
Research Article
Austin J Biotechnol Bioeng. 2016; 3(1): 1057.
Silver Nanoparticles an Accumulative Hazard in Silkworm: Bombyx mori
Jeyaraj Pandiarajan1,2, Venkatachalam Jeyarani¹, Sundaramahalingam Balaji² and Muthukalingan Krishnan¹*
¹Department of Environmental Biotechnology, School of Environmental Sciences, Bharathidasan University, India
²Department of Biotechnology, Ayya Nadar Janaki Ammal College, India
*Corresponding author: Muthukalingan Krishnan, Department of Environmental Biotechnology, School of Environmental Sciences, Bharathidasan University, India
Received: December 03, 2015; Accepted: March 01, 2016; Published: March 04, 2016
Abstract
To evaluate the lethal effects of silver nanoparticles on silkworm Bombyx mori, Morus alba (mulberry) leaf extract was used to synthesize bio-nanoparticles. Hence B. mori was a monophagous insect; the exact level toxicity can be evidenced through its own feed. Characterization of the nanoparticles was done by UV-Vis, FTIR spectroscopy and SEM. UV-Vis Spectroscopy illustrated the presence of silver nanoparticles at 422 nm. FTIR spectroscopy enabled the size of biosynthesized silver nanoparticles, ranging from 45 nm to 166 nm. SEM visualized the ranging particles with EDX value. The fifth instar larvae were dosed with silver nanoparticles at varying concentrations (1, 10 and 100 ppm). Observations revealed maximum larval weight was in 1ppm treatment, pupal weight was maximum in 100ppm. Also the cocoon weight and shell weight were moderately high in all the treated doses, when compared control. But there was lack of during larval pupal transition and pupal adult transition which lead to the high mortality rate at 100ppm and moderate mortality rate at 10ppm. The protein profile of treated hemolymph envisioned the expression of a 20 kDa protein, it was subjected to MALDI-TOF analysis and identified as Glutathione- S-transferase.
Keywords: Mulberry; Morus alba; Silver nanoparticles; Silkworm; Bombyx mori; Lethal toxicity; Glutothione-S-transferase
Abbreviations
AgNps: Silver Nanoparticles
Introduction
In recent decade, green synthesis of metallic nanoparticles has become a promising field of research. Metallic nanoparticles synthesized by various chemical, physical as well as biosynthetic approach mediated by numerous plants and microorganisms. Bio nanomaterials were feasible, scalable, non corrosive and economical when compared to synthetic nanoparticles, it can be achieved easily through the plant-mediated approach [1]. Silver is a renowned resource metal exploited for the common usage of mankind, which is well-known for its innate antimicrobial activity towards the microorganisms [2]. So this innate antimicrobial response of silver was employed in medical field for the preparation of topical ointments, creams containing silver to prevent infection of burns and open wounds [3], medical devices and implants prepared with silverimpregnated polymers [4]. In addition, silver-containing consumer products such as colloidal silver gel and silver-embedded fabrics are now used in sporting equipment.
Nanotoxicology is a new field established in the current decade, which emphasizes several risk factors to the human health and environment by the usage of nanomaterial in commercial goods and novel technologies [5]. Though the ingestion and toxicity level of nanoparticles was very low in the living systems, it leads to the accumulation in host and it could explicit adverse effect later. It has been already reported that, the nanomaterials such as carbon nanotubes, quantum dots and metal and metal oxide nanoparticles are having the adverse toxic effects when they are dosed even in low level [6]. The earlier reports insist that biologically synthesized nanoparticles are safer materials which do not have any danger in the living host. These assessments of toxicity mediated by bio nanoparticles will open up several questions raised against the risk assessment in human and cattle. The experimental evidence of toxicity generated by biological nanoparticles may bring out a decline in the use of nanomaterial in several fields such as nanomedicine, material safety, nano coupled drug and nano commercial goods. So the present investigation was sketched to analyze the toxicity created by biological silver nanoparticles, in the model animal silkworm, Bombyx mori.
Toxicity evaluation on silkworm, B. mori can be rationally comparable with those of other lepidopteran pest insects, so it is considered as a suitable model for exploring effects of any new synthetic formulations for the past two decades [7]. Silkworm rearing is a traditional business not only in India, but also in many developing countries and the life of many people is depended on it. Increase of larval growth, cocoon quality and quantity would result better economics for sericulture industry and meet out the production needs. Consequently, the enrichment of mulberry leaves by supplementary compounds with the aim of increasing the production of cocoon is a very important aspect. Many investigations have been done on this topic and various reports have been published [7-9]. Copiously the earlier reports have mentioned that the Silver Nanoparticles (AgNps) can be utilized as an ancillary complex which can boost up the growth and development of the larvae and also the quality and quantity of the cocoon. In another dimension, nanoparticles help to produce new pesticides, insecticides and insect repellents. Moreover, research on silkworm, B. mori clearly demonstrates that nanoparticles could stimulate more production of fibroin protein which can help in producing carbon nanotube in future [10]. Legay [11] has stated that silk production is dependent on the larval nutrition and the nutritive value of mulberry leaves which plays an effective role in producing good quality cocoons.
On the other hand the scientists were trying a lot to increase the yield of silk production by modern biotechnological approach. But unfortunately there is a decline in sericulture industry because of several threatening factors, in which pollution is the major issue. Since the nanoparticles are used in various applications such as pharmaceutical drug development and pesticide development relevance. Subsequently there is a need arise to study acute toxic effects of AgNps, therefore in the present investigation M. alba coupled nanoparticles was used to treat the B. mori larvae. The toxic effect of nanoparticles was measured using changes in biochemical, morphometric characters and proteomic level.
Materials and Methods
Biosynthesis of silver nanoparticles
Fresh mulberry leaves (Morus alba) were collected, surface cleaned with running tap water, followed by a thorough wash with double distilled water and shade dried for a week and powdered. Leaf extract was prepared by taking 5 g of dried leaf powder in 250 ml Erlenmeyer flask with 100ml distilled water and boiled the mixture for 20 minutes. The extract was then filtered through Whatman No.1 filter paper and used for further experiments. This extract was used as a reducing and stabilizing agent. For all experiments, the source of silver was silver nitrate in distilled water. 5 ml of leaf extract was added to 95 ml of 1mM aqueous silver nitrate for the reduction of Ag+ ions. The effect of temperature on particle size of the synthesized silver nanoparticles was studied by carrying out the reaction in water bath at 30°C to 95°C and incubated for 15 minutes. The silver nanoparticles thus obtained were purified by repeated centrifugation at 10000 rpm for 15 minutes followed by resuspension of the pellet in deionized water.
