
Research Article
Austin J Biotechnol Bioeng. 2016; 3(3): 1068.
Investigation the Compressive Strength of Glass Ionomer Cement Containing Hydroxyapatite Nano and Micro Particles
Khaghani M¹, Doostmohammadi A²* and Alizadeh S¹
¹Young Researchers and Elite Club, Islamic Azad University, Iran
²Materials Department, Shahrekord University, Iran
*Corresponding author: Ali Doostmohammadi, Materials Department, Shahrekord University, Iran
Received: June 14, 2016; Accepted: July 18, 2016; Published: July 21, 2016
Abstract
Glass Ionomer Cements (GICs) are one of the most important dental restorative materials. Improvement of biological and mechanical properties of these materials has been taken into consideration. The aim of this work was to preparation and characterization of GICs by melting method and evaluation of adding Hydroxyapatite (HA) micro and nanoparticles on compressive strength of GICs. In this research, the ceramic part of GIC was prepared using melting method, and micro and nano-hydroxyapatite were synthesized from natural bone. HA micro and nanoparticles were added to GICS in different weight percents (0, 1, 3, 5 and 7 wt.%). The microstructure of synthesized products, chemical composition of the ceramic part of glass ionomer cement and the size and shape of HA nanoparticles were studied by Scanning Electron Microscopy (SEM), X-ray Fluorescence (XRF) and Transmission Electron Microscopy (TEM), respectively. The phase analysis of GICs composite was carried out by X-ray Diffraction (XRD) technique. Finally, the compressive strength of composite samples were determined and compared. TEM confirmed the nanometric scale of hydroxyapatite particles. Results of the compression test showed that adding HA micro and nanoparticles with the values of less than 5% by weight had no distractive effect on compressive strength of GICs. The final result of this research was gaining GICs containing HA micro and nanoparticles with improved equivalent mechanical properties. The improvement of GICs properties in dentistry applications can be achieved by adding bioactive materials like HA micro and nanoparticles.
Keywords: Glass ionomer cement; Hydroxyapatite micro/nanoparticles; Compressive strength
Introduction
Glass Ionomer Cements (GICs) are of the most important toothcolored restorative materials used in dentistry. Since the GICs were introduced to the dental profession by Wilson and Kent in 1972, the use of these materials has been increased due to their exclusive properties such as proper biocompatibility with body’s hard tissue, long-term fluoride release, the capability of absorbing and restoring fluoride, low thermal expansion coefficient, good adhesion to moist enamel and dentin without necessitating an intermediate agent and low cytotoxicity [1-3]. Despite the above mentioned advantages, this group of substances has some disadvantages which mainly include low mechanical strength and sensitivity to humidity that limit their applications [4,5]. Some efforts have been dedicated to improve the mechanical properties of conventional GICs which include reinforcement with metal powders [6], modification with resin [7], Incorporation with SiC whiskers/ short fibers [8,9], HA and fluoroapatite nanobioceramics [10], forsterite nanoparticles [3], etc.
Hydroxyapatite (HA) is one of the most important calcium phosphate bioceramics (Ca10(PO4)6(OH)2 chemical formula; Ca/ P=1.67), and has a composition and crystal structure similar to apatite in the human dental structure and bone tissue [11,12]. In recent years, a number of studies have tried to assess the effect of HA addition to the restorative dental materials such as GICs [13]. It was found that glass ionomer cements were interacted with HA through the carboxylate groups in the polyacid. Hence, the incorporation of HA into GIC powder composition can not only improve the biocompatibility of GICs, but also increase the mechanical properties [10,14].
Lee et al. [15] investigated the physical properties of resinreinforced GIC modified with micro and nano-hydroxyapatite, and suggested that demineralization of enamel surface could be prevented by incorporating nano-HA into GIC.
On the other hand, studies on ceramic particles (or glass beads) reinforced polymers suggested that the particle size had effects on the mechanical properties of composites [16].
Therefore, the aim of this work was to synthesis micro and nano-hydroxyapatite from natural bone and via sol-gel method, respectively, and also investigate and compare the influence of HA particle size on the mechanical properties of GIC.
