
Research Article
Austin J Biotechnol Bioeng. 2016; 3(3): 1066.
Oxygen Uptake Rate Measurement by Modified Dynamic Method and Effect of Mass-Transfer Rates on Growth of Pichia Stipitis: Modeling and Experimental Data Comparison
Ghosalkar A¹ and Kashid MG1,2*
¹Department of Technology, University of Pune, Ganeshkhind, India
²Praj Matrix R&D Center, Division of Praj Industries Ltd., India
*Corresponding author: Kashid MG, Praj Matrix R&D Center, Division of Praj Industries Ltd., Urawade, Pirangut, Pune, India 412115
Received: May 09, 2016; Accepted: June 16, 2016; Published: June 22, 2016
Abstract
In the aerobic fermentation of xylose for the production of ethanol with the help of Pichia stipitis, optimum oxygen must be continuously supplied in order to achieve the desired yield and productivities. Oxygen Uptake Rate (OUR) and Oxygen Transfer Rates (OTR) for xylose fermentation with Pichia stipitis has been determined in a stirred tank bioreactor under different transport conditions using modified dynamic method. Logistic equation based kinetic model is applied to predict the cell biomass concentration during Pichia stipitis growth. The experimental values from the modified dynamic method were used for development of mathematical model based on oxygen uptake rate.
The proposed model is used for obtaining the kinetic growth parameters like Yield coefficient (Yox), Specific maintenance coefficient (m0). Model predicted values are compared with the experimental values with good agreement. Effect of mass transfer conditions are studied on the cell mass growth and ethanol formation. Overall maximum ethanol productivity (0.57 g/l/h) and maximum ethanol yield (0.45 g/g of xylose) has been observed at 200 rpm agitation rate. Maximum oxygen uptake rate of 8.6 x 10-6 moles of O2/ L/s was observed at 300 rpm. The proposed models can be used to predict the influence of oxygen on cell growth and ethanol productivity in industrial fermentation.
Keywords: Modified dynamic method; Pichia stipitis; Oxygen uptake rate; Growth model; Kinetic parameters
Abbreviations
OTR: Oxygen Transfer Rate (moles of O2 m-3 s-1); OUR: Oxygen Uptake Rate (moles of O2 m-3 s-1) a specific interfacial area (m-1); DO: Dissolved Oxygen; kL: Mass Transfer Coefficient (m s-1); kLa: Volumetric Mass Transfer Coefficient (s-1); qo2: Specific Oxygen Uptake Rate (mol O2 kg-1 s-1); CL: Concentration of Compound in Liquid Phase (kg m-3 or mol m-3); C*: Equilibrium Concentration (kg m-3 or mol m-3); h: Time (h); X: Cell Mass Concentration (g L-1); mo: Oxygen Maintenance Coefficient (mol O2 g-1 X s-1); Yox: Oxygen Consumption Coefficient (mol O2 g-1 X); P: Product Concentration (g L-1); Qp/s: Ethanol Volumetric Productivity (g L-1 h-1); Yp/s: Ethanol Yield On Xylose (g g-1); I.D: Internal Diameter (mm)
Introduction
Bio-ethanol which is the product of fermentation process is considered as a potential alternative fuel. Being a renewable energy source, bio-ethanol has important advantages when compared to gasoline. Being an oxygen rich fuel, the emission of green house gases and particulate materials from ethanol combustion is lower [1].
For ethanol fermentation, generally streams coming from agricultural products such as corn, sugarcane, sweet sorghum are used. In recent years, agriculture residues such as byproducts of corn, sugarcane and wood industry have also been identified as potential sources for ethanol production [2]. Most of these agricultural feedstock contains both hexose and pentose sugars. Though hexose sugars are used today for ethanol production, it is also possible to utilize xylose (pentose) for the ethanol fermentation, as xylose is the second major product of saccharification of lignocellulosic feedstocks [3].
