
Special Article - Biomaterials and Regenerative Medicine
Austin J Biotechnol Bioeng. 2017; 4(3): 1083
Akermanite Reinforced Wollastonteas Bioactive Ceramic Biomaterial
Eltohamy M1-3*, Hamzawy E2, Kundu B1 and Azooz M2
¹Institute of Tissue Regeneration Engineering (ITREN), Dankook University, Republic of Korea
²Department of Glass Research, National Research Centre, Egypt
³Department of Nanobiomedical Science, Dankook University, Republic of Korea
*Corresponding author: Eltohamy M, Department of Glass Research, National Research Centre, Dokki, Cairo 12622, Egypt
Received: June 22, 2017; Accepted: October 30, 2017; Published: November 06, 2017
Abstract
Despite substantial amount of research on ceramic biomaterials, the re-cycling of cement kiln dust is still a problem. Additionally, the quest of development of low cost, adequate amount of bioactive bone ceramic biomaterials in ease hastens the research focus to recycle the cement kiln dust for biomedical applications. Cement kiln dust is modified by addition of talc quarry and quartz fine sand to obtain the well-defined SiO2-MgO-CaO system. The glass ceramic is generated by melting all components and casting; followed by heat-treatment (1000 ºC) to prompt crystallization in presence of TiO2 or CaF2 served as nucleating agents to accelerate the crystallization. Akermanitewollastonite phases are created, which results hydroxyl-carbonate-apatite layer formation in stimulated body fluid. The akermanite-wollastoniteceramic exhibits microhardness of 440-680 Kg/mm2 with sustain degradability. The presence of Mg in akermanite is well known for bone regeneration performance; suggesting the promising candidature of the synthesized ceramic as bone implant in future.
Keywords: Cement kiln dust; Glass-ceramic; Akermanite; Wollastonite
Abbreviations
CKD: Cement Kiln Dust; [Ca2Mg (Si2O7)]: Akermanite; [CaSiO3]: Wollastonite; CaF2: Calcium Fluoride; TiO2: Titanium Oxide
Introduction
The disposal of Cement Kiln Dust (CKD) during Portland cement production is considered as significant financial loss to cement industries as well as environmental threatening that is ever increasing; approximately 30 million tons worldwide per year [1]. In reality, this jeopardize the production of Portland cement and non-recycling of by-products like CKD results resource depletion of many beneficial key ions. Enormous body of literatures represent the recycling of CKD as soil modifiers [2,3], in road construction [4,5] and solidification of hazardous wastes [6,7].
A class of Ca, Si and Mg containing bioceramic, gaining immense technological attention as promising bone scaffold, which stimulates apatite mineralization, osteoinduction [8] and osteo-differentiation of diverse cell types including human aortic endothelial cells [8], periodontal ligament cells [9] and bone marrow stem cells [10]. Alkermanite is of desirable ceramic in biomedical bone implant [8]; however, high contents of alkalis and chlorides in CKD [11] restricts the processing of akermanite from it. In addition, no single study exists that embraces the sustainable development of akermanitelike translational medicinal product by recycling CKD. Therefore, we hereby propose a facile, low-environmental impact, sustainable cost-effective method to obtain stable akermanite. Though, because of its brittleness, akermanite scaffolds alone are commonly referred as ‘poorly mechanically stable” in literature [12]. Therefore, to encompass the favorable osteogenic properties of akermanite, we utilize the reinforcement strategy that stabilizes akermanite [Ca2Mg (Si2O7)] within wollastonite [CaSiO3] phase.
Wollastonite possesses well osteoconductivity and bioresorbability [13], exhibits high bioactivity and fast rate of degradation compared with clinically used bone fillers [14]. The release of Ca2+ and SiO3 from wollastonite forms apatite layer on its surface imparting the bioactivity [15]. The incorporation of akermanite within wollastonite prompts us to investigate the chemical durability of the resultant synthesized material. Furthermore, we establish the relationship between the structure and its thermal - mechanical and bioactivity for biomedical applications.
Materials and Methods
Materials
Cement kiln dust (Domestic company, Egypt), was cleaned in dilute HCl and distilled water (to remove soluble impurities) for several time, dried at 120ºC prior to use. Talc (Hamata, Eastern Desert, Egypt) and Quartz sand (Abu Zenima, Sinai, Egypt) were used as neutral raw materials for glass ceramic preparation. Calcium fluoride (CaF2) and titanium oxide (TiO2) were purchased from Sigma Aldrich (USA) and were used as nucleating agents to promote crystallization of both akermanite and wollastonite phases.
