
Research Article
Austin J Biotechnol Bioeng. 2016; 3(2): 1064.
Ex Situ Conservation of an Endangered Fern Elaphoglossum Nilgiricum (Krajina Ex Sledge and Bonner) T. Moore Ex Alston & Bonner using In Vitro Spore Culture
Shibila T and Johnson M*
Centre for Plant Biotechnology, PG and Research Department of Botany, St. Xavier’s College, India
*Corresponding author: Johnson, M, Director, Centre for Plant Biotechnology, PG and Research Department of Botany, St. Xavier’s College (Autonomous), Palayamkottai, Tamilnadu, India
Received: April 23, 2016; Accepted: June 06, 2016; Published: June 08, 2016
Abstract
The present study was aimed to produce reproducible in vitro spore culture protocol for an endangered fern Elaphoglossum nilgiricum (Krajina Ex Sledge and Bonner) T. Moore Ex Alston & Bonner. Matured spores were sterilized with 0.1% (w/v) mercuric chloride for 10 min and washed with sterile distilled water for 20 min. The sterilized spores were inoculated onto various media for germination. After 86 days, the spore germination was noticed in KC Basal solid medium. Highest percentage of spore germination (86 ± 3.65) was observed in KC basal agar medium. KC liquid medium with 1.5% of sucrose illustrated highest percentage (56.66 ± 4.08) of sporophyte proliferation followed by KN (48.66 ± 3.80) combination with 1% of sucrose. In the present study we provided an alternative protocol to multiply an endangered fern Elaphoglossum nilgiricum through in vitro spore culture of the Western Ghats, India.
Keywords: Spore culture; Elaphoglossum nilgiricum; in vitro; Sporophyte proliferation
Abbreviations
KC: Knudson; KN: Knop’s; °C: Degree Celsius; %: Percentage; IUCN: International Union for Conservation of Nature; HgCl2: Mercuric Chloride; Mi: Mitra, MS: Murashige and Skoog’s
Introduction
Pteridophytes are an important factor of species-diversity, can be propagated only via spores or asexual method. The life cycle of a fern consists of two alternative generations (gametophyte & sporophyte). The world flora consists of 13,600 species of Pteridophytes concerning more than 1300 species into 70 families and 191 genera allocated in India and 270 fern species found in south India [1-3]. Bir [4] listed 49 endangered species of Pteridophytes from various regions of India. Nayar and Sastry [5-7] included 31 threatened pteridophytes in the volumes of the Botanical Survey of India’s Red Data Book of Indian Plants. There are 44 rare and endangered pteridophytes are reported from Western Ghats of [8]. A number of environmental factors, such as temperature, humidity and canopy cover influence the spore germination [9-11]. Due to various deforestation activities, nearly 7.7% of ferns under threaten including the epiphytic and lithophytic ferns native to Western Ghats [12]. The conservation process depends on geological distribution of the species, population ecology. Micropropagation is an efficient tool to preserve endemic, endangered and over exploited genotypes without defeated the mother traits and produces large number of plants for reintroduction and commercial delivery. The in vitro propagation is an influential technique to develop a protocol for the mass multiplication of the required plant species (fern and fern allies) (Figure 1). The common method of germination and growth requirements for all pteridophytes is not available. In this case, the in vitro culture methods provide an attentive knowledge about propagating sporophytes from spores and rhizomes and help us to understand the reproductive biology of ferns [13,14]. The jeopardized ferns such as Diplazium cognatum [15], Metathelypteris flaccida [16], Pteris gongalensis [17], Pteris confusa [18], Cheilanthes viridis [19], Pronephrium articulatum [20], Histiopteris incisa, Hypodematium crenatum, Thelypteris confluens, Athyrium nigripes, Pteris vittata, Cyathea crinita, and Nephrolepis multiflora have been reproduced through in vitro spore culture and organogenesis as part of ex situ conservation [21-23]. Elaphoglossum is one of the major and taxonomically generally multifaceted genera of ferns that contain about 600 species [24,25]. Kumar [26] considered the Elaphoglossum nilgiricum (Krajina Ex Sledge and Bonner) T.Moore Ex Alston & Bonner under the IUCN red list of Threatened species. With this background the present study was initiated to optimize the protocol for the mass multiplication of an endangered fern Elaphoglossum nilgiricum (Krajina Ex Sledge and Bonner) T.Moore Ex Alston & Bonner using in vitro spore culture.
