatory responses. They have been sub grouped into four different classes depending on the number and position of cysteine residues (CC, CXC, C, and CX3C) [15]. Among the chemokines, SDF-1α (stromal cell derived factor, CXCL12) is one of best studied chemokine for MSC recruitment. Other chemokines like IL-8 (CXCL8), MCP1 (CCL2), MIP-1 (CCL3), RANTES (CCL5), TARC (CCL17), SLC (CCL21) and MDC (CCL22) have also been proved to be effective in MSC recruitment and tissue regeneration. Further, lipids such as lysophospholipids, that includes Lysophosphatic Acid (LPA) and Sphingosine 1-Phosphate (S1P); and proteins including, non-histone DNA-binding cytokine, HMGB-1, tumor necrosis factor (TNF-α), TNF-stimulated gene 6 protein (TSG6), toll like receptors, and insulin etc, also aids in MSC migration [6]. TNF-α was reported to stimulate chemotaxis by rat bMSC and by human aMSC and bMSC in dose dependent manner [16,17]. It was also found that preincubations of aMSC with TNF-α result in increased migration response to chemokines and growth factors but preincubations of bMSC results in enhancement in migration response to chemokines but not to growth factors [18]. Furthermore, synthetic chemicals/drugs such as dexamethasone, isobutyl methyl xanthine, and indomethacin have also been reported to affect the lineage commitment of MSCs [10]. However, as a result of immunocompromised conditions, during several injuries, the endogenous cells do not produce sufficient quantities of these chemoattractants for long enough time periods thus hindering the healing process.
Several engineered strategies have been designed for accelerating the migration of MSCs to a particular tissue by the aid of specific compositions of exogenous chemoattractants. Variety of devices such as PLGA microspheres, hydrogels, bio-scaffolds, nanomaterials are being employed and exploited for the effective release of chemoattractants at the site of injury for a sustained recruitment process. These devices serve great purpose for locally applying/ injecting chemoattractants at site of injury and are becoming popular due to their effective low cost, ease of usage and labor requirements [19]. Many concerns needs to be addressed for efficient usage of chemoattractants as therapeutic molecules; that includes their (i) short half-life, (ii) rapid diffusion on bolus injection, (iii) amenability to cleavage by proteases, (iv) should be distributed in spatially defined gradients at precisely the right times to promote chemotaxis of endogenous stem cells, and (v) finally their inflammatory side effects [5].
Many delivery devices are being employed for both in-vitro and in-vivo trials to deliver several of the chemoattractants such as CXCL12, CCL2, CCL19, CCL20, CCL21, VGEF, BMP-2 etc, for in situ tissue regeneration [20,21]. Self-assembling peptides, covalent binding to PEGylated fibrin patch, heparinized collagen scaffold, mineralized collagen type 1 scaffold, poly (lactic-co-glycolic acid) (PLGA), poly (e-caprolactone), poly (lactideethylene oxide fumarate) hydrogel and chitosan/poly(gamma-glutamic acid) complexes etc., are few novel families of scaffolds as reviewed earlier [19]. PLGA is the most widely used synthetic polymer for the release of proteins, and was approved for several clinical applications by US and European medical agencies. The Glycosaminoglycan (GAG) based fabrications have been expended successfully in the form of heparin/hyaluronic acid based hydrogels, heparinized collagen scaffolds, heparin-coating, and poly (L-lysine)–hyaluronan multilayer films. These scaffolds add another layer of advantage for chemoattractant delivery as they mimic natural GAG-chemokine/growth factor/cytokine interactions and also efficiently protect them from proteolytic degradation. A more recent and novel approach is to use nucleic acids as a “super pharmaceutical” entities to thrust the biological responses of tissue regeneration using the gene delivery/therapy approaches [22].
Diversified biomaterial scaffolds are also being used to provide structural support for the immobilization of cells. Although assorted scaffolds are used, all these scaffolds share some common characteristics such as biological stability, biodegradable and temporal structural integrity. Moreover, they should be permeable to develop functional vasculation and provides enough space for cells to reside and for the entry of bioactive molecules that are involved in MSC migration, proliferation and differentiation effectively by using host microenvironment [23]. Scaffolds can be either prepared using natural or synthetic polymers. Natural polymers are made of either proteins like collagen or polysaccharides such as alginate, hyaluronic acid, heparin, chitin, starch, and dextran [24,25], and synthetic polymeric materials such as polyglycolic acid, polylactic acid and PLGA [26]. Such biodegradable polymers are non-toxic during degradation and are subsequently cleared out of the body in form of CO2 and water and can also be fabricated into different structural configurations owing to their thermoplastic properties [23].