Preparation of Akermanite-Wollastonite ceramic biomaterials
The chemical composition of cement kiln dust, talc and quartz sand used in the present investigation is summarized in Table 1 (all compositions in this study are given in Wt%, unless otherwise stated). Since the amount of nucleating agent in the raw materials were quite low, additional appropriate amounts of CaF2 and TiO2 were added into the batch and mentioned in Table 2. The raw powders were weighed and grinded using conventional ball mills for 24 h to obtain homogenous powders. The ceramic powder batches were then melted in electrical furnace using sealed Pt-crucibles at 1400ºC (melting temperature) for about 4 h to ensure complete homogenization.
Oxide%
Raw Material
CKD
Talc
Quartz
65.60
0.42
0.10
SiO2
16.64
65.17
99.59
K2O
5.42
0.12
0.00
Al2O3
4.68
1.68
0.28
Na2O
3.95
0.34
0.00
Fe2O3
2.73
0.42
0.03
MgO
0.77
31.85
0.00
TiO2
0.20
0.00
0.00
Table 1: Chemical analysis of the starting raw materials (wt. %).
Glass No.
Batch composition %
Raw Materials
Nucleating Agents
CKD
Talc
Quartz
TiO2
Cr2O3
CaF2
GB
62.48
15.03
22.49
0
0
0
GTiO2
6
0
0
GCaF2
0
0
6
Table 2: Glass batches and nucleating agents in (wt. %).
Following that, the melt was cast into pre-heated steel molds and annealed at 500ºC for 4 h to remove thermal residual stresses prior to the nucleation and crystallization step.
Characterization
Differential thermal analysis (DTA, Shimadzu DTG60, Japan) scans of as-cast glass with or without nucleating agents were carried out in order to determine the characteristic glass transition temperatures (Tg) and peak crystallization temperatures (Tp). About 100 mg of powdered sample of grain size 30μm were used in a platinum crucible. Equal amount of Al203 was used as inert reference in a temperature range between 25 to 1300ºC at a heating rate of 10ºC min-1 under N2 gas flow. Data for each run were collected directly from DTA. On the basis of dilatometer and DTA analysis of exothermic and endothermic temperature, nucleation experiments were conducted at 725ºC and holding time of 10h. Following nucleation, the temperature was raised to 1000ºC for 4h for crystal growth followed by air cooling gradually at furnace atmosphere.
Identification of precipitated crystals during the process of crystallization were investigated by X-ray diffraction carried out with a diffractometer (Brukur D8, Germany)adapting Ni-filtered CuKa radiation at 40 kV and 30 mA settings in the range from 20 to 80º at a scanning speed of 0.03º min-1. The phases were identified by comparing the peak positions and intensities with those listed in the JCPDS (Joint Committee on Powder Diffraction Standards).
The in vitro bioactivity of akermanite-wollastonite ceramic materials was studied by its apatite forming ability after immersing the ceramic discs (10mm x 5 mm) in SBF at 37ºC with a surface areato- volume ratio of 0.1 cm-1 in static water bath [16]. After specific period, the disks were gently removed from SBF solutions, rinsed with deionized water and acetone; followed by drying. The surface morphology was characterized by scanning electron microscopy (SEM, JEOL JSM-T20, Hitachi Japan). The infrared spectra of the discs were obtained using a Varian 640-IR Fourier Transform Infrared (FT-IR) spectrometer (Varian, Australia) with a resolution of 4 cm-1 in the range of 4000-400 cm-1.
Chemical durability study was carried out using 1.5 grams of the grains (0.25 mm < diameter < 0.50 mm) accurately weighed within sintered glass crucible of the Jena 1G4 type (average pore diameter is 5-10μm) and placed in a closed polyethylene container contain 200 ml SBF for 2 weeks at 37 ºC under continuous stirring. The percentage of weight loss was taken as measure of relative magnitude of rate of leaching and the released amount of cation was determined.
The microhardness properties of as-cast glass-ceramics crystallized at different temperatures were determined by Vickers and Knoop values using Shimadzu Microhardness Tests of M type under load of 100 g. The empirical relation formulated by Ponton and Rawlings, which utilizes fracture lines emanating from Vickers diamond indentation was used to determine the fracture toughness values [17].