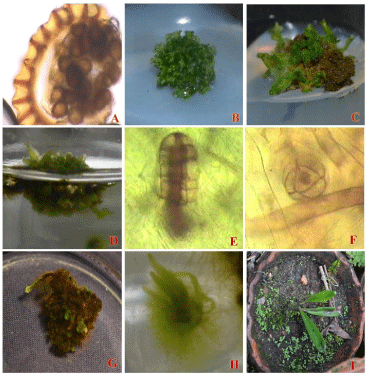
Figure 1: In vitro propagation of Elaphoglossum nilgiricum.
A - Spore with Sori; B - Young gametophyte of E. nilgiricum; C - Matured gametophyte of E. nilgiricum in KC Basal Solid medium; D - Matured gametophyte of
E. nilgiricum in KC Basal Liquid medium supplmented with sucrose; E - Female Sex organ of E. nilgiricum; F- Male Sex organ of E. nilgiricum; G - Sporophyte
proliferation of E. nilgiricum; H - 25 days old sporophytes of E. nilgiricum; I - Hadened sporophytes of E. nilgiricum.
Materials and Methods
Matured fronds of Elaphoglossum nilgiricum (Krajina Ex Sledge and Bonner) T. Moore Ex Alston & Bonner were collected from Kodaikanal Botanic Garden, St. Xavier’s College, Eettipallum, Tamil Nadu. The fronds were washed in running tap water for few minutes. The fronds were cut into small pieces and dried over white absorbent paper at room temperature (25°C). After drying the fronds at room temperature for 24 hrs, the liberated spores were passed through 40 μm nylon mesh to remove the sporangial wall materials and clean spores were collected and stored in refrigerator at 5°C.
The spores were surface sterilized with 0.1% HgCl2 solution for 5-15 min and washed with sterile distilled water for 15-30 min. The surface sterilized spores were inoculated in to different media viz. KC, KN, MS and Mitra [27-30] devoid of sugar and plant growth regulators using sterile Pasteur pipettes and incubated at 25°C ± 2°C under 12 h photoperiod (1500 lux). The pH of the media was adjusted to 5.8 before adding agar 0.5 % (w/v) and autoclaved at 121°C for 15 min. Both liquid and agar nutrient media were used for spore germination and sporophyte formation. Gametophytes regenerated from spores were sub-cultured on different basal media (KN, KC, Mi, MS and ½ MS medium) for sporophyte formation. Germination percentage of the spores and their development pattern were examined. Photomicrographs were taken with an Optika microscope. The culture tubes containing spore raised micropropagated plants of Elaphoglossum nilgiricum were kept at room temperature (30-32°C) for a week before transplantations. For acclimatization, the plants with well developed rhizoids (5-8 cms) were removed from culture tubes, washed in running tap water to remove the remnants of agar and each group was planted separately onto 10 cm diameter polycup filled with different potting mixtures river coconut husk; garden soil and farm yard manures (1:1:1). The plants were kept in mist chamber with a relative humidity of 70%. Plants were irrigated at 8 h intervals for 3-4 weeks and establishment rate was recorded. The plantlets established in community pots were transferred to shade net house for 3-4 weeks and then repotted in larger pots (20 cm diameter) with one plant in each pot.
Results and Discussion
The spores were sterilized with 0.1% (w/v) mercuric chloride for 10 min. The sterilized spores were washed with sterile distilled water for 20 min. The optimum concentration of mercuric chloride treatment (0.1%) continued existence of spores and low percentage of microbial contamination. The sterilized spores were inoculated onto various media for germination. After 86 days, the spore germination was noticed in KC Basal solid medium. The highest percentage of spore germination (86 ± 3.65) was observed in KC basal agar medium. The effect of medium on spore germination, gametophyte formation and sporophyte proliferations were illustrated in Table 1. Other tested mediums are failed to show the spore germination. Similar to the present observation Manickam et al. [15] also observed the highest percentage of spore germination in KC basal medium for Cheilanthes viridis. Archana et al. [31] also attempted to germinate the spores of Diplazium esculentum in Knop’s, Knudson and Murashige and Skoog’s medium. They observed the highest percentage germination in the Knop’s medium. Even though the Diplazium esculentum and Elaphoglossum nilgiricum belongs to the same group Aspidales they require unique nutritional condition for their growth and development. Bonomo et al. [32] observed spore germination of Alsophila odonelliana in all three studied nutritive (Dyer, KN and KC) medium. The result suggested that the universal media may not be employed for the spore germination of ferns. The spores require specific nutritional environment for germination and growth and development of ferns. To know the importance of nutritional composition on the growth and development of gametophyte, the spore derived prothalli were sub cultured on to fresh medium viz., KN, KC, Mi, MS and ½ MS basal medium. The spore derived gametophytes bestowed extensive gametophyte multiplication and sex organ development in the studied medium (Table 1). The appearance of female sex organs was observed on 41st day from the germination. The cordate gametophyte showed male sex organs on 67th day of germination. The appearance of sex organs on the gametophytes E. nilgiricum was noticed and results were recorded. The gametophytes bearing with sex organs were sub cultured in to the fresh nutrient basal medium (solid and liquid) for sporophyte proliferation. The basal media were failed to support the proliferation of E. nilgiricum sporophytes. The KC liquid medium supplemented with 1.5% of sucrose illustrated highest percentage (56.66 ± 4.08) of sporophyte proliferation followed by KN (48.66 ± 3.80) combination with 1% of sucrose. Similar to the present observation, Johnson and Manickam [33] also witnessed highest percentage of Cheilanthes viridis sporophyte emergence in KC nutrient medium supplemented with 1% sucrose.