Characterization of silver nanoparticles
UV-visible spectroscopy: The reduction of pure Ag+ ions was monitored by UV–visible spectrum (Perkin-Elmer Lambda-35 spectrophotometer operated at a resolution of 1 nm). The fine bio nanoparticles were aliquoted with 10 folds of deionized water for measuring the spectrum, Silver Nanoparticles (AgNps) were soluble in distilled water and the color change was observed visually. UV– visible spectroscopic analysis was performed by continuous scanning from wavelength 190 nm to 1190 nm.
FTIR & Particle size analysis: In order to determine the possible functional groups of the mulberry extract and their involvement in the synthesis of silver nanoparticles, FTIR analysis was carried out. Control and test samples were independently blended with KBr to obtain pellets. The FTIR spectra were collected at the resolution of 4 cm-1 in transmission mode (400-4000 cm-1) using FTIR (Perkin Elmer spectrum RX 1 model) spectrophotometer. The size distribution of the particles after dispersion in water was measured at room temperature by Dynamic Light Scattering (DLS) using a Nano- Zeta sizer (Malvern UK).
Scanning electron microscopy: SEM was performed to analyze the nanoparticles in the biological sample. The solution of the synthesized nanoparticles were lyophilized and recovered in powder form using Martin Christ lyophilizer (model Alpha 1-2). After freeze drying of the purified sample, the structure and composition of synthesized silver nanoparticles were analyzed through Scanning electron microscope equipped with Energy Dispersive X-ray Spectrophotometer (EDS). SEM was performed at various resolutions ranging from 1700X to 20000X.
Study design
The study was carried out with 5th instar larva of mulberry silkworm Bombyx mori (Lepidoptera: bombycidae). The study was designed to examine the effect of silver nanoparticles synthesized using mulberry leaf extract on the 5th instar larvae of B. mori. The 5th instar larvae were chosen for supplementation studies because the maximum synthesis of larval proteins occurs in that stage and it end in spinning of cocoon. Freshly ecdysed 5th instar larva were obtained from Sericulture field, Ariyavoor, Tiruchirapalli and used for all the experiments. The larvae were separated into three groups and two positive controls were also maintained. Each group contained 40 animals. Different concentrations of dose (silver nanoparticles) were prepared in distilled water by dissolving the lyophilized powder (1, 10 and 100 ppm). The mulberry leaves were washed thoroughly with double distilled water to remove the surface adhering substances and then air dried. For supplementation, selected concentration of the synthesized silver nanoparticles was spread evenly with the help of L-rod upon the leaves. The leaves were fed to the worms for the first time feed while rest of the 3 feedings was mulberry leaves alone. Two positive controls were maintained, in which, the first control larvae were fed with mulberry leaves alone and the second control larvae were fed with mulberry leaf extract. Mortality and larval weight were recorded daily for all the experimental and control groups.
Experimentation
Control 1 - Larvae were fed with mulberry leaves alone
Control 2 - Larvae were supplemented with mulberry leaf extract alone
Group 1 - Larvae were supplemented with 1 ppm silver nanoparticles through feed
Group 2 - Larvae were supplemented with 10 ppm silver nanoparticles through feed
Group 3 - Larvae were supplemented with 100 ppm silver nanoparticles through feed
Sample collection
Hemolymph was collected from 4th to 6th day of 5th instar larvae by making a slit in the proleg, whereas pupae were bled through an incision made at the extremities. Hemolymph that flowed from the wound without external pressure was collected into eppendorf tubes prerinsed with phenylthiourea solution to prevent tyrosinase activity. The samples were centrifuged at 3000 rpm for 10 minutes at 4°C to sediment hemocytes and cellular debris. Samples were either used immediately or stored at -85°C until further use.
Protein estimation and profiling
Amount of protein was determined according to Bradford protocol (1976) using bovine serum albumin as standard and the optical density was measured at 595 nm. One dimensional Polyacrylamide gel electrophoresis with the presence of Sodium dodecyl Sulphate was performed according to Laemmli 12 using Proviga (India) electrophoretic apparatus. A 10% linear resolving gel and 4% stacking gel were used to separate the proteins, 30μg of total protein was loaded per lane and consistently performed in the electric pulse of 50 V. Gels were stained with Coomassie Brilliant Blue R-250 staining solution for 4 hours and the destaining was carried out in the solution containing 10% glacial acetic acid and 30% methanol.
Ingel digestion
The band was excised with sterile blade and transferred to a sterile tube containing deionized water. Then the gel was destained with methanol in order to remove remaining commassie brilliant blue stain, and incubated with 200 mM ammonium carbonate. The gel piece was dehydrated with 100 μl of acetonitrile and dried in a vacuum. The gel piece was dried and then rehydrated with 20 μl of 50mM ammonium bicarbonate containing 0.2 μg trypsin (sigma). After 45 minutes, the solution was removed and 30μl of 50mM ammonium carbonate was added. Digestion was performed for overnight at 37°C.
Mass spectrometry
MALDI Mass spectra were obtained from utraflex MALDI-MS (Bruker Daltonics, Germany) in the reflection mode, using 200 ns time delay and a 25 kV accelerating voltage in the positive ion mode. The system utilizes 50 Hz pulsed nitrogen LASER, emitting at 337 nm. The matrix used was α-cyano-4-hydroxy cinnamic acid. In order to identify the proteins, all the MALDI MS spectra were recorded on tryptic peptides derived from the band and searched against the protein sequences from the NCBI-nr (organism: Bombyx mori) using the online MASCOT search program(www.matrixscience.com, Matrix Science, UK).
