
Research Article
Austin J Biotechnol Bioeng. 2017; 4(2): 1079.
Reduction of Hydrogen Peroxide-Induced Erythrocyte Damage by Leaf Extracts of Buchanania Lanzan Spreng as a Potential Natural Antioxidant
Shinde G, Patil AS* and Sheikh R
Department of Biotechnology, Sant Gadge Baba Amravati University, India
*Corresponding author: Anita Surendra Patil, Department of Biotechnology, Sant Gadge Baba Amravati University, Amravati, India
Received: May 10, 2017; Accepted: September 26, 2017; Published: October 03, 2017
Abstract
Buchanania lanzan Spreng belongs to family Anacardiaceae commonly known as priyal or Chironji. This species has high socio-economic value providing livelihood to tribal population within the area and has the high potential as commercial horticulture species. Unfortunately due to over-exploitation and indiscriminate harvesting (lopping and cutting), leading to the very severe threat to its extinction, which call for urgent conservation efforts of all levels. Besides this, the traditional indigenous knowledge reveals the immense value of almost all parts of the plant used in a drug in Ayurveda and the Unani system of medicine. It posses the antioxidant, antimicrobial and astringent, expectorant, diuretic and carminative and many more properties. To focus the positive sense to their antihaemolytic activity as it important biological property, present study was carried out. It has been found out that the plant leaves possessed haemoprotective capability potential, i.e. antihaemolytic activity, which was evaluated various concentration (20-100 mg/ml) using hydrogen peroxide dependent blood haemolysis method.
Keywords: Buchanania lanzan; Column chromatography; Antihaemolytic activity; Antioxidant assay
Introduction
Reactive Oxygen Species (ROS), such as hydrogen peroxide, super oxide anion and hydroxyl radical, capable of causing damage to DNA, have been associated with carcinogenesis, coronary heart disease, and many other health problems related to advancing age [1]. Antioxidant that scavenges these free radicals proves to be beneficial for these disorders as they prevent damage against cell proteins, lipids and carbohydrates [2]. Erythrocytes, which are the most abundant cells in the human body, possessing desirable physiological and morphological characteristics, are exploited extensively in drug delivery [3]. Oxidative damage to the erythrocyte membrane (lipid/ protein) may be implicated in haemolysis associated with some haemoglobinopathies, oxidative drugs, transition metal excess, radiation, and deficiencies in some erythrocyte antioxidant systems [4].
This assay is useful either for screening studies on various molecules and their metabolites, especially on one hand, molecule having an oxidizing or antioxidating activity or on the other hand, molecule having a long term action [5]. Several herbal secondary metabolites such as flavonoid have been found to protect cells from oxidative damage [6]. These compounds have been evidenced to stabilize RBC membrane by scavenging free radicals and reducing lipid peroxidation [7,8].
Herbs are a rich source of flavonoid, phenolic acid and alkaloids, some of which act as antioxidants [7-10]. B. lanzan Spreng plant is well known for its medicinal and therapeutic values in Indian folk medicine. It is being a vulnerable medicinal plant, is included in the Red Data Book published by International Union for Conservation of Nature and Natural Resources (IUCN). The plant parts i.e. roots, rhizome, leaves, fruits, seeds and gum are used for the treatment of various disorders. The seeds are also used as the expectorant and tonic. The oil extracted from kernels is applied on skin diseases and applied to remove spots and blemishes from the face. The root is used as an expectorant, in biliousness and also for curing blood diseases. The juice from the leaves is digestive, expectorant, aphrodisiac, and purgative. It is used as the form of decoction to treat intrinsic haemorrhage, diarrhea with blood and as tonic. Until now, sporadic reports have been published, reveals that specially leaf, bark, and seed are the major source of metabolites of great pharmaceutical value.
In the present study phytochemical characterization, qualitative and quantitative evaluation of antihaemolytic activity and antioxidant activity of methanolic extract of B. lanzan was performed.
Further to purify and identify the metabolites responsible for such activity, column chromatography was performed. The highly active fraction was screened further for its inhibition of erythrocyte haemolysis among the entire fractions. The compound accountable for such activity was again identified on TLC by antioxidant TLC autobiography (Figures 1 and 2). The band responsible for such activity was purified and further characterized by LC-HRMS and identified using library match.

Figure 1: Showing the antihaemolytic activity of soxhlet extract. In negative
control: Blood+ PBS+H202; Positive Blood + PBS + Sample.

Figure 2: Screening of most bioactive fraction collected from Column
Chromatography using antihaemolytic assay.