Medium
% of Spore Germination
% of Gametophyte multiplication
% of Sporophyte formation
% of Sporophyte established in KBG
KC basal
86 ± 3.65
86 ± 2.78
-
-
KC liquid
-
91.33 ± 1.82
56.66 ± 4.08
72 ± 4.47
KN basal
-
86.66 ± 2.35
-
-
KN liquid
-
95.33 ± 2.98
48.66 ± 3.80
-
Mitra basal
-
85.33 ± 2.98
-
-
Mitra liquid
-
89.33 ± 2.78
-
-
MS basal
-
80.66 ± 2.98
-
-
MS liquid
-
87.33 ± 2.78
-
-
½ MS basal
-
83.33 ± 2.35
-
-
Table 1: Effect of medium on spore germination of E. nilgiricum.
After fifty days, the in vitro derived sporophytes were transferred to the pot with a mixture of (1:2:1) sterile soil: coconut husk: farmyard manure for hardening. The sporophytes were kept in a culture room for 15 days. The pots were covered with polythene bags to maintain the humidity and irrigated with 10 x diluted KC liquid medium once in a week. After two weeks, the sporophytes were transferred to a green house holding regular physical parameters. After 45 days, the hardened sporophytes were distributed to the natural habitats at KBG, Kodaikannal, nearly 72 ± 4.47% of sporophytes were re-established in the KBG, Kodaikannal. In the present study, we developed the reproducible protocol for the spore germination, gametophyte development and sporophyte proliferations of Elaphoglossum nilgiricum.
Conclusion
The optimized protocol may be used for the large scale multiplication and conservation of an endangered fern E. nilgiricum.
Acknowledgment
The authors sincerely thank the SERB, New Delhi for the financial support through the Young Scientist research Scheme (Grant No. SERB/ LS- 240/ 2012 dated 14 May 2013.
References
- Dixit RD. A Census of the Indian Pteridophytes. Flora of India. Series 4, Botanical Survey of India, Government of India, Howrah. Ferns, Academic Press. London. 1984; 87-132.
- Perumal G. Ethnomedicinal use of Pteridophyte from Kolli Hills, Namakkal District, Tamil Nadu, India. Ethnobotanical leaflets. 2010; 14: 161-172.
- Chandra S, Fraser-Jenkins CR, Kumari Alka, Sivastava A. A summary of status of threatened pteridophytes of India. Taiwania. 2008; 53: 170-209.
- Bir SS. Pteridophytic flora of India. Rare and endangered elements and their conservation. Indian Fern Journal. 1987; 4: 101.
- Nayar MP, Sastry ARK. Red Data Books of Indian Plants. Botanical Survey of India, Calcutta. 1987; 1: 147-159.
- Nayar MP, Sastry A. Red Data Books of Indian Plants. Botanical Survey of India, Calcutta. 1988; 2: 109-118.
- Nayar MP, Sastry A. Red Data Books of Indian Plants. Botanical Survey of India, Calcutta.1990; 3.
- Manickam VS. Rare and endangered ferns of the Western Ghats of South India. Fern Gazette. 1995; 15: 1-10.
- Odland A. Size and reproduction of Thelypteris limbbosperma and Athyrium distentifolium along environmental gradients in Western Norway. Nordic Journal of Botany. 1998; 18: 311-321.
- Greer GK, Mc Carthy BC. Patterns of growth and reproduction in a natural population of the fern Polystichum acrostichoides. American Fern Journal. 2000; 90: 60-76.
- Crystal Arens N. Variation in performance of the tree fern Cyathea caracasana (Cyatheaceae) across a successional mosaic in an Andean cloud forest. Am J Bot. 2001; 88: 545-551.