Materials and Methods
Preparation of ceramic part of Glass Ionomer Cement (GIC)
The analytical-grade of Aluminum Oxide (Al2O3, Merck), Silicon Oxide (SiO2, Merck), Fluoride Strontium (SrF, Merck), Aluminum Phosphate (AlPO4, Merck) and Calcium Fluoride (CaF2, Merck) were used as the starting materials for producing the ceramic part of glass ionomer by melting method.
First, a defined weight percentage of the mentioned oxides was prepared and mixed in a ball mill with aluminum balls for homogenizing the powders’ mixture. Then the mixed materials were placed in an electric melting furnace, and heated for 3 hours with the rate of 5°C/min to reach the temperature of 1400°C. Finally, the asobtained melted glass was cooled at ambient temperature, underwent shattering process in the ball mill for 5 h, and passed through a 200 mesh sieve in order to be defined as glass powder according to ASTM [17].
Producing of hydroxyapatite micro and nanoparticles
Microparticles of hydroxyapatite were obtained from natural bone. A femur of an adult bovine was obtained from a slaughterhouse and boiled in water for 12 h to render it aseptic and loosen any attached soft tissues. Then it was washed and cleaned carefully to remove visible tissues, fats and any other impurities on the bone surface. To remove the internal organic content (e.g. collagen) and water, the bone was then heated in an electric furnace under ambient conditions, at 700°C with 2 h holding time. The resulting white solid specimens were first ground and crushed with a mortar and pestle to produce a powder, and then sieved to obtain particle-size distribution less than 50 µm. Bone powder was sterilized at 150°C for 1 h, rinsed in distilled water, and incubated in 1% phosphoric acid. Eventually, microparticles were rinsed again in sterile distilled water, and sterilized at 200°C.
A high energy planetary ball mill (Fretch Pulverisette 5), with a 125 ml zirconia via l and four 20 mm diameter zirconia balls at ambient temperature, was used to produce HA nanoparticles. Obtained HA microparticles in previous stage were ball milled for 20 h and finally sterilized at 200°C.
GIC-HA composites preparation
The glass ionomer- hydroxyapatite composites was fabricated through adding 1, 3, 5 and 7 wt.% of HA micro- and nanoparticles to the prepared glass powders. The specimens were transferred in to the aluminum mold after mixing of powder and liquid, and removed after 1 h.
Characterization
X-ray Fluorescence (XRF): X-ray fluorescence elemental analysis (XRF, Bruker, S4PIONEER, Germany) was used to confirm the presence of oxides in the final compound of glass ionomer powder.
Scanning Electron Microscopy (SEM): HA particles’ morphologies and glass ionomer cement containing HA were mounted on aluminum SEM pins and coated with Au, and were investigated with scanning electron microscope (SUPRA 40 VP FESEM, Carl Zeiss AG, Germany) operated at an acceleration voltage of 20 kV.
Transmission Electron Microscopy (TEM): Transmission Electron Microscopy (TEM) was also employed to investigate the size and morphology of HA nanoparticles. A universal method for the preparation of TEM samples was applied to bioceramic ones. In this method, special holders were used in which the sample is embedded using epoxy glues.
X-ray Diffraction (XRD): X-ray Diffraction (XRD) technique (Philips X’Pert-MPD system with a Cu Ka, 1.5418 Å) was used to analyze the structure of the prepared glass ionomer powder and the GIC-HA composites. The diffractometer was operated at 40 kV and 30mA at a 2θ range of 10–90° employing a step size of 5s per step.
Compressive strength measurements
Cylindrical specimens were prepared using aluminum cylindrical shaped molds 4 mm in diameter and 6 mm in height for compressive strength test. The molds were filled with the material, flattened and gently pressed by hand in order to remove air bubbles from uncured cement paste. The specimens were removed from the molds after 30 min and conditioned in distilled water at 37°C for 24 h [18]. Five specimens were made for each test. The different experimental groups of GICs and their abbreviations are tabulated in Table 1.