In the common bio-ethanol fermentation processes Saccharomyces cerevisiae yeast is widely used for ethanol production [4]. However, S. cerevisiae is only able to ferment glucose sugars, and cannot ferment pentose sugars like xylose [5]. Successful utilization of xylose would help in driving the process economics of lignocellulosic biomass to ethanol fermentation favorably. In several reports, yeast Pichia stipitis has been identified for efficient conversion of xylose to ethanol under micro-aerobic conditions. The dissolved oxygen concentration affects the cell mass growth and ethanol production rate in fermentation of xylose using Pichia stipitis [6-10].
Xylose to ethanol fermentation process could be further optimized by the development of realistic growth and fermentation models. In aerobic fermentation process oxygen should be continuously supplied in order to achieve higher productivities, as the role of oxygen in microorganism growth and metabolism is vital. The measurement of Oxygen Transfer Rate (OTR) and Oxygen Uptake Rate (OUR) by the cell can help in optimizing supply of oxygen to the fermenter.
Dissolved Oxygen Concentration (DO) in the fermentation broth depends on the balance of oxygen consumption rate for the cell growth and the oxygen transfer rate from the gas to the liquid phase [11]. Monitoring of DO in the fermentation broth is essential as oxygen often becomes the governing factor in the microbial metabolic pathways [12].
The OUR measurement has vital importance in biochemical processes to check the viability of the culture and maintain desired productivity. Different OUR measurement techniques currently being used in the bioprocess industry are: gas balancing method, dynamic method, and yield coefficient method. Dynamic method, which is mostly used for measurement of OUR is based on measurements of oxygen concentration in the broth and on the respiratory activity of the microorganism [13-15]. Modified dynamic method is an improvement to dynamic method, in which inlet air to the fermentation broth is replaced with the pure oxygen. After achieving the equilibrium oxygen concentration, pure oxygen is replaced with the air in the fermentation broth [16].
For better understanding and prediction of xylose to ethanol fermentation process, employment of mathematical model can allow us to predict the cell growth, oxygen uptake rate, substrate consumption and product formation rates [17-19]. In fact, mathematical modeling is requisite for the aerobic fermentation processes as it provides useful information related to OUR and OTR during cell growth and production process [20,21]. In xylose to ethanol production by Pichia stipitis, it is essential to understand Oxygen Uptake Rate (OUR) to supply optimum aeration for maximum yield and productivity. During fermentation, Pichia stipitis consumes oxygen as the substrate for cell growth and for cell maintenance. Hence OUR can be linked with the cell growth and cell maintenance parameter [22].
OUR measurement has recently received the due attention in different bio-process studies, such as in production of Xanthan gum [23] xylitol production and bio-desulphurization processes [24]. Previous studies have been carried out to determine the effect of oxygen uptake and mass transfer rates on the growth of Psedomonas putida CECT2579 as well as influence on bio-desulfurization capability [16].
This is the first report for the oxygen uptake rate measurement by modified dynamic method and the OUR model application which considers oxygen as a substrate for xylose to ethanol fermentation by Pichia stipitis. The focus of this work was to estimate the oxygen uptake rate to determine the optimum operating condition for maximum ethanol production taking in to account the rates of substrate consumption, oxygen uptake and ethanol production. Estimation of nonlinear growth kinetic parameters has been carried out and OUR pattern throughout the experiment was studied. Mathematical models such as logistic equation and Heijnen & Roelsmodel [25] were applied to provide model predicted values for cell biomass, oxygen uptake rate throughout the fermentation. Experimental values of OUR then compared with the model predicted values.
Material and Analytical Methods
Microorganism
Pichia stipitis ATCC 58784 was adapted by serial propagation in xylose hydrolysate. The adapted strain was designated as Pichia stipitis PSA30. It was maintained at -80°C in the form of glycerol stock in xylose rich hydrolysate.