- Marimuthu J, Manickam VS. Ex situ conservation of two threatened ferns of the Western Ghats through in vitro spore culture. Journal of Threatened Taxa. 2011; 3: 1919-1928.
- Fernandez H, Bertrand AM, Sanchez-Tames R. Biological and nutritional aspects involved in fern multiplication. Plant Cell, Tissue and Organ Culture. 1999; 56: 211-214.
- Cassanego MBB, Droste A, Windisch PG. Effects of 2, 4-D on the germination of megaspores and initial development of Regnellidium diphyllum Lindman (Monilophyta, Marsileaceae). Brazilian Journal of Biology. 2010; 70: 361-366.
- Manickam VS, Vallinayagam S, Johnson M. Micropropagation and Conservation of rare and endangered ferns of Western Ghats through in vitro culture. Chandra S, Srivastava M, editors. In: Pteridology in the New Millennium. Kluwer Academic Publishers, Netherlands. 2003; 497-504.
- Johnson M, Manickam VS. In vitro Studies on normal and abnormal life cycle of Metathelypteris flaccida (Bl.) Ching. Ethiopian Journal of Science and Technology. 2006; 4: 37-44.
- Johnson M, Manickam VS, Irudayaraj V. In vitro studies on agamospours fern Pteris gongalensis T.G. Walker. Ethiopian Journal of Science and Technology. 2005; 3:1-6.
- Irudayaraj V, Manickam VS, Johnson M. Incidence of Apospory in Pteris confusa T.G. Walker. Phytomorphology. 2003; 53: 73-77.
- Johnson M, Manickam VS. Influence of Plant Growth Regulators on in vitro raised gametophytes of Cheilanthes viridis (Forssk.) Swartz. Journal of Basic and Applied Biology. 2010; 4: 18-23.
- Johnson M, Manickam VS. In vitro multiplication of Pronephrium triphyllum (Sw.) Holtttum – An Endangered fern. Pulliah T, editor. In: Biotechnological Approaches for Sustainable Development Regency publications a division of Astral International Pvt. Ltd., New Delhi. 2015; 8: 169-178.
- Sara SC. Conservation of selected rare and endangered ferns of the Western Ghats through micropropagation and restoration. 2001.
- Johnson M. In vitro studies on some rare and endangered ferns of Western Ghats, south India. 2003.
- Vallinayagam S. Micropropagation of rare and endangered ferns of Western Ghats. 2003.
- Mickel. Elaphoglossum. Steyermark JA, Berry PE, Holst BK, editors. In: Flora of the Venezuelan Guayana. Timber Press, Portland, Oregon. 1995; 2: 89-105.
- Mickel JT, Smith AR. Elaphoglossum. Mickel JT, Smith AR, editors. In: The Pteridophytes of Mexico. Memoris of the New York Botanical Garden. New York Botanical Garden Press, New York. 2004; 88: 282-315.
- Kumar B. Elaphoglossum stelligerum. The IUCN Red List of Threatened species. 2013.
- Knops N. Quantitative Untersuchungan uber die Ernahrungsprozesse der pflangen Land wirtsch Vers. Stn. 1885; 7: 93-107.
- Knudson L. A nutrient solution for the germination of orchid seed. Bulletin of American Orchid Society. 1946; 15: 214-217.
- Mitra GC, Prasad RN, Chowdhury R. An inorganic salts and differentiation of protocornes, in seed callus of an orchid and correlated changes in free amino acids content. Indian Journal of Experimental Biology. 1976; 14: 350-351.
- Saxena G, Banerjee S, Rahman L, Mallavarapu GR, Sharma S, Kumar S. An efficient in vitro procedure for micropropagation and generation of somaclones of rose scented Pelargonium. Plant Sci. 2000; 155: 133-140.
- Archana GN, Pradeesh S, NIkhila GS, Sangeetha G, Mini I, Swapna TS. In vitro propagation of a rare medicinal fern of Western Ghats- Diplazium esculentum (Reytz.). Indian Journal of Experimental Biology. 2013; 51: 919-923.
- Bonomo MC, Martinez OG, Tanco ME, Cardozo R, Aviles Z. Spores germination and gametophytes of Alsophila odonelliana (Cyatheaceae) in different sterile media. International journal of experimental botany. 2013; 82: 119-126.
- Johnson M, Manickam VS. Influence of Sucrose on Morphogenetic Changes of Four Rare and Endangered Ferns of Western Ghats, South India. Biochemistry: An Indian Journal. 2007; 1: 18.