Results
Biosynthesis of nanoparticles
Synthesis of nanoparticles was carried out by mixing the mulberry leaf extract (5%) and 1mM silver mixture at the different temperatures (60°C, 90°C and 9°C (Figure 1)). No obvious color change was observed in the reaction mixture incubated at 30°C, whereas the aqueous solution incubated was turned to yellowishbrown (60°C), brown (90°C) and intense brown (95°C) within 10 minutes of incubation. With the increase in temperature, the reaction rate also got increased, where the color change (yellowish brown) was observed within 5 minutes of incubation and after 10 minutes complete reduction has taken place. Color change confirmed the synthesis of silver nanoparticles, which was due to the reduction of silver ions indicating the formation of nanoparticles. From the above observation, it was apparent that the Mulberry, M. alba leaf extract has the ability to synthesize silver nanoparticles (AgNps).

Figure 1: Colour change of AgNps synthesized using 5% Morus alba leaf (mulberry) extract.
UV-Visible spectrum
An UV-Visible spectrum is one of the most important and sensitive technique to observe the configuration of silver nanoparticles. The silver nanoparticles synthesized from the aqueous extract of M. alba was evaluated through UV-visible spectrophotometer at a wavelength ranges from 190–1100 nm. The UV-visible spectra of silver nanoparticles synthesized using mulberry leaf extract (5%) at various temperatures such as 30°C, 60°C, 90°C and 95°C were shown in Figure 2A-2D. The UV-visible spectra indicated the absorbance peak at 60°C (407 nm), 90°C (422 nm) and at 90°C (414 nm) respectively (Figure 3A-3D). But, Mulberry leaf extract (Control) showed no evidence of absorption peak in the range in 400-450 nm. The color change in reaction mixture was ass°Ciated with well-defined peaks with maximum absorption centered at 422 nm (90°C).
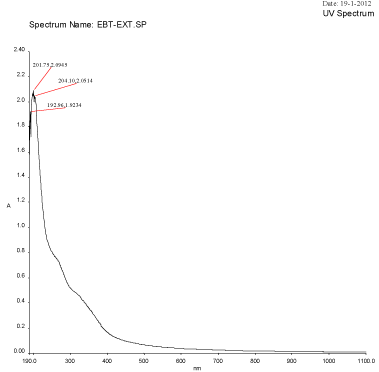
Figure 2A: UV-Visible spectra of Plant extract Mulberry Morus alba.
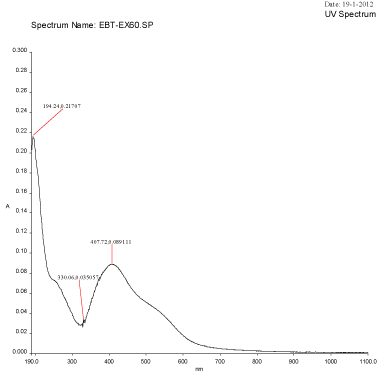
Figure 2B: UV-Visible spectra of silver nanoparticles synthesized using Mulberry Morus alba extract at 60°C
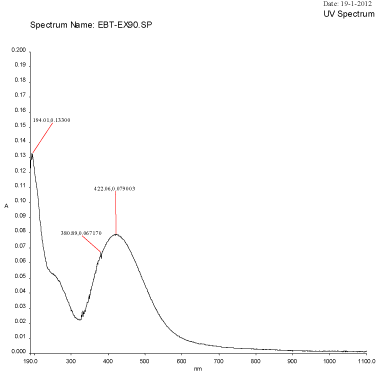
Figure 2C: UV-Visible spectra of silver nanoparticles synthesized using Mulberry Morus alba extract at 90°C.
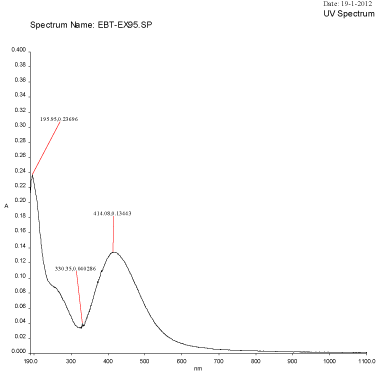
Figure 2D: UV-Visible spectra of silver nanoparticles synthesized using Mulberry Morus alba extract at 95oC.
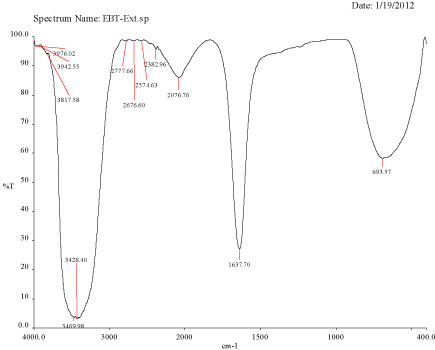
Figure 3A: FTIR spectra of Plant extract Mulberry, Morus alba.
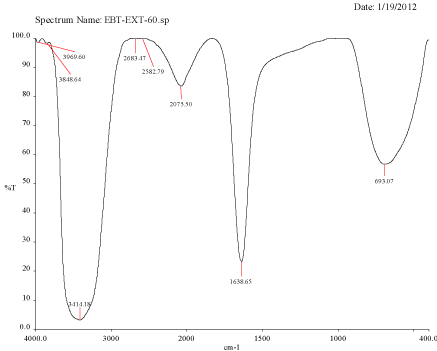
Figure 3B: FTIR spectra of silver nanoparticle synthesized using Mulberry, Morus alba extract at 60oC.
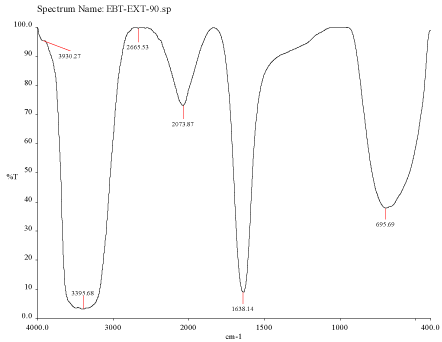
Figure 3C: FTIR spectra of silver nanoparticle synthesized using Mulberry,
Morus alba extract at 90°C.
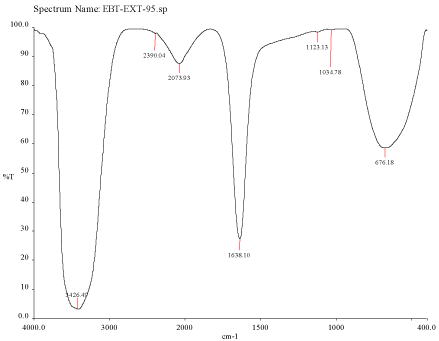
Figure 3D: FTIR spectra of silver nanoparticle synthesized using Mulberry,
Morus alba extract at 95°C.