Results and Discussion
Chemical composition of bioceramic materials are crucial for their therapeutic applications. The positive effects of released ions such as calcium, silicon on the formation of apatite layer is well known [18]. Magnesium is the fourth abundant intra-cellular element of human body [19] stimulating neo-bone formation; its depletion results bone resorption and osteoporosis [20]. Wollastonite bioceramic rationally tuned into a and β phase by using Mg content in small amount with varying hardness [13]. Wollastonite exhibits rapid rate of degradation compared to clinically used Ca-phosphate bone implants [14]; therefore, research interest focuses on optimizing wollastonite derivatives with suitable mechanical property. The synergistic effects of Ca and Mg ions facilitate osteogenesis in osteoporotic condition [21]. The released Mg of akermanite weakens the alkaline microenvironment in vivo, which stimulates osteoblastic anabolic effects; results in bone regeneration. Approximately 60% of Mg is stored in bone in human body [22]. Therefore, adulteration of akermanite within wollastonite is hypothesized to be beneficial of its application as biomedical materials for bone tissue engineering.
The chemical compositions of three investigated components including Base Glass (BG), created akermanite and wollastonite are represented in the ternary subsystem (Figure 1). According to the theories of phase equilibria, the assemblage of the thermodynamic stable phases should be the same for the investigated glass-ceramics (i.e., akermanite and wollastonite) [23, 24].
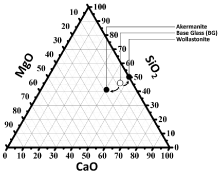
Figure 1: Triangle of CaO-MgO-SiO2 system; akermanite-wollastonite and
starting Base Glass (BG) composition location is clarified.
The well-ordered regular lattice crystal is necessity for withstanding the deformation under load for tissues such as bone. DTA data evidences the crystallization characteristics of glass from less ordered structure (Figure 2a). The glass softening temperature (Ts) was nearly similar to 735-755ºC confirming the viscosity was indifferent by the nucleation doped into the base glass composition. The addition of TiO2 to GTiO2 modified the local glass structures, pushed Ts to 762ºC; while incorporation of CaF2 in GCaF2 decreased it at 735ºC. In comparing the exothermic peaks with the BG at 930ºC, CaF2 decreased it to 877ºC in GCaF2; while TiO2 increased it to 935ºC in GTiO2. Because of, the DTA data highlighted intensive crystallization process around 877-935ºC interval, so crystallization temperature is selected at 1000ºC.
The crystalline phases of the heat-treated glasses at 1000ºC temperature range are showed in (Figure 2b). The resultant CaOMgO- SiO2 glass system exhibited the crystallization phases of wollastonite and akermanite; developed in all glass samples. However the intensity of both phases in the XRD patterns were in the order sample containing-CaF2GTiO2 BG.

Figure 2: (a) DTA and (b) X ray diffraction patterns of the present glasses
heat-treated at 1000ºC.
For understanding the structural characteristics of prepared glass, micro-architecture of glass was visualized under Scanning Electron Microscope (SEM) (Figure 3a). The SEM micrographs of BG (Base Glass) exhibited condensed granular crystals of nano-size along with some irregular micro-size fracture aggregates (Figure 3a). Incorporation of TiO2 increased the crystal growth rate and directed the growth of fibers in GTiO2 sample. The microstructure was irregular aggregates of nano- to micro-sized and dendritic-like growths were developed in high fluorine containing GF2 sample. The surfaces of all composite films after 7 and 14 days incubation in SBF were covered with layers rich in calcium and phosphorus (Figure 3a). The layers exhibited spherical flower-like morphology, typical of carbonated hydroxyapatite [25]. (Figure 3b) represents the FTIR spectra of the ceramic samples in the range 400-4000 cm-1, confirming the bands corresponding to PO4 3-(ν3-1027, ν4-601, ν4-561 cm-1) and CO3 2-(ν2-875 cm-1) respectively. The FTIR spectrum in the ν4 PO4 3- domain exhibited the bands at 607 and 569 cm-1, which are assigned to PO4 3-ions in apatite sites [24]. The ν2 CO3 2- domain exhibited the band at 875 cm-1, which is similar to the characteristic one observed in bone crystals [26] and may improve the bioactivity of HA.
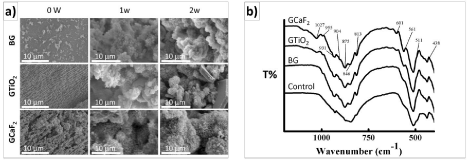
Figure 3: (a) SEM and (b) FTIR of akermanite-wollastonite after soaking in
stimulated body fluid.
