
Research Article
Austin J Biotechnol Bioeng. 2017; 4(3): 1082.
Synthesis and Antimicrobial Properties of Chitosan: A Case Study
Chauhan H¹, Dave G¹, Patel R¹, Shah M1,2*, Bishnoi A1,3 and Rai S1,4
¹Department of Polymer & Rubber Technology, Shroff S. R. Rotary Institute of Chemical Technology, India
²Department of Microbiology, Enviro Technology Limited, India
³Department of Chemistry, Indian Institute of Technology Hauz Khas, India
4GRP Limited, India
*Corresponding author: Maulin Shah, Department of Polymer & Rubber Technology, Shroff S. R. Rotary Institute of Chemical Technology Block no: 402, At: Vataria, Ankleshwar-Valia road, Ta: Valia, Dist: Bharuch, Gujarat, India
Received: June 19, 2017; Accepted: October 30, 2017; Published: November 06, 2017
Abstract
In the present study, an attempt was made to synthesis of chitosan from chitin extracted from crab shells. Chitosan is the second most abundant natural polymer. The different methods such as deproteinization, demineralization, and deacetylation respectively were used in synthesis of chitosan from chitin. Antimicrobial activity was studied and it was found that Chitosan is extremely good in inhibiting the growth of microorganisms; confirmed by the results obtained from experiments. In order to evaluate antimicrobial activity, the serial dilution method was used towards Escherichia Coli, Staphylococcus aureus, Bacillus subtillis.
Keywords: Chitin; Chitosan; FTIR spectroscopy; Biodegradability; Microbial activity
Introduction
Chitosan is a natural polymer and has numerous applications in biomedical and other industries. Due to high biodegradability and non-toxicity it has opened up many fields of application [1]. Studies have shown that it also has very good anti-microbial properties thus it can be widely-used as an antimicrobial agent either alone or blended with other natural polymers. Chitosan is consists of β-(1-4)-linked D-glucosamine and N-acetyl-D-glucosamine randomly distributed within the polymer (Figure 1). Chitosan is cationic in nature, while most of polysaccharides are either neutral or negatively charged in an acidic environment. This property of chitosan allows forming electrostatic complexes or multilayer structures with other negatively charged natural or synthetic polymers [2]. Chitosan is also having various biological properties, such as antitumor [3], antimicrobial [4,5] and antioxidant [6] activities. The degree of de-acetylation (Molar fraction of de-acetylated units or % de-acetylation) and the molecular weight of chitosan, significantly affect its biological properties [7]. Chitosan finds numerous biological and biomedical applications such as in water treatment [8] pharmaceutical excipient or drug carrier [9], and as a scaffold for tissue engineering [2,10]. Waste (crab, prawn shell) is generated from the seafood handling plant and the waste is directly dumps into the landfills and this have made a serious administration and natural issues. The production of chitosan from crab/prawn shells obtained from seafood handling plant is economically viable and environment friendly way to overcome this problem. The present work reports preparation of Chitosan using crab exoskeletons and subsequently this lab made Chitosan was evaluated for its antimicrobial properties against eleven most commonly found microorganism. In this study, we prepared a series of chitosan solution and checked them for antimicrobial activity against Escherichia Coli, Staphylococcus aureus, Bacillus subtillis [7]. Our main goal of this work is to combine the antimicrobial activity of chitosan in order to design biocompatible antimicrobial biomaterials.
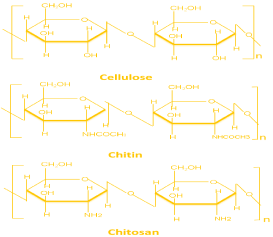
Figure 1: Chemical structures of cellulose, chitin, and chitosan [11].
Materials and Methods
Sample collection and preparation
The crab exoskeletons were obtained from local fish market (Bharuch). The crab exoskeletons were washed continuously with water. And they were sun dried for two days in order to remove moisture present in the crab shell. After which shells were crushed into small pieces and again oven dried for 4-5 hours at 100°C. The small pieces were then gridded into powder and stored in zip-lock bag.