X
Composition
Compressive Strength (MPa)
0 (GIC)
Liquid Compositions
Polyacrylic acid copolymer
XnHG#
XmHG*
Powder Compositions
Strontium containing aluminum
Fluorosilicate glass
46.10±3.39
46.10±3.39
Chemical Composition
SiO2
Al2O3
AlPO4
CaF2
SrF
39.00
25.50
16.50
12.00
7.00
1
GIC- 1% HA
74.76±6.50
49.54±1.95
3
GIC- 3% HA
61.7±0.40
50.95±0.35
5
GIC- 5% HA
56.8±0.1
65.56±1.62
7
GIC- 7% HA
37.75±1.46
41.31±3.64
*m: Microparticles: #n: Nanoparticles
Table 1: Compositions, the abbreviations used for various experimental groups and compressive strength values (mean values ± standard deviation) of micro and nano -HA-containing glass ionomer cement samples (mHG and nHG, respectively).
Compressive strength test was performed on a screw-driven testing machine (Hounsfield, Model H25KS, England) with a crosshead speed of 0.5 mm/min.
Cylindrical specimens with 4±0.1mm diameter and 6±0.1mm height were prepared according to ASTM D695 (Table 1), and their compressive strength (CS; MPa) was calculated by the following equation:
CS=4P/pd2 (1)
Where P is the load at fracture (N), and d is the diameter of the cylindrical specimen (mm).
Results and Discussion
Chemical composition of as-prepared glass ionomer
Glass ionomer powder composition analyzed by XRF is reported in Table 1. This result confirmed that the chemical compounds of the ceramic part of glass ionomer cement synthesized by the melting method were to a great extent similar to the expected weight rates.
The weight percentage of oxides was expressed by computer according to the elemental analysis and considering the assumption that all the elements are in oxidic form. These results showed that the composition of prepared GIC powder was in good agreement with reported composition for commercial type of GIC [19].
Scanning Electron Microscope (SEM)
SEM image of the ceramic part of GIC is shown in Figure 1a. From these images, it is found that the glass particles have irregular shapes and the size of less than 100 µm. Other researchers also reported the size of less than 100 micrometers for the glass particles produced through melting method [20].
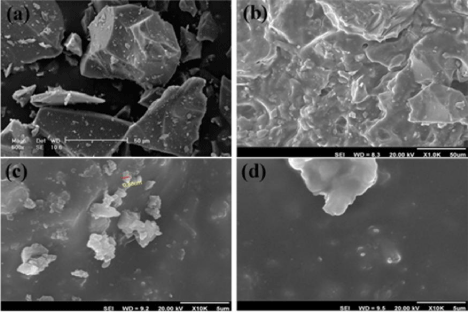
Figure 1: SEM images of (a) the ceramic part of glass ionomer cement, (b)
ceramic part of glass ionomer cement mixed with polyacrylic acid liquid, (c)
glass ionomer cement containing 5 wt.% hydroxyapatite microparticles and
(d) glass ionomer cement containing 5 wt.% hydroxyapatite nanoparticles.
Figure 1b shows the SEM micrograph of the integrated structure of glass ionomer (mixture of glass ionomer powder and polymer liquid (polyacrylic acid)). The relatively homogeneous and flat surface of glass ionomer is clearly observed in this image.
As it can be seen from the Figure 1c, hydroxyapatite microparticles were non-uniformly dispersed in glassy matrix of GIC.
Agglomerated hydroxyapatite semi-sphere nanoparticles (less than 150 nm) in a homogenous field of glass ionomer are observable in the SEM micrograph (Figure 1d). The same as many other nanocompounds, the agglomeration of hydroxyapatite nanoparticles is inevitable. These results showed a good agreement with the reports presented by other researches [10].
Transmission Electron Microscopy (TEM)
Results from TEM analyses for HA nanoparticles are shown in Figure 2. The TEM observations revealed that the powder particles of HA have prolate spherical and hexagonal morphology. However, the powders were not well dispersed, suggesting that the aggregation of powders occurred due to Vander Waals attraction [21].

Figure 2: TEM images of HA nanoparticles.
The size of the hydroxyapatite particles as estimated from the TEM images ranged from 50 to 150 nm.
Phase analysis (XRD)
The XRD patterns of GIC powder, 5nHG and 5mHG are shown in Figure 3. The pattern of GIC (Figure 3a) did not contain diffraction peaks, indicated the internal disorder and the glassy nature of this material.