Media and bioreactor assembly
Yeast extract (1%w/w), Peptone (2%w/w) and Xylose (3%w/w) media (YPX media) was used for inoculum development. Two stage inoculations were done to prepare inoculum for main fermentation. First stage inoculum (Seed-I) was grown by transferring cells from glycerol stock to 100 mL conical flask containing 50 mL YPX media. The inoculated flasks were incubated at 32°C in a rotary shaker at 250 rpm for 24 h. After incubation, a sample was removed to measure the cell concentration. Culture from seed-I was used to inoculate second seed stage (Seed-II) in the two 500 mL flask containing 250 mL YPX media. Seed-II flasks were incubated for 24 h under same conditions as seed-I. This culture was used as inoculum for main fermentation of 5 L working volume in YPX media. The concentration of D-xylose was increased to 4%w/w in the main fermentation.
The fermentation trials were performed in New Brunswick BioFlow 7 L fermenter with pH, temperature, agitation, aeration control with 5 L working volume. During the fermentation temperature and pH was set at 30°C and 5.2 respectively. Aeration rate kept at 0.2 vvm and agitation rates were operated in the range of 150-300 rpm.
Analytical methods
Sugars (cellobiose, glucose, xylose), fermentation products (ethanol, xylitol, glycerol) and inhibitors (formic acid, acetic acid) were analyzed by High Performance Liquid Chromatography (HPLC) using an Agilent 1100 system, refractive index detector and Bio-Rad Aminex HPX-87H column (300×7.8 mm I.D.) for separation of compounds at 55°C Sulfuric acid (0.005 M) was used as the mobile phase at flow rate of 0.6 mL/min. Cell growth was measured by using spectrophotometer, in terms of optical density at 600 nm. Dry cell weights were measured by keeping samples overnight in vacuum oven at 60°C.
Data processing-model fit
Data fitting to the model were performed by linear regression using the least-squares method, based on the assumption that the distribution of errors is normal. Estimates for parameters were obtained by minimizing the Residual Sum of Squares (RSS).
Where n is the number of data points, yi is the observed value, and yi’ is the fitted value. The performance of the models was evaluated by using correlation coefficients (r).
Where, y’ is the mean value
Model is accurate when value of correlation coefficient (r) is around 1.
Measurement of OUR
In previous studies, oxygen transport and consumption have been mostly measured by the dynamic method [16-18]. The mathematical model for the description of OUR and OTR by dynamic method is given by,
Where,
= Oxygen accumulation rate in the liquid
kLa.(C* - C) = Oxygen Transfer Rate (OTR)
qO2 . X = Oxygen Uptake Rate (OUR)
Though, dynamic method has a lot number of applications, it has challenges with regard to obtaining accurate measurements [26]. In the case of aerobic fermentation of xylose by Pichia stipitis, oxygen uptake rate is very high. When we cut off the aeration to fermenter, DO quickly decreases below the critical levels very fast and DO do not increases when aeration is resumed.
In case of modified dynamic method pure oxygen is introduced in the fermenter instead of cutting airflow during absorption stage. After achieving steady state oxygen concentration in the fermentation broth, pure oxygen flow to the fermenter is interrupted. In the desorption phase pure oxygen is replaced with air and rate of depletion of DO is used to measure the OUR (Figure 1).
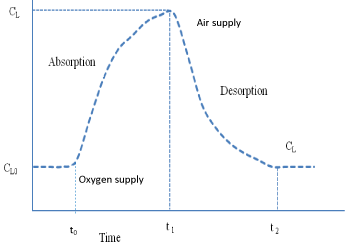
Figure 1: Evolution of oxygen concentration in the liquid phase when the air
is replaced with the oxygen rich stream for modified dynamic method.
On integration of equation (3) for the absorption phase (supply of pure oxygen) for the limit of t=0, C=CL0 for initial conditions and t=t1, C=CL, will generate the following equation:
m 4Equation (4) shows the impact on oxygen concentration in the liquid phase when the air is replaced with the pure oxygen at concentration of CL0.