Fourier transform infra red spectroscopy
FTIR spectra for the mulberry leaf extract (control) and the silver nanoparticles synthesized using mulberry leaf extract were recorded in order to identify the possible functional groups involved in the capping and efficient stabilization. The control spectrum showed number of peaks. The FTIR spectrum of mulberry leaf extract showed peaks at 3470, 2077, 1638 and 693.57 cm-1 whereas the IR spectrum of silver nanoparticles showed intense bands at 3414, 2076, 1638.65 and 693 cm-1. The peak present at 1638 was attributed to N-H bending vibrations. A minor peak shift was observed from 1637.70 to 1638.65 cm-1 (Figure 4) which implied that these groups (amines) may be involved in the reduction process helping in the synthesis of nanoparticles. The peak located at 3469.98 could be attributed to the O-H strong stretching vibrations. A major vibrational shift from 3470 to 3414.18 cm-1 indicated the possible involvement of (O-H group) polyphenolic compounds of the mulberry, which may act as reducing agents in the nanoparticles synthesis. The peak around 693 cm-1 was assigned to the -C-C-H group of alkynes (Figure 4).
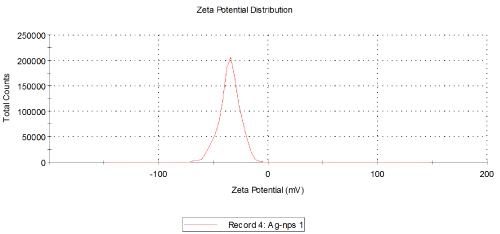
Figure 4: Particle size distribution curve for the AgNps synthesized using
Morus alba extract at 90°C.
Particle size analysis
Temperatures at which the reaction mixtures were incubated also affects the size and shape of the synthesized nanoparticles. The average size distribution of the reaction mixture incubated at 60°C was 45.85 nm (Figure 4) whereas the size of the particles incubated at 90°C and 95°C were 163.4 nm and 166.2 nm (Figure 3) respectively. The currently synthesized nanoparticles using mulberry extract showed peaks at 407.72 nm, 414.08 nm and 422.06 nm incubated at 60°C, 90°C and 95°C respectively. When there was very slight increase in wavelength; the average size of the particles too increased, this show parallel results were obtained in both FTIR and Particle size analysis.
SEM with EDX (Energy dispersive X-ray spectroscopy)
The morphology of the synthesized silver nanoparticles was observed using Scanning Electron Microscope. According to the results observed in the UV-vis spectroscopy and Particle size analysis, the temperature at which the small size being synthesized were taken for the structural characterization using SEM. Most of the particles were irregular in shape and some were spherical (Figure 5). The EDS helps in the elemental analysis and compound characterization of the sample, which illustrate the interaction between the sample and the light source. The EDS profile revealed strong silver signal which confirmed the presence of silver nanoparticles, along with a weak oxygen, chloride, potassium peak were also obtained, that may originate from the biomolecules bound to the surface of the silver nanoparticles and it may serve as encapsulating agents. A distinct signal and high atomic percent values of silver was obtained. Generally, metallic nanosilver showed optical absorption peak approximately at 3 keV due to the surface Plasmon resonance (Figure 6).

Figure 5: Scanning electron micrograph of silver nanoparticles magnified at
1700X and 5000X synthesized using Morus alba extract, 1mM silver nitrate
at 90oC.
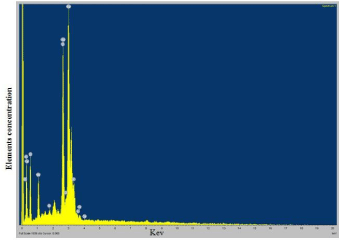
Figure 6: EDAX spectrum of silver nanoparticle synthesized using Morus
alba. Strong silver signals from the atom in nanoparticles are visible at 3KeV.
Toxicity assessment
Freshly molted fifth-instar larvae of silkworm, B. mori were selected for the study. Larvae were fed with different concentration of silver nanoparticles once in a day (1, 10 and 100 ppm) from day 3 to day 5 of fifth-instar larvae. The growth of the larvae was continuously monitored after oral feeding (through leaves). The weight of the larvae after treatment was taken and shown in Table 1. From the Table 1, it was obvious that the mean weight of the control larvae had its maximum weight of 2.72 ± 0.1 g on day 5 of fifth-instar larvae and reduced on the consecutive day to 2.695 ± 0.1 g. A similar prototype was also observed in the treated groups. Larvae fed with 1 ppm showed mean weight of 2.719 ± 0.3 g on day 4, 3.088 ± 0.2 g on day 5 and 2.844 ± 0.1 g on day 6. The mean weight of fifth-instar larvae of group treated with 10 ppm was 2.52 ± 0.3 on day 4, 2.866 ± 0.01 g on day 5 and 2.680 ± 0.2 g on day 6. The mean weight of fifth-instar larvae group treated with 100 ppm was 2.373±0.1 on day 4, 2.531 ± 0.2 on day 5 and 2.58 ± 0.3 on day 6. The larvae treated with silver nanoparticles showed increase in its body weight when compared to the two positive controls (control and leaf extract). Among the treated groups, larvae fed with 1 ppm gained maximum weight which fed on the mulberry leaves ferociously. Larvae fed with 10 ppm and 100 ppm were also shown to weight gain but lesser than the larvae fed with 1 ppm. The decrease in mean larval body weight was observed on day 6 which may be due to the shrinkage (for building cocoon). Both the control and treated groups fed on mulberry leaves continuously without any toxic symptoms. No mortality was observed during the larval days. But during larval pupal transition the mortality rate started for 10 ppm and reached the maximum at 100 ppm, the same was seen in the pupal adult transition also (Figure 7).
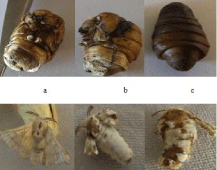
Figure 7: Lethal effects of silver nanoparticles (10 ppm and 100 ppm) on
pupation and adult development in silkworm, Bombyx mori subjected to oral
feeding from day 3 to day 5 of fifth-instar larvae a & b – 10 ppm ; c – 100 ppm.