Materials and Methods
Preparation of chemicals
Double distilled water was used in all the experiments. Different concentrations of Methanolic soxhlet leaves extract of B. lanzan were prepared in methanol from 20 mg/mL to 100mg/mL. The chemicals used during this experiment were of analytical grade. Phosphate buffer saline (pH 7.4), Hydrogen Peroxide, Butanol, Glacial acetic acid, Methanol, Dichloromethane, Ethyl acetate, Dragendroff reagent, Mayer’s reagent, Wagner’s reagent, Benedict’s reagent, Picric acid, sulphuric acid, Fehling’s solution, Ferric chloride, Ninhydrin, HCL, Gallic acid. Glucose, Silver chloride, Sodium nitroprusside, Sodium carbonate, Silica gel G (TLC) purchased from Himedia Laboratories, India. Silica gel 120 mesh size for column chromatography (SD FINEChem limited), 2,2, diphenyl- 2-picryl-hydrazyl (Sigma®) (DPPH) in methanol, Glass plates used were customs made, prepared according to necessity.
Instrumentation and reagents
The plant extract metabolites were separated on TLC and observed under UV Transilluminator. Metabolites showing antioxidant activities were confirmed by TLC-direct bioautography. Partial structure of these bioactive metabolites was elucidated by High Resolution Liquid Chromatography by mass spectroscopy (LC-HRMS). The analysis was done using Q-TOF LCMS coupled instruments (Q-TOF B. 05. 01) (Bs12s.1) (6200 series, Agilent).
Collection of Sample
The plant leaves material of B. lanzan was collected from the forest of Yavatmal District, MS, India in 2015.The Herbarium specimen was prepared, authenticated by its morphological characters by the Sr. taxonomist Prof. S. R. Manik, Department of Botany, SGBAU Amravati, MS, INDIA and submitted to Department of Biotechnology, Sant Gadge Baba Amravati University with Accession number SGBAU-DBT-10. Leaves of B. lanzan were thoroughly washed in tap water and allowed to dry completely in shade for 15 days, and it was powdered using the grinder.
Preparation of extract
100 g powdered plant leaves sample of B. lanzan was extracted in 1000 mL methanol using soxhlet apparatus at 55-65 °C for 3 days in order to extract polar compounds. The solvent was allowed to evaporate under vacuum condition in rota evaporator, after drying, dark-green semisolid mass was obtained and stored in deep freeze and kept for further analysis.
Qualitative phytochemical screening
The different qualitative chemical tests were performed for establishing profile of extract for its chemical composition. Qualitative phytochemical analysis was done using the standard procedures [11].
Column chromatography of B. lanzan extract for purification of compound
The dried soxhlet methanolic leaves extract (SMLE) of B. lanzan (50 g) was subjected to column chromatography on silica gel (120 mesh) using solvent’s gradient of petroleum ether, chloroform, ethyl acetate and methanol; the fractions were collected in 50 mL centrifuge tube and dried to get purified form. The entire 42 fraction collected were air dried and stored for future use. 1mg/mL Stock solutions of all fractions were prepared and tested for antihaemolytic activity. The screened fraction (Column extract- CLE) was analyzed for its antihaemolytic activity in different concentration, i.e. 20μl, 40μl, 60μl, 80μl and 100 μl. Ascorbic acid was used as standard (1 mg/mL).
Assay for Inhibition of Erythrocyte Haemolysis
Preparation of RBC suspension
Antihaemolytic activity was assessed by following the spectrophotometric method. From a normal healthy individual 5 ml of blood was taken and centrifuged at2000 rpm for 10 min. Pellet of blood was washed three times in sterile phosphate buffer saline solution (pH 7.2). The pellet was resuspended in normal 0.5% saline solution. Obtained RBC pellets were washed twice and finally diluted with saline to have a cell density of 2 × 104 RBC/ml suspensions and processed for studying H2O2 induced haemolysis [12].
Screening the potential antihaemolytic activity of SMLE and CLE of B. lanzan
Soxhlet Methanolic Leaves Extract [SMLE] of B. lanzan were used for the antihaemolytic assay. Extract was taken in different concentration from the stock of 1 mg/mL i.e. 20μl, 40μl, 60μl, 80μl and 100μl in each tube and the volume make up to 500μl by using phosphate buffer (pH 7.4). After that 100μl Hydrogen peroxide was added to each tube. Reaction mixture was incubated the mixture at 37°C for 20 min it was centrifuged at 2000 rpm for 10 min. Antihaemolytic activity was assessed by measuring the absorbance at 540 nm. For positive and negative control distilled water and phosphate buffer saline were used respectively. Increase in absorbance indicates greater haemolysis [13].