Considerable progress has also been made in the areas of nanotechnology, nanomedicine and nanobiotechnology, which aim at the development of materials specifically for tissue regeneration. This field promises many exciting nanomaterials such as nanoparticles, nanoclusters, nanocrystals, nanotubes, nanofibers, nanowires, nanorods, and nanofilms etc, for tissue engineering [27]. Nanomaterials like peptide amphiphiles, self-assembling peptides, electrospun scaffolds, layer-by-layer complexes, nanotubes and nanocomposites have been successfully applied to cell culture, encapsulation and delivery [28]. Due to biomimetic features and astonishing physiochemical properties of nanomaterials, they plays an essential role in enhancing the cell growth and assist in tissue regeneration. They can imitate well the nanometer sized natural tissues and organs and interactions of cells with nanosized Extracellular Matrix (ECM). Many of the nanomaterial scaffolds wrapping different types of cells like chondrocytes, osteoblasts and other stem cells have been fabricated and proven to be cytocompatible biomimetic nanomaterial scaffolds [27]. They have also contributed towards highly challenging nervous system by helping the repair of damaged nerves. Carbon nanotubes/fibers are being used for regeneration of axon, because of their nanoscale dimensions which are similar to neurites having excellent electrical conductivity and mechanical strength [29]. As the usage of nanomaterials is increasing at high pace in tissue engineering applications, there is a pressing need to delineate the adverse effects of these nanomaterials on human health and environment. Few studies have already reported nanomaterials toxicity to humans due to their capacity to form aggregates with biomolecules [30], thus this field demands an in depth understanding of behaviors of these nanomaterials for their exploitation in biomedical applications. Currently several research groups across the globe are devoted towards therapeutic applications and toxic effects of these delivery devices for tissue regeneration.
Even though, tissue regeneration sector has made tremendous progress, the field is still at its infancy to address issues like immunoregulatory contribution of MSCs in situ, chemoattractant performance in tissue repair, species dependent functionality of MSCs, effect of inflammatory cytokines on multi-potency and differentiation of MSCs. For the effective tissue regeneration, it is essential to expand our knowledge about chemoattractants and their molecular recognition and signaling principles. Thus this field appeals for a comprehensive and concerted knowledge of evolutionary, molecular and structural features of all the biomolecules such as the interactions of glycosaminoglycans with chemokines and growth factors that are participating in tissue regeneration process, along with unique biocompatible scaffolds/devices to throw light on this jigsaw puzzle.
Acknowledgement
This work is supported by the Grants, DBT-IYBA – No.BT/07/ IYBA/2013-19, SERB - No.SB/YS/LS-380/2013 and IITR-BIOFIG- 100637 from Government of India to KMP.
References
- Karp JM, Leng Teo GS. Mesenchymal stem cell homing: the devil is in the details. Cell Stem Cell. 2009; 4: 206-216.
- Badylak SF, Nerem RM. Progress in tissue engineering and regenerative medicine. Proc Natl Acad Sci USA. 2010; 107: 3285-3286.
- Newman RE, Yoo D, LeRoux MA, Danilkovitch-Miagkova A. Treatment of inflammatory diseases with mesenchymal stem cells. Inflamm Allergy Drug Targets. 2009; 8: 110-123.
- Anitua E, Sánchez M, Orive G. Potential of endogenous regenerative technology for In situ regenerative medicine. Adv Drug Deliv Rev. 2010; 62: 741-752.
- Chen FM, Wu LA, Zhang M, Zhang R, Sun HH. Homing of endogenous stem/progenitor cells for In situ tissue regeneration: Promises, strategies, and translational perspectives. Biomaterials. 2011; 32: 3189-3209.
- Vanden Berg-Foels WS. In situ tissue regeneration: chemoattractants for endogenous stem cell recruitment. Tissue Eng Part B Rev. 2014; 20: 28-39.
- Laird DJ, von Andrian UH, Wagers AJ. Stem cell trafficking in tissue development, growth, and disease. Cell. 2008; 132: 612-630.
- da Silva Meirelles L, Caplan AI, Nardi NB. In search of the in vivo identity of mesenchymal stem cells. Stem Cells. 2008; 26: 2287-2299.