Group
Day 4 After 24 hrs
Day 5 After 48 hrs
Day 6 After 72 hrs
Control
2.623±0.2
2.720±0.1
2.695±0.1
1 ppm
2.719±0.3
3.088±0.2
2.844±0.1
10 ppm
2.520±0.3
2.866±0.1
2.680±0.2
100 ppm
2.373±0.1
2.531±0.2
2.580±0.3
Leaf extract
2.644±0.2
2.794±0.1
2.694±0.3
Table 1: Morphometric data on various concentrations of silver nanoparticles treated mulberry leaves fed on 5th instar larvae weight of Bombyx mori.
Morphometric characters
The mature fifth instar larvae were picked up from rearing trays and released on Netrikas for spinning cocoons. The cocoons were harvested after 3 days of spinning. After the completion of spinning, the cocoon, pupae and shell were weighed separately. The mean cocoon weight of the control, 1 ppm, 10 ppm, 100 ppm and mulberry extract larvae were found to be 1.319 ± 0.2 g, 1.465 ± 0.3 g, 1.425 ± 0.3 g, 1.463 ± 0.3 g and 1.375 ± 0.2 g respectively. The weight of the cocoon of B. mori fed with silver nanoparticles treated mulberry leaves were found to be more than that of the larvae left untreated (control). In these five observations, the 1 ppm AgNps treated larvae produced cocoon weight were appreciably got increased than the other four groups.
Randomly selected 20 shells were weighed using Sartorius weighing balance. The yield of shell from control, 1 ppm, 10 ppm, 100 ppm and mulberry extract were found to be 4 ± 0.3 g, 4.97 ± 0.2 g, 4.77 ± 0.2 g, 4.76 ± 0.1 g and 4.52 ± 0.3 g. From the above Table 2, it was clear that the yield (shell weight) from 1 ppm treated silkworm, B. mori was 4.97 which was noticeably got increased when compared to other two treated and control groups. The shell weight of 10 ppm, 100 ppm treated groups also got increased but comparatively less than that of group treated with 1 ppm. Table 2 represent mean weight of the control and silver nanoparticle treated group, from which it was clear that the mean weight of the pupae treated with 100 ppm which showed 1.06g whereas the other two treated groups showed decreased pupal weight i.e, 1 ppm & 10 ppm showed 1.14g and 0.95g respectively. The control and 1 ppm treated larvae did not show any wing deformities. The larvae treated with 100 ppm, successfully completed spinning, after that many of them were died (Figure 7), which may be due to the accumulation of silver nanoparticles. The adult emergence rate in 10 ppm was low when compared to other control and treated groups. Even the emerged pupae treated with 10 ppm showed malformed wings. Some of the pupae in 10 ppm concentration were also died, however many of the pupae emerged but some with malformed and vestigial wings (Figure 7).
Group
Mean weight of the larvae
Mean weight of the pupae
Mean weight of the cocoon
Mean weight of the shell
Control
2.679
1.067
4.00
1.319
1 ppm
2.88
1.146
4.97
1.465
10 ppm
2.422
0.959
3.87
1.225
100 ppm
2.261
0.806
3.76
1.163
Leaf extract
2.644
1.132
4.52
1.375
Table 2: Morphometric data on various concentrations of silver nanoparticles treated pupa of Bombyx mori.
Proteomic analysis
The proteomic profile was also performed in 10% SDS PAGE; the profile also uttered the same effects, as the toxicity of nanoparticles was reflected in the 10 ppm of the pupae samples (Figure 8). There was complete shearing occurred in the treated sample at the concentration of 10 ppm, though there was a clear band visualized in the 100 ppm (Figure 9A and 9B). The hemolymph samples were collected from the day 4 to day 6. They were subjected to SDS PAGE (10%), the band pattern of hemolymph sample revealed a complete disintegration in the treated samples when compared to the control. Surprisingly the day 5 the banding pattern was visualized clearly in all the concentrations with the over expression of 70 kDa (SP1 and SP2) and 30 kDa proteins. Interestingly, during the protein profile of hemolymph a band ranging 20 kDa was observed in treated samples of pupal hemolymph, which was observed only in treated samples. The band was sliced and subjected to MALDI analysis, which expressed only in the experimental samples. The data obtained was alleviated through MASCOT analysis to validate the novel peptide based on the mass/charge (m/z) values obtained through peaks calibrated in the MALDI analysis (Table 3). Amusingly the short peptide sequences obtained through mascot analysis revealed the presence of Glutathione-S-transferase subunits (Figure 10).
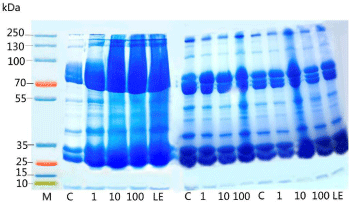
Figure 8: Effect of various concentrations of silver nanoparticles on
hemolymph of B. mori subjected to oral feeding from day 3 to day 5 of fifthinstar
larvae. It represents the hemolymph protein profile in 10% SDS-PAGE
stained with coomassie blue from day 4 of fifth-instar larvae till pupation. Each
lane was loaded with 30μg of protein extracted from hemolymph.
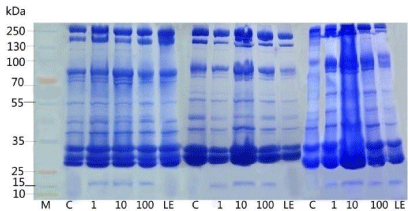
Figure 9A: Represents the hemolymph protein profile of pupae in 10%
SDS-PAGE stained with coomassie blue from day 1 to day 3. Each lane was
loaded with 30μg of protein extracted from hemolymph.
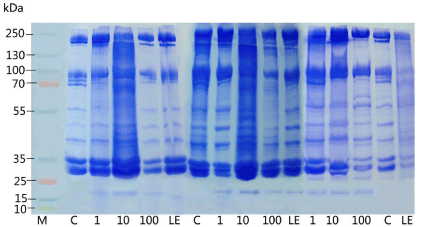
Figure 9B: Represents the hemolymph protein profile of pupae in 10%
SDS-PAGE stained with coomassie blue from day 4 to day 6. Each lane was
loaded with 30μg of protein extracted from hemolymph.