Percentage inhibition = ([Absorbance of control - Absorbance of sample]) / (Absorbance of control) x 100
Determination of in vitro antioxidant assay of SMLE and CLE of B. lanzan
The antioxidant potential can be assessed by finding their free radicals scavenging capacity or their potential to reduce the compounds using the Radical scavenging activity assay.1,1- Diphenyl-2-picrylhydrazil (DPPH) is a stable free radical that accepts an electron or hydrogen radical to become a stable diamagnetic molecule. The model of scavenging the stable DPPH radical is widely used for relatively rapid evaluation of antioxidant activities compared with other methods [14]. The capacity to scavenge the stable free radical DPPH was done with slight modifications in 96 well microtitreplate. Various concentrations of SMLE and CLE of B. lanzan i.e. (10, 20 to 100μL) were mixed with 150μL of methanolic solution containing DPPH radicals (0.1mM) respectively. The mixture was shaken vigorously and left to stand for 30 min at room temperature. The reduction of the DPPH radical was measured by monitoring continuously the decrease of absorption at 517 nm. Following formula was applied to determine the DPPH radical scavenging activity;
(%) DPPH scavenging effect = ([Absorbance of control - Absorbance of sample]) / (Absorbance of control) x100
IC50 is the concentration value which scavenged 50% of the DPPH radicals. Ascorbic acid was used as reference compounds.
Preparative TLC for active CLE extract of B. lanzan
The number of compounds present in the sub-fractions obtained by column chromatography has been identified by Thin Layer Chromatography (TLC). A streak of crude fraction was applied manually on a preparative TLC glass plate (8 cm × 12 cm; 1.5mm thickness). After air drying, the plate was developed in a pre-saturated glass chamber with optimized mobile phase i.e., toluene: ethyl acetate: Toluene: ethyl acetate: methanol (10:8:2) was used.
Antioxidant TLC autobiography of active CLE extract of B. lanzan
Many TLC techniques have been developed and successfully applied for qualitative and quantitative analysis of antioxidants [15,16] and the stable free radical 2,2- diphenyl-1-picrylhydrazyl (DPPH) was often used as a derivatization reagent for this purpose [17]. In the screening of antioxidants, the TLC bioautography assay is the method of choice due to several advantages that include flexibility, simplicity and high throughput. The mixed CLE extract of active fraction were used for the antioxidant autobiography.
Antihaemolytic and antioxidant activity of purified TLC bioactive band of CLE of B. lanzan
Bioactive band of TLC was isolated and then re-dissolved in methanol, later supernatant was collected and used as purified compounds for inhibition of erythrocyte haemolysis and antioxidant activity.
Microscopic examination of RBC viability and morphological changes after induced H2O2 treatment
Primostar, Carl Zeiss Axiocam attached image analysis system was used for microscopic analysis. RBC viability and morphological changes after induced H2O2 treatment were observed under the microscope. Assay for inhibition of erythrocyte haemolysis was performed of purified TLC band and after reaction 10μL sample was loaded on microscopic slides to check the viability of cells. In negative control there is no role of the leaf extract and hence the pellets are disrupted and hence dead. Whereas, in positive control quercetin/ ascorbic acid there is fully control of RBC lysis due to presence of leaf extract.
LC-HRMS analysis of bioactive fraction
The bioactive band was purified by preparative TLC. The fraction was isolated, dissolved in 20 mL methanol and centrifuged at 4000 rpm. The supernatant was taken in fresh tube and evaporated. The dried samples were used for identification of compounds by using LC-HRMS analysis.
The partial structure elucidations of these bioactive compounds were performed by LC-HRMS by SAIF, IIT, Mumbai, MS, India. The analysis was done using Q-TOF LCMS coupled instruments (Q-TOF B.05.01) (Bs12s. 1) (G6550A series, Agilent); the following HPLC method was used. A binary gradient elution at flow rate of 0.2 mL/ min was employed using aqueous solution of phosphoric acid 0.1% (v/v) as solvent A and acetonitrile as solvent B, the run was completed as follows: 5–100% B at 0–35 min using ZORBAX C-18-2.1 × 50mm × 1.8 micron column.
Statistical analysis
In vitro antioxidant and antihaemolytic assay were performed in triplicate and results are shown as mean ± SD. Antioxidant potential of different assays was determined as IC50 values and antihaemolytic potential as IC90 value by applying Graph pad prism 5-software. Statistical significance was determined among various treatments with one way ANOVA test. A statistical significance of P < 0.05 or P < 0.01 was considered to be significant.