Synthesis
Initially crushed exoskeletons were heated in an oven at 100°C for 30 minutes. Sample was then taken in 500 ml beaker and 100 ml of (4%) NaOH solution was added into it. The solution was heated with the help of a hot plate at 60-70°C for 1 hour. And it was allowed to cool for 1 hour. Solution was then filtered and the washed with demineralise water. Then it was soaked in 1% HCl solution for 20 hours. After filtering the solution, the residue was washed with demineralised water and the process was followed by deacetylation (Figures 2 and 3).
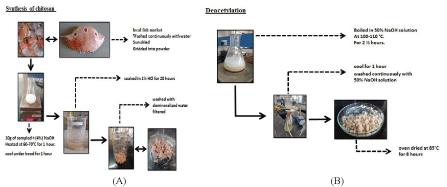
Figure 2: (A,B): Pictorial representation of Synthesis of Chitosan.
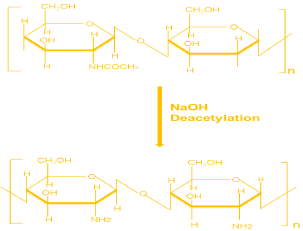
Figure 3: Chemical Reaction to synthesize Chitosan from Chitin [11].
The residue was boiled in 50% NaOH solution at 100-110°C and 70-80 RPM with the help of hot plate magnetic stirrer for 3 hours [11]. Then the sample was allowed to cool for 1 hour. Then the sample was washed continuously with 50% NaOH solution. The chitosan obtained by above method was of pale white colour. After which it was oven dried at 85°C for 8 hours [12-14].
Characterization of chitosan
Chitosan was characterized with help of Fourier Transform Infrared spectroscopy and Nitrogen content. Experiments were carried out at Ankleshwar Research and Analytical Infrastructure Ltd., Ankleshwar.
Antimicrobial activity of chitosan
Test organisms: The bacteria tested used (Staphylococcus auresus, E. Coli, Bacillus subtillis) in this study were obtained from Microbiology Lab of Enviro Technology Limited, Ankleshwar, India. All the cultures were incubated at 37°C and all were activated by incubation for 24 hours in nutrient broth (Hi-Media, India). Staphylococcus aureus is a gram-positive bacterium that is commonly associated with a food product as a result of human treatment. Escherichia coli is a gram-negative bacterium and has been selected based on its infectious characteristics than other genera. The choice of Bacillus subtillis (gram-positive bacterium) was made because it is frequently isolated and bearing in mind the increased resistance of Gram-positive bacteria to the bactericidal action of chitosan compared to Gram-negative ones excluding E. coli.
Antibacterial activity determination: Bacterial Cultures were emulsified in normal saline solution and turbidity was observed and matched with 0.5 McFarland opacity standards. The agar cup method [15] was followed to examine the antibacterial activity of the extracts. 0.1 ml broth culture of the test organisms were strongly seeded over the Mueller-Hinton agar plates. Wells of 6 mm diameter were punched over the agar plates with a sterile cork drill. The floors of the wells were formed by casting 50-100 μL of molten muller-hilton in the broken wells. With a micropipette, solution of chitosan was added to various wells in the plate. These plates were then kept at low temperature (4°C) for exactly 3 hours and then incubated at 37°C for 24 hours. After the incubation period, formation of zones around the well, confirms the antibacterial activity of the respective extracts. All results were compared with the standard antibiotic slice.
Results and Discussion
Characterization of chitosan
FTIR study of chitosan was performed to characterise the chemical structure. FTIR spectrums of chitosan are shown in (Figure 4). In this spectrum, a band of 3467 cm-1 was obtained which corresponds to the peak of OH overlapped on N-H (strong). A 2923 cm-1 peak of C-H group was obtained. A 1578 cm-1 peak of N-H was obtained. A 1073 cm-1 peak of C-O-C was obtained.
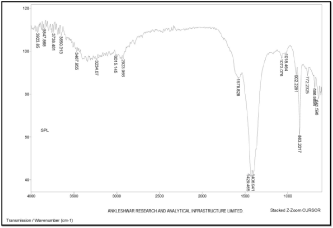
Figure 4: FTIR spectrum of Chitosan.