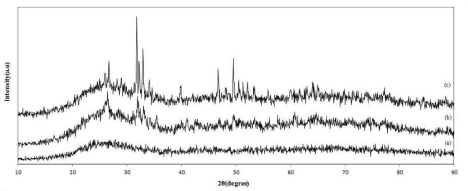
Figure 3: The XRD spectra of (a) GIC, (b) 5nHG and (c) 5mHG.
In addition, the XRD patterns of 5nHG and 5mHG showed the peaks related to HA particles in glass ionomer structure (Figure 3b and 3c). These results had a good agreement with the results reported by Moshavernia et al. [10].
Compressive strength measurements
The results of 7 days compressive strength values of the microand nano-HA containing glass ionomers are shown in Figure 4 and Table 1, and compared with GIC ones as the control group. The results showed that there was a significant difference between the compressive strength values of the micro- and nano-HA containing GIC samples and the control group.
In micro-HA-containing glass ionomer cement samples (mHG), the higher compressive strength value was observed for 5mHG sample (42% increases) (Figure 4). The same trend was observed for the nano-HA-containing glass ionomer samples (nHG) and the higher compressive strength value was observed in 1nHG sample (62% increases).
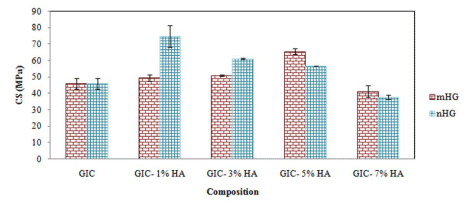
Figure 4: Compressive strength values of micro and nano -HA-containing
glass ionomer cement samples (mHG and nHG, respectively).
Increasing of compressive strength in presence of hydroxyapatite particles is due to the formation of crystal phases in the amorphous structure of glass ionomer. In both micro- and nano-HA containing glass ionomers, with increasing of HA, the compressive strength of GIC is increased. In micro-HA containing glass ionomers, the maximum strength was observed in 5 %wt and in nano-HA containing glass ionomers the maximum strength was observed in 1 %wt of HA particles. Decrease of compressive strength in hydroxyapatite weight percentages upper than 5 and 1, is due to the decrease in bonding forces between the ceramic part and the polymeric matrix of GICs. In fact, the added hydroxyapatite particles act as a barrier and prevents from the complete bonding of the parts of glass ionomer cement [3,10,14].
Results also showed that the enhancement in compressive strength of nano-HA containing glass ionomers is more than micro- HA containing glass ionomers. In addition, when compared to micro- HA containing glass ionomers, in presence of HA nanoparticles, more compressive strength was observed in lesser amounts of HA. Large specific surface area can easily lead to particle agglomeration, which makes nanoparticles more difficult to be evenly dispersed into polymer matrix, thus resulting in a strength reduction [22].
Conclusion
Micro and nano-hydroxyapatite particles were successfully synthesized from natural bone. The addition of micro- and nano- HA into prepared conventional composition of GIC enhanced the compressive strength of the resulting cements. These bioceramics are therefore considered promising additives for glass ionomer restorative dental materials. However, the compressive strength increase in nano-HA containing glass ionomers was more than micro-HA containing glass ionomers. Moreover, in presence of HA nanoparticles, more compressive strength was observed in lesser amounts of HA.
Acknowledgment
The authors are thankful to Nikceram Razi Company for the excellent technical assistance during the study.
References
- Zraikat HA, Palamara JEA, Messer HH, Burrow MF, Reynolds EC. The incorporation of casein phosphopeptide–amorphous calcium phosphate into a glass ionomer cement. Dental Materials. 2011; 27: 235-243.
- Zoergiebel J, Ilie N. Evaluation of a conventional glass ionomer cement with new zinc formulation: effect of coating, aging and storage agents. Clin Oral Invest. 2013; 17: 619-626.
- Sayyedan FS, Fathi MH, Edris H, Doostmohammadi A, Mortazavi V, Hanif A. Effect of forsterite nanoparticles on mechanical properties of glass ionomer cements. Ceram Int. 2014; 40: 10743-10748.