Results
Qualitative phytochemical screening
B. lanzan has been subjected for Soxhlet Methanolic Extraction (SMLE) and preliminary phytochemical evaluation. The concentrated SMLE of B. lanzan was greenish, sticky and had pungent smell; it was stored in cold conditions for further study. The phytochemical analysis shown the existence of the number of phyto-constituents including Alkaloid, Carbohydrate, Phenol, Tannin, Saponin, Glycosides whereas Gum and Mucilage, Proteins and Amino acid were found to be absent. The results are shown in (Table 1). Earlier reports also support the presence of phytochemicals of Glycosides, phenolic compound and flavonoids in the different leaf extracts of B. lanzan [18,19].
Name of Test
Type of Test
Inference
Alkaloids
Wagner’s Test
+ +
Mayer’s Test
+ +
Hager’s Test
+ +
Dragendorff’s Test
+
Carbohydrates
Molisch’s Test
+ +
Benedict’s Test
++
Fehling’s Test
++
Phenols & Tannins
Ferric Chloride Test
+ +
Gelatin Test
++
Lead Acetate Test
+ +
Glycosides
Borntragers Test
+
Proteins & Amino Acids
Biuret Test
-
Ninhydrin Test
+
Saponins
Foam Test
+
Gum & Mucilage
-
Table 1: Phytochemical screening of SMLE of B. lanzan.
Screening the potential Antihaemolytic activity of SMLE, CLE and Purified TLC bioactive band of CLE of B. lanzan.
Hemolytic activity of B. lanzan leaves extract and its various fractions were screened against normal human erythrocytes. From the (Graph 1), results showed that RBC treated with H2O2 along with extracts of SMLE, CLE and Purified TLC bioactive band of CLE of B. lanzan marked reduction in haemolysis. Scavenging of H2O2 by B. lanzan leaves extract may be attributed to their antioxidant and other active components, which can donate electrons to H2O2, thus neutralizing it by water molecule. The extracts were capable of scavenging H2O2 in a concentration dependent manner. SMLE, CLE showed antihaemolytic activity with IC90 value of 79.56mg/ ml, 68.41mg/ml and R2 value of 0.865, 0.7989 respectively. The lower value of IC90 indicates higher antihaemolytic activity [20], and hence the purified TLC bioactive band of CLE of B. lanzan extract exhibited the highest antihaemolytic activity with IC90 value of 4.915mg/ml compared to SMLE and CLE. Similarly it has been compared with heamolytic stability of 100 μl of 1mg/mL of quericitin and ascorbic acid showing 0.008 and 0.165 optical density at 540nm.
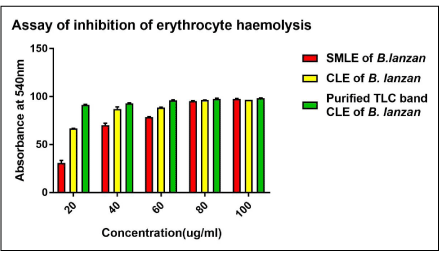
Graph 1: Graph showing Comparative activity of inhibition of erythrocyte
haemolysis of SMLE, CLE and Purified TLC bioactive band of CLE of B. lanzan.
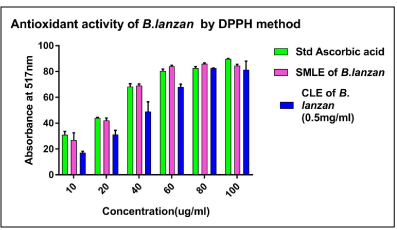
Graph 2: Graph showing Comparative antioxidant activity of Standard
Ascorbic acid, SMLE of B. lanzan and CLE of B. lanzan (0.5 mg/ml).
Determination of in vitro antioxidant assay of SMLE, CLE and purified TLC band of CLEB. lanzan
Antioxidant molecules can quench DPPH-free radicals (providing hydrogen atoms or by electron donation, conceivably via a free-radical attack on the DPPH molecule) and convert them to a colorless product (2,2-diphenyl-1-hydrazine, or a substituted analogous hydrazine), resulting in a decrease in absorbance at 517 nm. Hence, the more rapidly the absorbance decreases, the more potent the antioxidant activity of the extract. Free radical scavenging is one of the known mechanisms by which antioxidants inhibit lipid oxidation [21,22].
In DPPH radical scavenging method, the activity of tested extracts was expressed as Percent Inhibition of Standard ascorbic acid, SMLE of B. lanzan and CLE of B. lanzan (0.5mg/ml). Graph 2 represents the comparative DPPH percent inhibition potential of Ascorbic acid, SMLE of B. lanzan and CLE of B. lanzan (0.5mg/ml extract. The IC50 (50 percent Inhibition) of Ascorbic acid, SMLE of B. lanzan and CLE of B. lanzan (0.5mg/ml was 26.42 μg/ml, 27.99 μg/ml, 45.20 μg/ml respectively. It was calculated using equation, Y = 0.6364*X + 33.18, Y = 0.6523*X + 31.74, Y = 0.751* X+16.05 relatively. The R2 value of Ascorbic acid, SMLE of B. lanzan and CLE of B. lanzan were found to be 0.8906, 0.813, and 0.9361 respectively.CLE of B. lanzan exhibits low DPPH antioxidant activity compared to SMLE of B. lanzan and Ascorbic acid. A lower value of IC50 indicates a higher antioxidant activity. The highest free radical scavenging activity was exerted by SMLE of B. lanzan i.e.27.99 μg/ml compared to CLE of B. lanzan.