- Kuhn NZ, Tuan RS. Regulation of stemness and stem cell niche of mesenchymal stem cells: implications in tumorigenesis and metastasis. J Cell Physiol. 2010; 222: 268-277.
- Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999; 284: 143-147.
- Shi Y, Su J, Roberts AI, Shou P, Rabson AB, Ren G. How mesenchymal stem cells interact with tissue immune responses. Trends Immunol. 2012; 33: 136-143.
- Liu ZJ, Zhuge Y, Velazquez OC. Trafficking and differentiation of mesenchymal stem cells. J Cell Biochem. 2009; 106: 984-991.
- Ingber DE, Mow VC, Butler D, Niklason L, Huard J, Mao J, et al. Tissue engineering and developmental biology: going biomimetic. Tissue Eng. 2006; 12: 3265-3283.
- Li L, Jiang J. Regulatory factors of mesenchymal stem cell migration into injured tissues and their signal transduction mechanisms. Front Med. 2011; 5: 33-39.
- Moser B, Willimann K. Chemokines: role in inflammation and immune surveillance. Ann Rheum Dis. 2004; 63: ii84-ii89.
- Fu X, Han B, Cai S, Lei Y, Sun T, Sheng Z. Migration of bone marrow-derived mesenchymal stem cells induced by tumor necrosis factor-alpha and its possible role in wound healing. Wound. Repair Regen. 2009; 17: 185-191.
- Zhang A, Wang Y, Ye Z, Xie H, Zhou L, Zheng S. Mechanism of TNF-α-induced migration and hepatocyte growth factor production in human mesenchymal stem cells. J Cell Biochem. 2010; 111: 469-475.
- Baek SJ, Kang SK, Ra JC. In vitro migration capacity of human adipose tissue-derived mesenchymal stem cells reflects their expression of receptors for chemokines and growth factors. Exp Mol Med. 2011; 43: 596-603.
- Andreas K, Sittinger M, Ringe J. Toward In situ tissue engineering: chemokine-guided stem cell recruitment. Trends Biotechnol. 2014; 32: 483-492.
- Jay SM, Shepherd BR, Andrejecsk JW, Kyriakides TR, Pober JS, Saltzman WM. Dual delivery of VEGF and MCP-1 to support endothelial cell transplantation for therapeutic vascularization. Biomaterials. 2010; 31: 3054-3062.
- Zhao X, Jain S, Benjamin Larman H, Gonzalez S, Irvine DJ. Directed cell migration via chemoattractants released from degradable microspheres. Biomaterials. 2005; 26: 5048-5063.
- Pacheco DP, Reis RL, Correlo VM, Marques AP. The Crosstalk between Tissue Engineering and Pharmaceutical Biotechnology: Recent Advances and Future Directions. Curr Pharm Biotechnol. 2015; 16: 1012-1023.
- Ko IK, Lee SJ, Atala A, Yoo JJ. In situ tissue regeneration through host stem cell recruitment. Exp Mol Med. 2013; 45: e57.
- Cen L, Liu W, Cui L, Zhang W, Cao Y. Collagen tissue engineering: development of novel biomaterials and applications. Pediatr Res. 2008; 63: 492-496.
- Lee KY, Jeong L, Kang YO, Lee SJ, Park WH. Electrospinning of polysaccharides for regenerative medicine. Adv Drug Deliv Rev. 2009; 61: 1020-1032.
- Lutolf MP, Hubbell JA. Synthetic biomaterials as instructive extracellular microenvironments for morphogenesis in tissue engineering. Nat Biotechnol. 2005; 23: 47-55.
- Zhang L, Webster TJ. Nanotechnology and nanomaterials: promises for improved tissue regeneration. Nanotoday. 2009; 4: 66-80.
- Wan AC, Ying JY. Nanomaterials for In situ cell delivery and tissue regeneration. Adv Drug Deliv Rev. 2010; 62: 731-740.
- Mattson MP, Haddon RC, Rao AM. Molecular functionalization of carbon nanotubes and use as substrates for neuronal growth. J Mol Neurosci. 2000; 14: 175-182.
- Lam CW, James JT, McCluskey R, Hunter RL. Pulmonary toxicity of single-wall carbon nanotubes in mice 7 and 90 days after intratracheal instillation. Toxicol Sci. 2004; 77: 126-134.