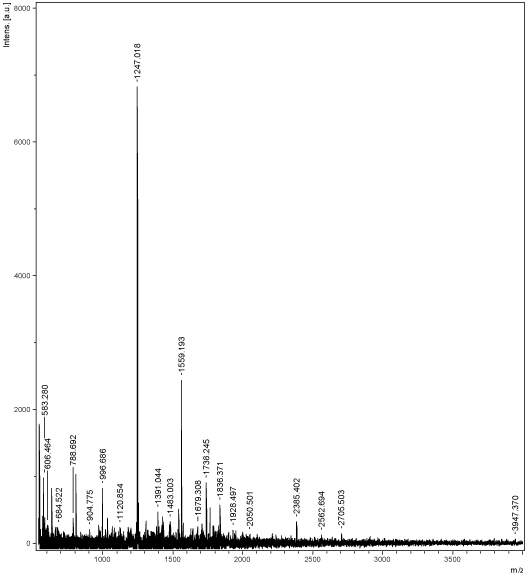
Figure 10: MALDI peaks of the 20 kDa ranging protein expressed in the
hemolymph of nanoparticles treated insect protein samples.
Start-End
Observed
Mr (expt)
Mr (calc)
Delta
Miss Sequence
Similarity hits obtained through BLAST
393-399
809.7160
808.7087
808.3861
0.3226
1
R.MKSNSSR.G
Chain A, Glutathione S-Transferase Unclassified 2 from Bombyx mori
435-443
1240.9150
1239.5594
1239.9077
0.3483
0
R.MWYEQLQDK.L
Chain A, Glutathione S-Transferase Unclassified 2 from Bombyx mori
452-464
1537.1610
1536.1537
1535.8056
0.3482
1
R.AALDQLRQEHVEK.L
Chain A, Crystal Structure of Glutathione S-Transferase
475-483
1179.8990
1178.8917
1179.5488
-0.657
1
R.QRQMQMAQK.L
Glutathione S-Transferase
477-488
1541.2060
1540.1987
1539.7207
0.4780
1
R.QMQMAQKLDIMR.K
Glutathione S-Transferase
Table 3: MALDI MS peaks cured by MASCOT and searched sequences.
Discussion
Silver nanoparticles (AgNps) are being extensively studied because of its smaller size and its superior activity. AgNps are basically of two major types; chemically synthesized Silver nanoparticles and biologically synthesized Silver nanoparticles. Quantum and semiquantums are the best example of the chemically synthesized nanoparticles. Biological silver nanoparticles are derived from sources such as plants, bacteria, fungi and algae. Recently in sericulture, the scientists are attempting to improve the silk production by using the nanoparticles synthesized from mulberry leaves (M. alba), which may be exploited to devise an artificial diet during the scarcity of leaf production. Also the scientific community was very much interested to study the physiological and biochemical impact of nanoparticles on silkworm B. mori.
In the present work, we have attempted a detailed investigation on the effects of silver nanoparticles in vitro. Such studies on toxic effects of nanomaterials are very few and there is no clear guidelines are available to quantify these effects. Silkworm B. mori was a perfect and universally accepted model to evaluate the lethal toxic effects. Hence the silver nanoparticles were synthesized by following the standard Ag reduction through heating process. Surface Plasmon Resonance (SPR) principle was responsible for the excitation of silver nanoparticles when it was combined with biological extracts [13]. From that it was very obvious that the maximum reduction of Ag+ ion into nanoform has taken place within 10 minutes of incubation period. With the increase in temperature, the reaction rate also got increased, where the color change (yellowish brown) was observed within 5 minutes of incubation. In metal nanoparticles such as in silver and gold, the conduction band and valence band lie very close to each other in which electrons move freely. These free electrons give rise to a Surface Plasmon Resonance (SPR) absorption band [14], occurring due to the collective oscillation of electrons of silver nano particles in resonance with the light wave. In UVVisible spectrophotometer, the beam interact with the sample which emits continuous changing wavelength, when the frequency of the field becomes resonant with the coherent electron motion; a strong absorption will takes place, (energy is being transferred from the incident light to the nanoparticles, which is the origin of the observed color. The magnitude, peak, wavelength and the spectral bandwidth of the Plasmon resonance associated with nanoparticles are dependent on particle’s size, shape, and material composition, as well as the local environment [15].
Terpenoids are believed to play an important role in the reduction of ionic form to nano silver. Shankar [16] reported the biological reduction of gold, from ionic form to nanoform (nanogold) using geranium leaf extract, mediated by terpenoids in which the alcoholic group of terpenoids was oxidized to carboxyl group and N-H group were involved in the stabilization of nanoparticles. The leaf extract of cashew was used in the silver nanoparticles synthesis, where water soluble tannin was found to be responsible for the reduction and stabilization of Ag+ ion [17]. Bar [18] synthesized nanoparticles from Jatropha curcas latex , where curcacyclines A & B and curcain act as reducing and stabilizing agents in which particle size ranges from 20- 40nm. Apiin, a secondary metabolite present in the henna (air-dried leaves) interacted with the silver nitrate and reduced the silver present to nanoparticles [19]. FTIR analysis confirmed the involvement of carbonyl group of terpenes involved in nanoparticles synthesis which thereby imparts the negative charge and acted as stabilizing agent in Tansy fruit (T. vulgare) [20]. Philip [21] produced silver nanoparticles from Hibiscus rosasinensis, in which the carboxylate (COO-) group of terpenoids played a key role in stabilizing the nanoparticles was confirmed through FTIR. Prathna [13] predicted that in addition to ascorbic acid and citric acid in lemon juice, phenolic compounds in the form of glycosides quercetin responsible for the reduction.
Smitha [22] synthesized gold nanoparticles using Cinnamonum seylanicum leaf extract as reducing agent and they proposed that the important secondary metabolites such as eugenol, chinnamaldehyde and tannin present in the extract helps in the reduction and stabilization of silver nanoparticles. It has been already reported that mulberry was rich in polyphenolic compounds especially tannin content, (one of the major and important secondary metabolite) which might have played a crucial role in the reduction and stabilization of the silver nanoparticles synthesized in the present study.