Preparative TLC for active CLE extract of B. lanzan
The number of compounds present in the sub-fractions obtained by column chromatography has been identified by Thin Layer Chromatography (TLC). A streak of crude fraction was applied manually on a preparative TLC glass plate (20 cm × 20 cm; 1.5mm thickness). After air drying, the plate was developed in a pre-saturated glass chamber with optimized mobile phase, i.e., Toluene: Ethyl acetate: Methanol (10:8:2) was used and the results shown in (Table 2 and Figure 3).
Rf of Bands observed
Color under UV light
Color under visible light
Band Rf - 0.81 cm
Band Rf-0.68 cm
Yellow
Orange
Light Yellow
Table 2: Rf of Purified bioactive TLC compound of CLE of B. lanzan.
Sr no
Peak name
Name of compound
Retention time
Mass
Formula
1
A
C16Sphinganine
12.517
273.26
C16H35NO2
2
B
Unknown
12.607
546.33
-
3
C
Unknown
12.686
217.10
-
4
D
Phytosphingosine
12.607
317.29
C18H39NO3
5
E
Leukotriene F4
12.818
568.27
C28H44NO8S
6
F
Doxapram
13.259
378.22
C24H30N2O5
7
G
Dihdrospingosine
13.8
301.30
C18H39NO2
8
H
Cuscohygrine
14.44
224.19
C13H24N2O
9
I
Unknown
15.3
329.33
Table 3: The LC-HRMS characterization of selected peaks chromatogram of B. lanzan.
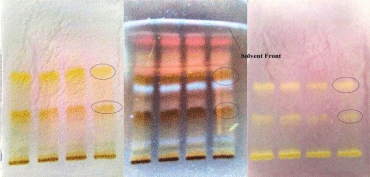
Figure 3: TLC showing DPPH antioxidant autobiography of CLE of B. lanzan.
Antioxidant TLC autobiography of active CLE extract of B. lanzan
To screen the antioxidant capacity of soxhlet methanolic leaves of B. lanzan, a TLC bioautography method was performed. After separation on TLC plates, the compounds with radical scavenging activity were determined in situ with DPPH reagent. The TLC plate was observed in visible light. As shown in (Figure 3), the samples producing yellowish bands on the red background were considered as antioxidants. Usually, the purple background color was visualized after spraying the plate with DPPH reagent. The background color on the plate changed from purple to red after 12 hr in darkness. The red background makes the yellowish bands clearly visible, which benefits the next image processing.
Microscopic examination of RBC viability and morphological changes after induced H2O2 treatment
Erythrocytes are considered as major target for the free radicals owing to the presence of both high membrane concentration of Polyunsaturated Fatty Acids (PUFA) and the oxygen transport associated with redox active hemoglobin molecules, which are potent promoters of activated oxygen species. The extent of haemolysis was found to be much greater, when red blood cells were treated with hydrogen peroxide (toxicant).This could be attributed to the oxidizing nature of hydrogen peroxide with respect to the destruction of cell membrane and subsequent liberation of hemoglobin from the cells. Due to these cells viability decreases, which were observed under the microscopic examination? The results were shown in the (Figure 4 and Figure 5).

Figure 4: Confirmation of RBC pellets stabilization of bioactive band
(metabolites) from TLC plates.
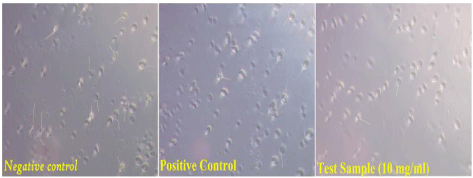
Figure 5: Microscopic examination of antihaemolytic test (A. In negative
control maximum disrupted and dead cell were observed. B. Positive control
(Standard Quericitin) less disrupted cells were observed. C. Test sample (B. lanzan) less disrupted cells was observed).