Figure 4a shows the weight loss as a function of time for BG, GTiO2 and GCaF2 before and after crystallization. It was noticed that, in general, the glass ceramics were more durable than corresponding glass, however, the chemical durability increased in the order of GCaF2 > GTiO2 > BG. The chemical durability can be explained in terms of composition and structural point of view. In terms of composition, the incorporation of Ca, and Mg to silicate glasses is accepted to increase the chemical durability of the resultant glasses [27,28]. Furthermore, the possible formation of sparingly soluble or precipitates during the progress of the corrosion process (e.g. sparingly soluble or insoluble hydroxides). In case of structure, the glass ceramic form additional structural building units which strengthen the silicate network and hence retard the corrosion process [29-32].
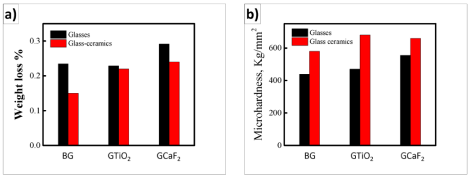
Figure 4: (a) Weight loss of glass and glass ceramic in stimulated body fluid
at 37ºC for 2 weeks under orbital stirring condition; (b) Microhardness before
and after crystallization.
It is worth noticing the microhardness of the base glass and akermanite-wollastonite ceramics for its load bearing tissue engineering applications and were ranged between 440-680 kg/ mm2. The synthesized glass ceramics were more hard than the corresponding base glasses attributed to highly ordered internal network arrangement during crystallization processes; imparting improved mechanical stability of the produced ceramics. Improved mechanical strength with modulating degradability facilitates the applicability of akermanite-wollastonite ceramics in tissue engineering and regenerative therapy.
Conclusion
The substantial need of osteogenic biomaterials are not only confined to high efficacy but also cost-effectiveness, ability to produce in large scale to address the widespread global need and safety. With akerminite-wollastonite composition; a safe, bioactive, clinically applicable composite ceramic is demonstrated which is processed by an eco-friendly green process by recycling cement kiln dust. Results indicate cement kiln dust can be considered as valuable resource for successful making of bioactive glass-ceramic materials with controllable microstructure and microhardness.
Acknowledgment
This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF), Ministry of Education [2014R1A1A2009025] and National Research Centre, Cairo, Egypt.
References
- Dyer T, Halliday J, Dhir R. An investigation of the hydration chemistry of ternary blends containing cement kiln dust. Journal of Materials Science. 1999; 34: 4975-4983.
- Ismail AIM, Belal ZL. Use of Cement Kiln Dust on the Engineering Modification of Soil Materials, Nile Delta, Egypt. Geotechnical and Geological Engineering. 2016; 34: 463-469.
- AlZubaidi RM, AlRawi KH, AlFalahi AJ. Using cement dust to reduce swelling of expansive soil. Geomechanics and Engineering. 2013; 5: 565-574.
- Baghdadi Z, Rahman M. The potential of cement kiln dust for the stabilization of dune sand in highway construction. Building and Environment. 1990; 25: 285-289.
- Freer-Hewish RJ, Ghataora GS, Niazi Y. Stabilization of desert sand with cement kiln dust plus chemical additives in desert road construction. Proceedings of the Institution of Civil Engineers: Transport. 1999; 135: 29-36.
- Park C-K. Hydration and solidification of hazardous wastes containing heavy metals using modified cementitious materials. Cement and Concrete Research. 2000; 30: 429-435.
- Morgan DS, Novoa JI, Halff AH. Oil Sludge Solidification Using Cement Kiln Dust. Journal of Environmental Engineering. 1984; 110: 935-948.
- Wu C, Chang J, Ni S, Wang J. In vitro bioactivity of akermanite ceramics. J Biomed Mater Res A. 2006; 76: 73-80.
- Xia L, Zhang Z, Chen L, Zhang W, Zeng D, Zhang X, et al. Proliferation and osteogenic differentiation of human periodontal ligament cells on akermanite and beta-TCP bioceramics. Eur Cell Mater. 2011; 22: 68-82.
- Huang Y, Jin X, Zhang X, Sun H, Tu J, Tang T, et al. In vitro and in vivo evaluation of akermanite bioceramics for bone regeneration. Biomaterials. 2009; 30: 5041-5048.
- Maslehuddin M, Al-Amoudi O, Rahman MK, Shameem M, Ibrahim M. Use of cement kiln dust in blended cement concretes. Proceedings of the Institution of Civil Engineers-Construction Materials. 2010; 163: 149-156.
- Chen L, Zhai D, Wu C, Chang J. Poly (d, l-lactic)-reinforced akermanite bioceramic scaffolds: preparation and characterization. Ceramics International. 2014; 40: 12765-71275.