These results suggested that silver nanoparticles synthesized using mulberry leaf extract were surrounded by some secondary metabolites which have functional groups like amines, alcohols, aldehydes and carboxylic acids. The observed peaks were characteristics of polyphenolic compounds which were very abundant in mulberry leaves. From the FTIR analysis of the mulberry leaf extract and silver nanoparticles, it was clear that they were reduced to nano silver and being encapsulated by secondary metabolites, which was contrast to the silver nanoparticles synthesized using Trichoderma viridae [23], E. coli, Magnolia kobus [24] where the size of the particles decreased with increase in temperature. Initially with the increase in temperature, the reaction rate too was maximum; hence the enormous amount of small sized particles might have been formed, after that the nanoparticles may have chance to aggregate, (when aggregated) which reflects in increased size. Kim [25] reported that the optical absorption spectra of metal nanoparticles with longer wavelengths correspond to increase in particle size. Wu [26] assumed and reported that with the increase in intensity of absorbance band, i.e UV spectra slightly shifted from 423 to 435 leads to increase in size distribution. In the present study too, size variation may be due to the largely synthesized nanoparticles got agglomerated which gives maximum Plasmon absorption from 407 nm to 422 nm. The obtained results were consistent with above and earlier reports on silver nanoparticles synthesized using banana peel extract and Trichoderma viridae.
Legay [11] stated that silk production is dependent on the larval nutrition and nutritive value of mulberry leaves plays a very effective role in producing good quality cocoons (Akhtar and Asghar [27] found that vitamins and mineral salts played an important role in the nutrition of silkworm. The effect of vitamin supplementation on the growth of B. mori has been investigated by many researchers [8,28- 31]. Seki and Oshikane [32] observed better growth and development of silkworm larvae as well as good quality cocoons when fed on nutritionally enriched leaves. Silkworm obtains its entire nutritional requirement from mulberry leaves because this insect is monophagous and can complete the life cycle on mulberry leaves exclusively. The quality of mulberry leaves plays an important role in the success of the sericulture industry and directs its economics [33], and hence much effort and research have been carried out to improve the quality and quantity of mulberry-leaf production for silkworm rearing and then cocoon production. Also he reported that varietal improvement of mulberry through breeding not only gives superior leaf yield for feeding the B .mori but also better raw silk and its adaptability to climatic conditions and resistance against diseases. Treatments of B. mori larvae with propolis extracts (a honeybee product) yielded heavier cocoons and cocoon shells, a higher silk content, and females laid more eggs than controls [34]. In B. mori GST is common but in our study this criteria was expressed to rescue the insects from the oxidative stress induced by the silver nanoparticles.
Oxidative stress is an important factor in nanoparticle-induced toxicity [35]. GSH levels have been observed to increase in various cells and animal tissues exposed to oxidative stress-inducing xenobiotics including nanoparticles [36]. The increase in GSH levels is in agreement with previous studies addressing the effect of oxidative stress on induction of GSH-synthesizing enzymes [37]. It has been reported that many organisms over expressed GSTs on exposure to oxidative stress inducing chemicals [38]. C. elegans exposed to PQ exhibited 40-fold increases in the steady-state mRNA level expressions of GST genes [39]. In a previous report, several D. melanogaster GSTs were observed to participate in defense mechanisms against oxidative stress [40].
Conclusion
We have synthesized biological silver nanoparticles using aqueous extracts of mulberry, Morus alba leaves to evaluate the candidate drug toxicity through its feed. Elemental silver obtained was characterized using spectroscopy and microscopy, to emphasize the efficacy of nanoparticles production by Morus alba. Treatment bias of silver nanoparticles to Bombyx mori larvae elucidated the physiological traits. Toxicity assessment created by silver nanoparticles with the comparative efficacy of graded doses of silver nanoparticles. The protein profile of silkworm hemolymph in revealed the occurrence of stress in response to silver nanoparticles treatment also expressed the GST protein in reflection to the stress created by AgNps.
Acknowledgement
Author J.P. gratefully acknowledges the UGC, India for the award of a UGC Non SAP meritorious fellowship (No. F.4-3/2006 (BSR) 11- 73/2008/ BSR, 24 February 2009).
References
- Gan PP, Li SFY. Potential of plant as a biological factory to synthesize gold and silver nanoparticles and their applications. Rev Environ Sci Biotechnol. 2012; 11: 169-206.
- Jiang H, Manolache S, Wong ACL, Denes FS. Plasma enhanced deposition of silver nanoparticles onto polymer and metal surfaces for the generation of antimicrobial characteristics. J Appl Polym Sci. 2004; 93: 1411-1422.
- Becker RO. Silver ions in the treatment of local infections. Met Based Drugs. 1999; 6: 311-314.
- Silver S. Bacterial silver resistance: molecular biology and uses and misuses of silver compounds. FEMS Microbiol Rev. 2003; 27: 341-353.
- Pompa PP, Vecchio G, Galeone A, Brunetti V, Sabella S, Maiorano G, et al. In Vivo Toxicity Assessment of Gold Nanoparticles in Drosophila melanogaster. Nano Res. 2011; 4: 405-413.
- Asharani PV, Hande MP, Valiyaveettil S. Anti-proliferative activity of silver nanoparticles. BMC Cell Biol. 2009; 10: 65.
- Rajathi A, Pandiarajan J, Krishnan M. Effect of RH-2485 on development, metamorphosis and synthesis of major proteins in female silkworm Bombyx mori. Biologia. 2010; 65: 903-913.
- Etebari K, Kaliwal B, Matindoost L. Supplementation of mulberry leaves in sericulture, theoretical and applied aspects. Int J Indust Entomol. 2004; 9: 14-28.
- Islam MR, Ali MO, Paul DK, Sultana S, Banu NA, Islam MR. Effect of salt, nickel chloride supplementation on the growth of silkworm Bombyx mori L. J Biol Sci. 2004; 4: 170-172.
- Bhattacharya R, Mukherjee P. Biological properties of "naked" metal nanoparticles. Adv Drug Deliv Rev. 2008; 60: 1289-1306.
- Legay JM. Recent advances in silkworm nutrition. Ann Rev Ent. 1958; 3: 75-86.