LC-HRMS analysis of bioactive fraction
The LC-HRMS characterization results in chromatogram profile (Figure 6) show ~9 high prominent peaks in bioactive fraction from B. lanzan. The LC-HRMS analysis results were resolved with chromatograms along with their mass characterization compared with database results in (Figure 6 and Table 3). Analyzing the complete report, it was found that Peaks A,D,E,F,G represents C16 Sphinganine, Phytosphingosine, Leukotriene F4, Doxapram, Dihdrospingosine, Cuscohygrine respectively. Peak D represents Phytosphingosine which is a sphingolipid metabolites, such as sphingosine and ceramide, are highly bioactive compounds and are involved in diverse cell processes, including cell-cell interaction, cell proliferation, differentiation, and apoptosis [23]. In same analysis report the peaks, i.e. B, C and I remain unidentified.
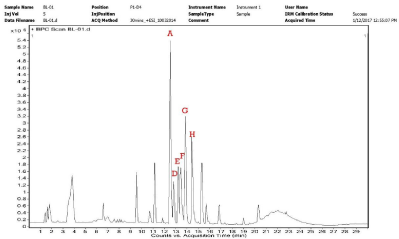
Figure 6: Chromatogram of selected peaks from purified TLC compound of
B. lanzan of LC-HRMS analysis.
Discussion
Phytochemicals present in medicinal plants are normally nontoxic and have the potential of preventing diseases. The multiple properties of these photochemical have made them more attractive, as they can modulate and variety of disease conditions. Plant ingredients are an important source of natural products, which differ broadly in their structures, biological properties and mechanism of action. Phytochemical screening provides basic information about medicinal importance to a plant extract. In this study evaluation for qualitative nature of the chemical constituents of B. lanzan extracts showed the presence of secondary metabolites. In the present study, phytochemical analysis of Soxhlet Methanolic Leaves Extract (SMLE) of B. lanzan revealed the presence of bioactive constituents such as Alkaloid, Carbohydrate, Phenol, Tannin, Saponins and Glycosides. Different phytochemical components especially polyphenols, flavonoids; phenolic acids are responsible for the free-radical scavenging and antioxidant activity of the plants.
Antioxidant potential of plant is directly related to the scavenging of hydroxyl radical and consequently, the inhibition of lipid peroxidation [24]. Generation of lipid hydro-peroxides can cause damage to every molecule of the biological system and have the capacity to bind with DNA causing strand breaks, carcinogenesis and mutation [25]. The erythrocytes intrinsically are more prone to peroxidation due to the heavy accumulation of polyunsaturated fatty acids and hemoglobin. During respiration, erythrocytes are continuously exposed to high tension of oxygen, which can induce oxidative damage [26]. Further, exposure of erythrocytes to toxicants leads to the generation of free radicals resulting in potential damages to the membranes and consequently hemolysis [27]. All these factors together cause destabilization of cell membrane, which is probably the key event of the lysis of the cell. Lipid peroxidation is regarded as one of the primary rationales in cellular damage. There were no previous reports on anti haemolytic activity and antioxidant activity of B. lanzan leaves. Hence in the experimental condition as red blood cells were treated with extracts along with hydrogen peroxide marked reduction in haemolysis was found. This may be because of radical scavenging activity of the bioactive components presents in the extracts showing potent anti haemolytic nature of the extracts [28-30]. Extract and fractions used in this experiment exhibit the scavenging of hydroxyl radicals suggesting the presence of primary antioxidants, which possess anti hemolytic as well as anti-lipid peroxidation potential.
In the present study, the LC-HRMS characterization of B. lanzan is the first report of presence of isolated compounds such as C16Sphinganine, Phytosphingosine, Leukotriene F4, Doxapram, Dihydrospingosine, Cuscohygrine respectively. Compound leukotriene, F4 (LTF4) and LTF4 sulfone have been synthesized and their biological activities determined in the Guinea pig showed antiinflammatory nature [31] Cuscohygrine is the important examples of pyrrolidine alkaloids, and a lot of research is being carried out on this compound, and it has shown to possess exceptional antibacterial, antifungal and antitubercular properties [32]. Polyphenol of medicinal plants possesses antioxidant activity because the hydroxyl groups and protects more efficiently against some free radicalrelated diseases [33]. B. lanzan plant is rich in polyphenol and total flavonoids. The free-radical attack on erythrocyte membrane induces lipid peroxidation leading to hemolysis. Hemolysis has a long history of use in measuring free-radical damage and its inhibition by antioxidants [13]. B. lanzan extracts showed strong antioxidant activity along with antihaemolytic activity (Figure 7). The extract can neutralize the free radicals liberated in cell, due to its antioxidant activity, supposed to protect the erythrocyte membrane from damage, thus prevent hemolysis. Therefore, both the activities can be linked to the presence of phytochemicals viz. polyphenols and flavonoids [34,35], seems to have the exacerbation of the van der Waals contacts inside the lipid bilayer, influencing on the structural stability to the erythrocyte membrane [36].