In case of desorption phase (replacement of pure oxygen with the air), the integration of equation (3) for the limit of t= t1, C=CL1 for initial conditions and t= t2, C= CL, will generate the following equation:
M 5Equation (5) shows the impact on oxygen concentration in the liquid phase when the pure oxygen is replaced with the air at concentration of CL1
OUR values were determined by above equations during the xylose fermentation by Pichia stipitis at different time intervals. Model parameters were estimated by fitting experimental data to the mathematical equations using nonlinear regression analysis.
Mathematical modeling for OUR estimated by modified dynamic method
A logistic equation based model was used under optimal growth conditions and when the inhibitory effects of substrate and product played no role to describe the cell growth [25]. The rate of cell growth followed the well-known exponential relationship.
dX/dt = μm.X. (1 - X/Xm) (6)
Where,
μm = Maximum specific growth rate
X = Cell mass concentration
Xm = Maximum Cell mass concentration.
Prit model proposed that substrate requirement for growth includes a substrate for maintenance and substrate for biomass production. By using this principle OUR can be related to the cell mass concentration. According to this OUR can be divided into two terms, Oxygen necessary for cell mass production rate and other is for oxygen required for cell maintenance [13,18,19,23, 24,27].
Equation for OUR for Pichia stipitis cell mass in xylose fermentation can be described by,
OUR= mo.X + YOX .dx/dt (7)
Where,
mo = Specific maintenance constant
X = Cell mass concentration
YOX = Yield coefficient for oxygen consumed for cell mass
On substituting Logistic equation (6) in equation (7) gives the following equation for OUR.
OUR = mo.X + YOX. μm. X. (1-X/Xm) (8)
The fitting of the experimental values of OUR in the equation (8) estimates the values of oxygen consumption factors related to the cell mass growth (YOX), cell mass maintenance (mo).
Results and Discussion
Fermentation of xylose to ethanol in presence of Pichia stipitis has been carried out at constant aeration rate of 0.2 vvm and at different agitation rate i.e. 150, 200, 250, 300 rpm. Biomass concentration, xylose consumption, ethanol production and oxygen uptake rate was measured at various time intervals.
The fermentation profiles for cell growth, xylose consumption and ethanol production are shown in Figure 2 & Figure 3. It was observed that xylose consumption rate increased with increase in agitation at constant aeration rate. At higher rpm of 250 and 300, the initial xylose was consumed within 26-28 h of fermentation time. Slower xylose consumption rate was observed under low agitation of 150 and 200 rpm and the total xylose consumed in between 34-36 h. From the experimental data it has been observed that with increase in agitation the cell mass concentration increases. Higher cell mass growth at higher rpm can be attributed to increase in mass transfer coefficient.
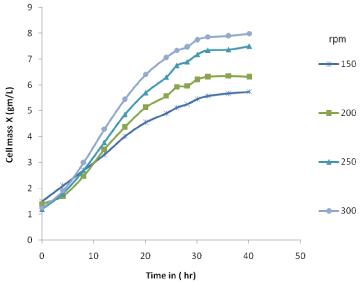
Figure 2: Experimental cell mass production variation at different rpm.
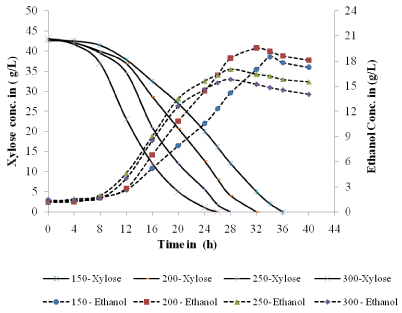
Figure 3: Experimental xylose consumption and ethanol production behavior
at different rpm.
Ethanol yield and productivity at different rpm and kLa are shown in Table 1. For the experimental trials kLa value for xylose fermentation by Pichia stipitis has been found to be in the range of 6.48 to 14.5 h-1.Overall maximum ethanol productivity (0.57 g/L/h) and maximum Ethanol yield (0.45 g/g of xylose) was observed at 200 rpm agitation speed and at kLa value of 10 h-1. At the agitation speed of 300 rpm, although the biomass concentration was high, the ethanol yield was lower, as at higher agitation more xylose was consumed for cell growth and which leads to decrease in ethanol yield.