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970; 227: 680-685.
- Prathna TC, Chandrasekaran N, Raichur AM, Mukherjee A. Biomimetic synthesis of silver nanoparticles by Citrus limon (lemon) aqueous extract and theoretical prediction of particle size. Colloids Surf B Biointerfaces. 2011; 82: 152-159.
- Kreibig U, Vollmer M. Optical properties of metal clusters. Springer-velrag. Newyork. 1995.
- Mulvaney P. Surface plasmon spectroscopy of nanosized metal particles, Langmuir. 1999; 12: 788-800.
- Shankar SS, Ahmad A, Sastry M. Geranium leaf assisted biosynthesis of silver nanoparticles. Biotechnol Prog. 2003; 19: 1627-1631.
- Sheny DS, Mathew J, Philip D. Phytosynthesis of Au, Ag and Au-Ag bimetallic nanoparticles using aqueous extract and dried leaf of Anacardium occidentale. Spectrochim Acta A Mol Biomol Spectrosc. 2011; 79: 254-262.
- Bar H, Bhui DK, Sahoo GP, Sarkar P, De SP, Misra A. Green synthesis of silver nanoparticles using latex of Jatropha curcas. Colloids Surf A. 2009; 339: 134-139.
- Kasthuri J, Veerapandian S, Rajendiran N. Biological synthesis of silver and gold nanoparticles using apiin as reducing agent. Colloids Surf B Biointerfaces. 2009; 68: 55-60.
- Dubey SP, Lahtinen M, Sillanpaa M. Tansy fruit mediated greener synthesis of silver and gold nanoparticles. Process Biochem. 2010; 45: 1065-1071.
- Philip D. Green synthesis of gold and silver nanoparticles using Hibiscus rosa sinensis. Physica E. 2010; 42: 1417-1424.
- Smitha SL, Philip D, Gopchandran KG. Green synthesis of gold nanoparticles using Cinnamomum zeylanicum leaf broth. Spectrochim Acta A Mol Biomol Spectrosc. 2009; 74: 735-739.
- Fayaz M, Tiwary CS, Kalaichelvan PT, Venkatesan R. Blue orange light emission from biogenic synthesized silver nanoparticles using Trichoderma viride. Colloids Surf B Biointerfaces. 2010; 75: 175-178.
- Song JY, Kim BS. Rapid biological synthesis of silver nanoparticles using plant leaf extracts. Bioprocess Biosyst Eng. 2009; 32: 79-84.
- Kim Y, Johnson RC, Li J, Hupp JT, Schatz GC. Synthesis, linear extinction, and preliminary resonant hyper-Rayleigh scattering studies of gold-core/silver-shell nanoparticles: comparisons of theory and experiment. Chem Phys Lett. 2002; 352: 421-428.
- Wu J, Zhang F, Zhang H. Facile synthesis of carboxymethyl curdlan-capped silver nanoparticles and their application in SERS. Carbohydr Polym. 2012; 90: 261-269.
- Akhtar M, Asghar A. Nutritional requirement of silkworm Bombyx mori. Pakistan J Zool. 1972; 4: 101-107.
- Babu M, Swamy MT, Rao P, Rao MS. Effect of ascorbic acid-enriched mulberry leaves on rearing of Bombyx mori L. Ind J Seric. 1992; 31: 111-114.
- Faruki SI, Khan AR, Mannan A. Fecundity and fertility of the silkworm, Bombyx mori L. fed on mulberry leaves supplemented with para-amino benzoic acid. Bangladesh J Zool. 1992; 20: 351-353.
- Rajabi R, Ebadi R, Fazilati M, Mirhoseini SZ. Nutritive effects of mulberry leaves enrichment with riboflavin vitamin on bio-economic characters of silkworm, Bombyx mori L, 9th Arab Congress of Plant Protection. Damascus, Syria. 2006a.
- Rajabi R, Ebadi R, Fazilati M. The effects of mulberry leaves enrichment with pyridoxine-HCl on economic traits and biological parameters of silk worm Bombyx mori. L, 17th Iranian Plant Protection Congress. Teheran. 2006b; 391.
- Seki K, Oshikane K. Res Reports Fac. Textile and Sericulture, Shinshu University. 1959.
- Choudhury PC, Shukla P, Ghosh A, Mallikarjuna B, Sengupta K. Effect of spacing, crown height and method of pruning on mulberry leaf yield, quality and cocoon yield. Indian J seric. 1991; 30: 46-53.
- Nour ME, El-Maasarawy SA, Mahmoud S. Propolis in the nutrition of the silkworm Bombyx mori. L, influence on biology, silk yield and biochemical changes in haemolymph. Proceedings of the 1st International Conference on silk "ICSAI". 1997.
- Nel A, Xia T, Mädler L, Li N. Toxic potential of materials at the nanolevel. Science. 2006; 311: 622-627.
- Arora S, Jain J, Rajwade JM, Paknikar KM. Interactions of silver nanoparticles with primary mouse fibroblasts and liver cells. Toxicol Appl Pharmacol. 2009; 236: 310-318.
- Mulcahy RT, Wartman MA, Bailey HH, Gipp JJ. Constitutive and betanaphthoflavone- induced expression of the human gamma-glutamylcysteine synthetase heavy subunit gene is regulated by a distal antioxidant response element/TRE sequence. J Biol Chem. 1997; 272: 7445-7454.
- Shi MM, Kugelman A, Iwamoto T, Tian L, Forman HJ. Quinone-induced oxidative stress elevates glutathione and induces gamma-glutamylcysteine synthetase activity in rat lung epithelial L2 cells. J Biol Chem. 1994; 269: 26512-26517.
- Tawe WN, Eschbach ML, Walter RD, Henkle-Dührsen K. Identification of stress-responsive genes in Caenorhabditis elegans using RT-PCR differential display. Nucleic Acids Res. 1998; 26: 1621-1627.
- Sawicki R, Singh SP, Mondal AK, Benes H, Zimniak P. Cloning, expression and biochemical characterization of one Epsilon-class (GST-3) and ten Delta-class (GST-1) glutathione S-transferases from Drosophila melanogaster, and identification of additional nine members of the Epsilon class. Biochem J. 2003; 370: 661-669.