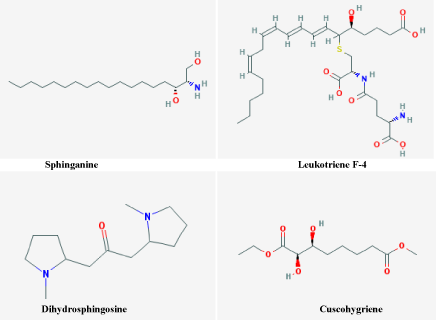
Figure 7: Most probable compounds identified as antihaemolytic and
antioxidant (A) C16Sphinganine; Dihydrosphingosine (B) Phytosphingosine
(C) Leukotriene F4 (D) Cuscohygrine of LC-HRMS results.
Conclusion
Strong antioxidant capacity and antihaemolytic potential of the extract and the purified band of B. lanzan has been assessed in vitro bio assays. Thus the results confirm its therapeutic application in traditional medicine. However further studies by in vivo, models are still needed to confirm the medicinal potential of plant.
Acknowledgement
The authors would like to Department of Biotechnology, Sant Gadge Baba Amravati University, Amravati (M.S) India for providing the necessary research facilities. Authors would also like to thank Mr. Ankit Kale and Mr. Gaurav Ajane for their help in experimental setup and corrections in manuscript. The partial structure elucidations of these bioactive compounds were performed by LC-HRMS by SAIF, IIT, Mumbai, MS, India.
References
- Beris H. Antioxidant affects a basis of drug selection. Drugs. 1991; 42: 569- 605.
- Marnett L. Oxyradicals and DNA damage. Carcinogenesis.2000; 21: 361- 370.
- Hamidi H, Taferzadeh H. Carrier erythrocytes: an overview. Drug Delivery. 2003; 10: 9-20.
- KO, Hsiao F, Kuo F. Protection of oxidative haemolysis by demethyl dineisoeugenol in normal and beta –thalassemia red blood cells. Free Radic Biol Med. 1997; 22: 215-222.
- Djeridane A, Yous M, Nadjemi B, Vidal N, Lesgards J, Stocker P. Screening of some Algerian medicinal plants for the phenolic compounds and their antioxidant activity. European Food Res Technol. 2007; 224: 801-809.
- Kumar S, Pandey A. Chemistry and Biological Activities of Flavonoids: An Overview. SCI World J. 2013; 1-16.
- Yu L. Free radical scavenging properties of conjugated linoleic acids. J Agric Food Chem. 2001; 49: 3452-3546.
- Ebrahimzadeh M, Nabavi S, Nabavi S, Eslami B. Antihemolytic and antioxidant activities of Allium paradoxum. Cent Eur J Biol. 2010; 5: 338-345.
- Djeridane A, Yousfi M, Nadjemi B, Vidal N, Lesgards JF, Stocker P. Screening of some Algerian medicinal plants for the phenolic compounds and their antioxidant activity. Eur Food Res Technol. 2006; 224: 801-809.
- Ebrahimzadeh M, Nabavi S, Eslami B, Nabavi S. Antioxidant and antihaemolytic potentials of Physosperum cornubiense (L.) DC. Pharmacology online. 2009; 3: 394-403.
- Herborne JB. Phytochemical Methods, Chapman and Hall. London.1973; 110-113.
- Khalili M. Antihaemolytic activity of herbal extracts in mouse RBCs. ArhHigRadaToksikol. 2014; 65: 399-406.
- Ramchoun M, Sellam K, Harnafi H, Alem C, Benlyas M, Khallouki F, et al. Investigation of antioxidant and antihemolytic properties of Thymus satureioides collected from Tafilalet Region, south-east of Morocco. Asian Pac J Trop Biomed. 2015; 5: 93-100.
- Yokozawa T, Chen C, Dong E, Tanaka T, Nonaka G, Nishioka I. Study on the inhibitory effect of tannins and flavonoids against the 1,1-diphenyl-2 picrylhydrazyl radical. Biochem Pharmacol. 1998; 56:213-222.
- Jasprica I, Bojic M, Mornar A, Besic E, Lucan K, Medic-Saric M. Evaluation of antioxidative activity of Croatian propolis samples using DPPH? and ABTS?+ stable free radical assays. Molecules. 2007; 5: 1006–1021.
- Zhao J, Zhang J, Yang B, Lv G, Li S. Free radical scavenging activity and characterization of sesquiterpenoids in four species of curcuma using a TLC bioautography assay and GC-MS analysis. Molecules. 2010; 11: 7547-7557.