Agitation speed
(rpm)
Aeration rate
(vvm)
kLa
(h-1)
P
(g/L)
QP/S
(g/L/h)
YP/S
(g/g)
150
0.2
6.48
18.5
0.50
0.43
200
0.2
9.96
19.5
0.57
0.45
250
0.2
12.0
17.0
0.56
0.39
300
0.2
14.5
15.8
0.52
0.36
P=Maximum ethanol concentration; QP/S=ethanol productivity; YP/S=Ethanol Yield on xylose basis (g of ethanol / g of xylose).
Table 1: Experimental data for evaluation of the agitation rate on ethanol production from xylose by Pichia stipitis.
Variable
Yoxx 10-2
(Moles of O2/gof cell mass)
mox 10-7
(Moles of O2/g of cell mass/s)
Constants
2.07
7.83
Table 2: Estimated oxygen uptake rate parameters from fitting of equation (6) to experimental data.
Xylose consumption rate has been found to be increase with Oxygen Transfer Rate (OTR). However, at higher OTR more xylose was utilized for cell mass production. The ethanol productivity increased with OTR but the overall ethanol yield has been decreased due to consumption of xylose for cell growth by Pichia stipitis. Maximum ethanol yield and volumetric productivity was achieved at 200 rpm agitation and 6.66 x 10-7 moles of O2/ L/s OTR.
Experimental & modeled predicted values of OUR with respect to biomass concentration at different agitation rate is shown in Figure 4. OUR values have been found to increase during the initial stage of growth of Pichia stipitis. It has been observed that after achieving cell mass concentration of 3-4 g/L, OUR started decreasing till the end of fermentation. The decrease in oxygen uptake rate after reaching a maximum value can be attributed to the shift in metabolism of Pichia stipitis cell towards ethanol production. As shown in Figure 3, the rate of ethanol production increases at approximately 12 h when cell mass concentration reached to 3-4 g/L. With increase in cell concentration in fermentation broth, the shift of Pichia stipitis metabolism from growth to ethanol production could be attributed to limitation in oxygen transfer rate at respective agitation and aeration rate.
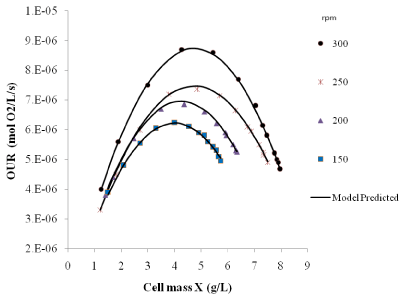
Figure 4: Variation of experimental values (symbol) and model predicted
values (lines) of OUR with biomass concentration at different agitation rate.
Maximum OUR has been observed in between 12-16 h fermentation time. OUR values observed during fermentation are in the range of 3.3 x 10-6 to 8.6 x 10-6 moles of O2/ L/s depending on the agitation rate and biomass concentration. It has been observed that Oxygen Uptake Rate (OUR) is dependent on Oxygen Transfer Rate (OTR) as OUR increases with the agitation speed.
The parametric constants for cell maintenance rate and constant for cell growth of Pichia stipitis were obtained by fitting the equation (8) with the experimental data and by using nonlinear regression technique. Maintenance Coefficient (mo) & growth yield constant (Yox) i.e. oxygen consumption per g cell growth values has been found 7.83 x 10-7moles of O2/ (g of cell. s) and 2.07 x 10-2 respectively for Pichia stipitis. As shown in the Figure 4 both experimental and model estimated values of oxygen uptake rate are in good agreement with each other. Experimental values of oxygen consumption parameters for xylose fermentation by Pichia stipitis has not been reported in the literature. Emillio Gomez et al. reported values of OUR, mo and Yox for Pseudomonas Putida for biodesulfurization capability as 3.3 x 10-6 to 8.6 x 10-6 moles of O2 / L/s, 5.16 x 10-7 moles of O2/ (g of cells), 5.26 x 10-2 moles of O2/(g of cell mass) respectively [16,28]. In our work Heijnen and Roels et al. model was used for prediction of mo and Yox constants [25]. Both predicated parameters were in well agreement with the available data in the literature for other yeasts and bacteria.