Nitrogen content
Nitrogen content of the prepared chitin as well as chitosan was measured. Nitrogen content of chitin was found to be 7.96 and that of chitosan was 8.06.
Antimicrobial activity of chitosan
As per research reports available, the anti-microbial potential of chitin, chitosan and their derivatives were considered to be a bacteriocidal (kills the live bacteria or some fraction therein) or bacteriostatic (hinders the growth of bacteria but does not imply whether or not bacteria are killed). Several studies show that the effect of Degree of Acetylation and molecular weight is significant on the anti-microbial properties [5]. The effect of the required chitosan was evaluated against Staphylococcus aureus, Bacillus subtillis and Escherichia coli and the results are shown in (Table 1) S. aureus can cause skin infections such as pimples, impetigo, boiling, cellulitis folliculitis, carbuncles, scalded skin syndrome, and abscesses, lifethreatening diseases such as Pneumonia, meningitis, osteomyelitis, endocarditis, toxic shock syndrome, bacteremia and sepsis. It is still one of the five most common hospital infections [16]. Escherichia coli is one of the most common causes of numerous common bacterial infections including cholecystitis, bacteria, cholangitis, urinary tract infection and traveler’s diarrhea as well as other clinical infections such as neonatal meningitis and pneumonia [17]. The most common inhibition zone against S. aureus, B. subtillis was observed at 12 mm and 14 mm at 800 and 1000 ppm/well. Many hypotheses have been proposed to determine the mechanism of antibacterial activity of chitosan. Chitosan contains three types of reactive functional groups, an amino / acetamido group, both primary and secondary hydroxyl groups, respectively, in C-2, C-3 and C-6 positions. Amine content is the main reason for differences in their structures and physicochemical properties and their correlation with their chelation, flocculation and biological functions [15]. They are designed with three models that are the most beneficial interactions with the positively charged chitosan molecule and the negatively charged microbe cell membranes. In this model, the interaction is mediated by the protonated NH3+ and negative groups of electrostatic forces [18] probably compete with Ca2+ electroegativní sites on the surface of the membrane [19]. Electrostatic interaction results in two interventions: (i) supporting permeability changes in the properties of the membrane wall, resulting in internal osmotic imbalance and hence reducing microorganism growth and (ii) hydrolysis in the peptidoglycan wall to microorganisms that result in leakage intracellular electrolytes, Such as potassium ions, and other proteins with low molecular weight components [20] Dislodging produces ions and water flow that causes a decrease in internal pressure bacteria [21]. The visual enhancement effective film lysis has also been reported against gram- negative and gram-positive bacteria [22] Given that this mechanism is based on electrostatic interaction suggest that a higher amount of amines katonizovanÝch, the greater is the antibacterial activity [23].
Concentraion (µg/mL)
100
200
500
800
1000
Zone of inhibition in diameter (mm)
Test Organisms
E.coli
8
9
10
12
13
S. aureus
7
10
10
12
14
B. subtillis
8
10
11
12
14
Table 1: Antimicrobial activity and zone of inhibition of Chitosan (mm).
Conclusion
This study revealed that crab chitosan could be used to inhibit S. aureus, Bacillus subtillis and E. coli in food products involving human handling where there is possibility of contamination of S. aureus, Bacillus subtillis and E. coli. Hence, we suggest that the chitosan matrix will be a promising material for biomedical as well as food science applications.
References
- Rinaudo M. Chitin and chitosan: Properties and applications. Prog Polym Sci. 2006; 31: 603-632.
- Venkatesan J, Kim SK. Chitosan composites for bone tissue engineering-an overview. Mar Drugs. 2010; 8: 2252-2266.
- Karagozlu MZ, Kim SK. Chapter Twelve-Anticancer effects of chitin and chitosan derivatives. In Advances in Food and Nutrition Research. Academic Press: Waltham MA. USA. 2014; 72: 215-225.
- Martins AF, Facchi SP, Follmann HD, Pereira AG, Rubira AF, Muniz EC. Antimicrobial activity of chitosan derivatives containing N-quaternized moieties in its backbone: a review. Int J Mol Sci. 2014; 15: 20800-20832.