- Kusznierewicz B, Piekarska A, Mrugalska B, Konieczka P; Namiesnik J, Bartoszek A. Phenolic composition and antioxidant properties of polish blue-berried honeysuckle genotypes by HPLC-DAD-MS, HPLC post column derivatization with ABTS or FC, and TLC with DPPH visualization. J Agric Food Chem. 2012; 7: 1755–1763.
- Siddiqui M, Choudhary A, Prasad N, Thomas M. Buchanania lanzan: a species of enormous potentials. World J Pharma Sci. 2014; 2: 374-379.
- Kapoor S, Mukherjee S, Jaiprakash B. Preliminary phytochemical investigation on leaves of Buchanania lanzan (Chironji). Int J PharmaSci Review and Res. 2010; 3: 2.
- Shabbir M, Khan M, Saeed N. Assessment of phytochemicals, antioxidant, anti-lipid peroxidation and anti-hemolytic activity of extract and various fractions of Maytenus royleanus leaves. BMC Complement Altern Med. 2013; 13: 143.
- McDonald S, Prenzler P, Antolovish M, Robards K. Phenolic content and antioxidant activity of olive extract. Food Chemistry. 2001; 73: 73-84.
- Mehra M, Pasricha V, Gupta R. Estimation of nutritional, phytochemical and antioxidant activity of seeds of muskmelon (Cucumis melo) and watermelon (Citrullus lanatus) and nutritional analysis of their respective oil. J Pharmacognosy and Phytochem. 2015; 3: 98-102.
- Friedman A, Kan C, Ehleiter D, Persaud R, McLoughlin M, Fuks Z, et al. Ionizing radiation acts on cellular membranes to generate ceramide and initiate apoptosis. J Exp Med. 1994; 180: 525-535.
- Orabi K, Al-Qasoumi S, EI-Qlemy M, Mossa J, Muhammad I. Dihydroagarofuran alkaloid and triterpenes from Maytenus heterophylla and Maytenus arbutifolia. Phytochem. 2001; 58: 475–480.
- Sharififar F, Dehghan-nudeh G, Mirtajaldini M. Major flavonoids with antioxidant activity from Teucrium polium L. Food Chem. 2009; 112: 885–888.
- Babu B, Shylesh B, Padikkala J. Antioxidant and hepatoprotective effect of Alanthusicicifocus. Fitoterapia. 2001; 72: 272–277.
- Chakraborty D, Verma R. Ameliorative effect of Emblica officinalis aqueous extract against ochratoxin – induced lipid peroxidation in the kidney and liver of mice. Int J Occup Med Environ Health. 2010; 23: 1–11.
- Thephinlap C, Pangjit K, Maitree S, Srichairatanakoo S. Anti-oxidant properties and anti-hemolytic activity of Psidium guajava, Pandanous odorus and Rhinacanthus nasutus. J Medicinal Plants Res. 2013; 7: 2001-2009.
- Avaligbe C, Joachim D, Kpoviessi D, Accrombessi G, Moudachirou M, Gbeassor M. Antihemolytic Properties of Extracts of Six Plants Used in the Traditional Treatment of Sickle Cell Disease in Benin. J Applied Pharma Sci. 2012; 2: 08-13.
- Ramchoun M, Sellam K, Harnafi H, Alem C, Benlyas M, Khallouki F, et al. Investigation of antioxidant and antihemolytic properties of Thymus satureioides collected from Tafilalet Region, south-east of Morocco. Asian Pac J Trop Biomed. 2015; 5: 93-100.
- Denis D, Charleson S, Rackham A, Jones TR, Ford-Hutchinson AW, Lord A, et al. Synthesis and Biological Activities of Leukotriene F4 and Leukotriene F4 Sulfone. Prostaglandins. 1982; 24: 801-814.
- Kaur R, Arora S. Alkaloids-important therapeutic secondary metabolites of plant origin review article. J Critical Review. 2015; 2: 2394-5125.
- Vaya J, Mahmood S, Goldblum A, Aviram M, Volkova N, Shaalan A, et al. Inhibition of LDL oxidation by flavonoids in relation to their structure and calculated enthalpy. Phytochemistry. 2003; 62: 89-99.
- Yanagimoto K, OchiH, Lee KG, Shibamoto T. Antioxidative activities of fractions obtained from brewed coffee. J Agric Food Chem. 2004, 52: 592– 596.
- Zhu QY, Hackman RM, Ensunsa JL, Holt RR, Keen CL. Antioxidant activities of oolong tea. J Agric Food Chem. 2002, 50; 6929–6934.
- de Freitas MV, Netto RM, da Costa Huss JC, de Souza TM, Costa JO, Firmino CB, et al. Influence of aqueous crude extracts of medicinal plants on the osmotic stability of human erythrocytes. Toxicol In Vitro. 2008, 22: 219–224.