Conclusion
The suitability of non-linear growth kinetic parameter estimation was demonstrated for avoiding time consuming experiments or large experimental errors. The oxygen uptake model together with the measurements of cell oxygen utilization rate for Pichia stipitis was useful in predicting the optimum conditions for producing ethanol with higher yield and productivity.
From the oxygen uptake rate model we could predict the actual oxygen uptake rate at any time interval in fermentation at industrial scale. Based on the model predicted values of OUR, we can regulate the aeration and agitation rate in the fermenter after shift of metabolism of Pichia stipitis cells towards ethanol production. This can minimize the operating costs associated with aeration required for xylose to ethanol fermentation.
In fermentation of xylose by Pichia stipitis, oxygen concentration diminishes very quickly in the fermentation broth. Therefore dynamic method was not applicable and modified dynamic method was applied to measure the OUR and OTR. OUR during the fermentation was modeled and kinetic parameters Yox and mo were determined. The oxygen uptake rate simulations together with cell growth model were found to be in agreement with experimental values. The proposed model can contribute to the development of an optimal and cost effective process for ethanol production from xylose by Pichia stipitis.
Acknowledgment
The authors gratefully acknowledge Praj industries Ltd. Pune for financial support to conduct the research work. The authors also thank analytical team of Praj Matrix- R&D Center for providing assistance with analytical measurements. We also thank Dr. Sasisanker for support in reviewing the manuscript.
References
- Sensoz S, Angin D, Yorgun S. Influence of particle size on the pyrolysis of rapeseed (Brassica napus L.): fuel properties of bio-oil. Biomass Bioenergy. 2000; 19: 271-279.
- Dawson L, Boopathy R. Use of post-harvest sugarcane residue for ethanol production. Bioresour Technol. 2007; 98: 1695-1699.
- Wright L. Worldwide commercial development of bioenergy with a focus on energy crop-based projects. Biomass Bioenergy. 2006; 30: 706-714.
- Dombek KM, Ingram LO. Ethanol production during batch fermentation with Saccharomyces cerevisiae: changes in glycolytic enzymes and internal pH. Appl Environ Microbiol. 1987; 53: 1286-1291.
- van Maris AJ, Abbott DA, Bellissimi E, van den Brink J, Kuyper M, Luttik MA, et al. Alcoholic fermentation of carbon sources in biomass hydrolysates by Saccharomyces cerevisiae: current status. Antonie Van Leeuwenhoek. 2006; 90: 391-418.
- Agbogbo FK, Coward-Kelly G, Torry-Smith M, Wenger KS. Fermentation of glucose/xylose mixtures using Pichia stipitis. Process Biochemistry. 2006; 41: 2333-2336.
- Skoog K, Hahn-Hägerdal B. Effect of Oxygenation on Xylose Fermentation by Pichia stipitis. Appl Environ Microbiol. 1990; 56: 3389-3394.
- Silva JP, Mussato SI, Roberto IC, Teixeira JA. Ethanol production from xylose by Pichia Stipitis NRRL-7124 in a stirred tank bioreactor. Brazilian Journal of Chemical Engineering. 2011; 28: 151-156.
- Rouhollah H, Iraj N, Giti E, Sorah A. Mixed sugar fermentation by Pichia stipitis, Saccharomyces cerevisiaea, and an isolated xylose fermenting Kluyveromyces marxianus and their cocultures. African Journal of Biotechnology. 2007; 6: 1110-1114.