- Shiekh RA, Ab Rahman I, Masudi SM, Luddin N. Modification of glass ionomer cement by incorporating hydroxyapatite-silica nano-powder composite: Sol– gel synthesis and characterization. Ceram Int. 2014; 40: 3165-3170.
- Brito CR, Velasco LG, Bonini GAVC, Imparato JCP, Raggio DP. Glass ionomer cement hardness after different materials for surface protection. J Biomed Mater Res. 2010; 93: 243-246.
- Irie M, Nakai H. Mechanical properties of silver-added glass ionomers and their bond strength to human tooth. Dent Mater J. 1988; 7: 87-93.
- Farrugia C, Camilleri J. Antimicrobial properties of conventional restorative filling materials and advances in antimicrobial properties of composite resins and glass ionomer cements-A literature review. Dent Mater. 2015; 31: e89-e99.
- Kobayashi M, Kon M, Miyai K, Asaoka K. Strengthening of glass ionomer cement by compounding short fibers with CaOP2O5–SiO2-Al2O3 glass. Biomaterials. 2000; 21: 2051-2058.
- Arita K, Nakajima H, Nishino M, Okabe T. Effect of reinforcements on mechanical properties of glass ionomer. J Dent Res. 1992; 72: 631.
- Moshaverinia A, Ansari S, Moshaverinia M, Roohpour N, Darr JA, Rehman I. Effects of incorporation of hydroxyapatite and fluoroapatite nanobioceramics into conventional Glass Ionomer Cements (GIC). Acta Biomaterialia. 2008; 4: 432-440.
- Goenka S, Balu R, Kumar TSS. Effects of nanocrystalline calcium deficient hydroxyapatite incorporation in glass ionomer cements. J Mech Behav Biomed Mater. 2012; 7: 69-76.
- Arita K, Yamamoto A, Shinonaga Y, Harada K, Abe Y, Nakagawa K, et al. Hydroxyapatite particle characteristics influence the enhancement of the mechanical and chemical properties of conventional restorative glass ionomer cement. Dent Mater J. 2011; 30: 672-683.
- Moshaverinia A, Roohpour N, Chee WWL, Schricker SR. A review of powder modifications in conventional glass-ionomer dental cements. J Mater Chem. 2011; 21: 1319-1328.
- Gu YW, Yap AUJ, Cheang P, Khor KA. Effects of incorporation of HA/ZrO(2) into glass ionomer cement (GIC). Biomater. 2005; 26: 713-720.
- Lee JJ, Lee YK, Choi BJ, Lee JH, Choi HJ, Son HK, et al. Physical properties of resin-reinforced glass ionomer cement modified with micro and nanohydroxyapatite. J Nanosci Nanotechnol. 2010; 10: 5270-5276.
- Wang M, Joseph R, Bonfield W. Hydroxyapatite-polyethylene composites for bone substitution: effects of ceramic particle size and morphology. Biomater. 1998; 19: 2357-2366.
- Fathi MH, Hanifi A. Evaluation and characterization of nanostructure hydroxyapatite powder prepared by simple sol–gel method. Mater Lett. 2007; 61: 3978-3983.
- Doostmohammadi A, Monshi A, Salehi R, Fathi MH, Karbasi S, Pieles U, et al. Preparation, chemistry and physical properties of bone-derived hydroxyapatite particles having a negative zeta potential. Mater Chem Phys. 2012; 132: 445-446.
- Smith DC. Development of glass-ionomer cement systems. Biomater. 1998; 19: 467-478.
- Milne KA, Calos NJ, O’Donnell JH, Kennard CHL, Vega S, Marks D. Glassionomer dental restorrative: part I: A structural study. J Mater Sci: Mater Med. 1997; 8: 349-356.
- Ruksudjarit A, Pengpat K, Rujijanagul G, Tunkasiri T. Synthesis and characterization of nanocrystalline hydroxyapatite from natural bovine bone. Curr Appl Phys. 2008; 8: 270-272.
- Zhao J, Xie D. Effect of nanoparticles on wear resistance and surface hardness of a dental glass-ionomer cement. J Comp Mater. 2009; 43: 2739- 2752.