- Rejane C, Goy, Britto, Douglas de, Assis, Odilio BG. A review of the antimicrobial activity of chitosan. Polímeros São Carlos. 2009;19: 241-247.
- Ngo DH, Kim SK. Chapter Two-Antioxidant effects of chitin, chitosan, and their derivatives. In Advances in Food and Nutrition Research. Academic Press: Waltham MA. USA. 2014; 73: 15-31.
- Bagheri-Khoulenjani S, Taghizadeh S, Mirzadeh H. An investigation on the short-term biodegradability of chitosan with various molecular weights and degrees of deacetylation. Carbohydr Polym. 2009; 78: 773-778.
- Onsosyen E, Skaugrud O. Metal recovery using chitosan. J Chem Technol Biotechnol. 1990; 49: 395-404.
- Felt O, Buri P, Gurny R. Chitosan: A unique polysaccharide for drug delivery. Drug Dev Ind Pharm. 1998; 24: 979-993.
- Zhang Y, Zhang M. Three-dimensional macroporous calcium phosphate bioceramics with nested chitosan sponges for load-bearing bone implants. J Biomed Mater Res. 2002; 61: 1-8.
- Sandeep RK, Kumar S, Mistry J, Singh N, Rai A. Chitosan: Chemistry, Applications & Economics of Production-A Review. International Journal of Analytical and applied chemistry. 2016; 2: 23-34.
- Younes I, Rinaudo M. Chitin and Chitosan Preparation from Marine Sources. Structure, Properties and Applications. Marine Drugs. 2015: 13: 1133-1174.
- Yuan Y, Chesnutt BM, Haggard WO, Bumgardner JD. Deacetylation of Chitosan: Material Characterization and in vitro Evaluation via Albumin Adsorption and Pre-Osteoblastic Cell Cultures. Materials. 2011; 4: 1399- 1416.
- Paul S, Jayan A, Sasikumar CS, Cherian SM. Extraction and purification of chitosan from chitin isolated from Sea prawn (fenneropenaeusindicus). 2014; 7: 0974-2441.
- Barry AL. Procedure for testing antimicrobial agents in agar media. In: Antibiotics in laboratory medicine. Williams Wilkins Co. Baltimore. USA. 1980; 1-23.
- Luytmans J, Van Belkum A and Verbrugh H. Clin Microbiol Rev. 1997; 10: 505-520.
- Kappeli U, Hachler H, Giezendanner N, Beutin L and Stephan R. Emerg Infect Dis. Human Infections with Non-O157 Shiga Toxin–producing Escherichia coli, Switzerland, 2000–2009. 2011; 17:180-185.
- Amiri A, Zardini HZ, Shanbedi M, Maghrebi M, Baniadam M, Tolueinia B. Efficient method for functionalization of carbon nanotubes by lysine and improved antimicrobial activity and water-dispersion. Mat L. 2012; 72: 153- 156.
- Aslan S, Deneufchatel M, Hashmi S, Li N, Pfefferle LD, Elimelech M, Pauthe E, et al. Carbon nanotube-based antimicrobial biomaterials formed via layerby- layer assembly with polypeptides. JCIS. 2012; 388: 268-273.
- Aziz MA, Cabral JD, Brooks HJ, Moratti SC, Hanton, LR. Antimicrobial properties of a chitosan dextran-based hydrogel for surgical use. Antimicrob Agents Chemother. 2012; 56: 280-287.
- Reinheimer JA, Demkov MR, Condioti MC. Inhibition of coliform bacteria by lactic cultures. Aust J Dairy Technol. 1990; 45: 5-9.
- National Committee for Clinical Laboratory Standards (NCCLS). Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically. National Committee for Clinical Laboratory Standards (NCCLS). Villanova Pa USA. 2006; 26.
- Hernandez D, Cardell E, Zarate V. Antimicrobial activity of lactic acid bacteria isolated from Tenerife cheese: Initial characterization of plantaricin TF711, a bacteriocin-like substance produced by Lactobacillus plantarumTF711. Journal of Applied Microbiology. 2005; 99: 77-84.