- Chandel AK, Gajula C, Konakalla R, Rudravaram R, Pogaku R. Bioconversion of pentosesugars into ethanol: A review and future directions. Biotechnology and Molecular Biology Review. 2011; 6: 8-20.
- Garcia-Ochoa F, Gomez E, Santos V, Merchuk J. Oxygen uptake rate in microbial processes: An overview. Biochemical engineering journal. 2010; 49: 289-307.
- Sivaprakasam S, Mahadevan S, Gopalaraman S. Oxygen mass transfer studies on batch cultivation of P. aeruginosa in a biocalorimeter. Electronic journal of Biotechnology. 2008; 11: 1-13.
- Sanchez M, Francisco G, Gomez A, Grima E, Chisti Y. Bubble column and airlift Photo bioreactors for algal culture. AIChE J. 2000; 46: 1872-1887.
- Puthli M, Rathod V, Pandit A. Gas-liquid mass transfer studies with triple impeller system on laboratory scale bioreactor. Biochemical Engineering. 2005; 23: 25-30.
- Calik P, Yilgor P, Ayhan P, Demir A. Oxygen transfer effects on recombinant benzaldehyde lyase production. Chemical Engineering sciences. 2004; 59: 5075-5083.
- Gomez E, Santos V, Alcon A, Martin A, Garcia-Ochoa F. Oxygen uptake and mass transfer rates on growth of Pseudomonas putida CECT5279: influence on biodesulfurization (BDS) capability. Energy fuels. 2006; 20: 1565-1571.
- Ensari S, Lim HC. Kinetics of L-lysine fermentation: a continuous culture model incorporating oxygen uptake rate. Appl Microbiol Biotechnol. 2003; 62: 35-40.
- Koutinas A, Wang R, Kookos I, Webb C. Kinetic parameters of Aspergillus awamori in submerged cultivations on whole wheat under oxygen limiting conditions. Biochemical engineering journal. 2003; 16: 23-34.
- Garcia-Ochoa F, Gomez E. Bioreactor scale-up and oxygen transfer rate in microbial processes: an overview. Biotechnol Adv. 2009; 27: 153-176.
- Deshpande R, Heinzle E. On-line oxygen uptake rate and culture viability measurement of animal cell culture using microplates with integrated oxygen sensors. Biotechnology Letters. 2004; 26: 763-767.
- Schafer S, Schrader J, Sell D. Oxygen uptake rate measurements to monitor the activity of terpene transforming fungi. Process Biochemistry. 2004; 39: 2221-2228.
- Pirt SJ. Maintenance energy: a general model for energy-limited and energysufficient growth. Arch Microbiol. 1982; 133: 300-302.
- Garcia-Ochoa F, Castro E, Santos V. Oxygen transfer and uptake rates during xanthan gum production. Enzyme Microb. Technology. 2000; 27: 680- 690.
- Santos V, Galdeano C, Gomez E, Alcon A, Garcia-Ochoa F. Oxygen uptake rate measurements both by the dynamic method and during the process growth of Rhodococcus erythropolis IGTS8: Modeling and differences in results. Biochemical engineering journal. 2006; 32: 198-204.
- Heijnen J, Roels J. A macroscopic model describing yield and maintenance relationships in aerobic fermentation processes. Biotechnology and Bioengineering. 1981; 23: 739-773.
- Gomez E, Santos V, Alcon A, Garcia-Ochoa F. Oxygen transport rate on Rhodococcus erythropolis cultures: Effect on growth and BDS capability. Chemical Engineering Science. 2006; 61: 4595-4604.
- Rowe G, Margaritis A, Wei N. Specific oxygen uptake rate variations during batch fermentation of Bacillus thuringiensis subsoecies kurstaki HD-1. Biotechnology Progress. 2003; 19: 1439-1443.
- Olmo C, Santos V, Alcon A, Garcia-Ochoa F. Production of a Rhodococcus erythropolis IGTS8 biocatalyst for DBT biodesulfurization: influence of operational conditions. Biochemical engineering journal. 2005; 22: 229-237.