- Liu A, Sun M, Shao H, Yang X, Ma C, He D, et al. The outstanding mechanical response and bone regeneration capacity of robocast dilute magnesiumdoped wollastonite scaffolds in critical size bone defects. Journal of Materials Chemistry B. 2016; 4: 3945-3958.
- Reichert JC, Cipitria A, Epari DR, Saifzadeh S, Krishnakanth P, Berner A, et al. A tissue engineering solution for segmental defect regeneration in loadbearing long bones. Sci Transl Med. 2012; 4: 141-193.
- Ni S, Chang J. In vitro degradation, bioactivity, and cytocompatibility of calcium silicate, dimagnesium silicate, and tricalcium phosphate bioceramics. J Biomater Appl. 2009; 24: 139-158.
- Kokubo T, Takadama H. How useful is SBF in predicting in vivo bone bioactivity? Biomaterials. 2006; 27: 2907-2915.
- da Silva Dias AM. Análise Numérica do Processo de Fratura no Ensaio de Indentaç&aTilde;o Vickers em uma Liga de Carboneto de tungstênio com Cobalto. UFMG. 2004.
- Lin Q, Li Y, Lan X, Lu C, Chen Y, Xu Z. The apatite formation ability of CaF2 doping tricalcium silicates in simulated body fluid. Symposium on Biomaterials and Bionanotechnology (SBB), part of the International Conference on Multifunctional Materials and Structures (MFMS2008); 2008 July 28-July 31; Hong Kong. Biomed Mater. 2009; 4: 045005.
- Staiger MP, Pietak AM, Huadmai J, Dias G. Magnesium and its alloys as orthopedic biomaterials: a review. Biomaterials. 2006; 27: 1728-1734.
- Ren N, Li J, Qiu J, Sang Y, Jiang H, Boughton RI, et al. Nanostructured titanate with different metal ions on the surface of metallic titanium: a facile approach for regulation of rBMSCs fate on titanium implants. Small. 2014; 10: 3169-3180.
- Liu W, Wang T, Zhao X, Dan X, Lu WW, Pan H. Akermanite used as an alkaline biodegradable implants for the treatment of osteoporotic bone defect. Bioactive Materials. 2016; 1: 151-159.
- Green J, Kleeman CR. The role of bone in the regulation of systemic acidbase balance. Osaka MH, editor. In: Calcium-Regulating Hormones II. Karger Publishers. 1991; 91: 61-76.
- Huang W, Hillert M, Wang X. Thermodynamic assessment of the CaO-MgOSiO 2 system. Metallurgical and Materials Transactions A. 1995; 26: 2293- 22310.
- Jung I-H, Decterov SA, Pelton AD. Critical thermodynamic evaluation and optimization of the CaO–MgO–SiO2 system. Journal of the European Ceramic Society. 2005; 25: 313-333.
- Li W, Nooeaid P, Roether JA, Schubert DW, Boccaccini AR. Preparation and characterization of vancomycin releasing PHBV coated 45S5 Bioglass®- based glass-ceramic scaffolds for bone tissue engineering. Journal of the European Ceramic Society. 2014; 34: 505-514.
- Padilla S, Izquierdo-Barba I, Vallet-Regí M. High specific surface area in nanometric carbonated hydroxyapatite. Chemistry of Materials. 2008; 20: 5942-5944.
- Hamilton JP, Pantano CG. Effects of glass structure on the corrosion behavior of sodium-aluminosilicate glasses. Journal of non-crystalline solids. 1997; 222: 167-174.
- Bunker BC, Tallant DR, Headley T, Turner G, Kirkpatrick R. The structure of leached sodium borosilicate glass. Physics and Chemistry of Glasses. 1988; 29: 106-120.
- Deng W, Cheng J-S, Tian P-J, Wang M-T. Chemical durability and weathering resistance of canasite based glass and glass-ceramics. Journal of Non- Crystalline Solids. 2012; 358: 2847-2854.
- Kim BH, Kang BA, Yun YH, Hwang KS. Chemical durability of β-wollastonitereinforced glass-ceramics prepared from waste fluorescent glass and calcium carbonate. Materials Science- Poland. 2004; 22: 83-91.
- Szabó I, Nagy B, Völksch G, Höland W. Structure, chemical durability and microhardness of glass-ceramics containing apatite and leucite crystals. Journal of Non-Crystalline Solids. 2000; 272: 191-199.
- Anusavice KJ, Zhang NZ. Chemical durability of Dicor and fluorocanasitebased glass-ceramics. J Dent Res. 1998; 77: 1553-1559.