
Research Article
Austin J Biotechnol Bioeng. 2017; 4(3): 1084.
Comparative Phytochemical Screening and Analysis of Different Vignaspecies in Organic Solvents
Shukla S* and Tyagi B
¹Amity Institute of Biotechnology, Amity University, India
*Corresponding author: Susmita Shukla, Amity Institute of Biotechnology, Amity University, Noida, Uttar Pradesh, India
Received: July 27, 2017; Accepted: December 07, 2017; Published: December 14, 2017
Abstract
Vigna belongs to the family Fabaceae. They are used traditionally as appetizer, diuretic, laxatives, and anthelmintic. This research work provides an insight into the phytochemical components of various Vigna species viza- viz Vigna radiata, Vigna angularis, Vigna mungo, Vigna unguiculata, and Vigna acontifolia. Phytochemical analysis of five different varieties of seeds of Vigna is done and a comparative account is prepared. The comparison is made in order to find out the best possible way to extract the maximum amount of required phytochemicals. This study shows the presence of different phytochemical constituents in seeds of Vigna species. This will provide us a better understanding of the potential applications of Vigna species in drug and cosmetic industry.
Phytochemical constituents that are isolated from Vigna species are flavonoid, steroid, glycoside, terpenoids, phlobatannins, tannins, alkaloid, protein, reducing sugar and coumarin. Phytochemicals were extracted from the sample using three different solvents i.e., methanol, ethanol and chloroform. The study reveals that the extract made by using chloroform showed the best result as the presence of maximum phytochemicals was seen here as compared to the other two extracts.
Keywords: Vigna radiata; Vigna angularis; Vigna mungo; Vigna unguiculata; Vigna acontifolia; Phytochemicals; Methanolic; Diuretic; Laxative; Anthelmintic
Introduction
In recent years, phytochemicals have received a great deal of attention mainly on their role in preventing diseases. Plant food extracts and phytochemicals constitute a variety of compounds that are part of our diet. In recent years, they have also been highlighted as QSI (QSI: Quorum Sensing Inhibitors) [1]. In this context, evaluation of plant phytochemicals becomes fundamental. Phytochemicals are the bioactive compounds that occur naturally in plants. Leguminous seeds are important source of proteins and source of natural antioxidants [2]. Legumes contain a number of phenolic compounds such as flavonoids, phenolic acids, and tannins. There is a considerable interest in finding natural phytochemicals and antioxidants from plants due to their role in the treatment and prevention of various diseases [3].
Characterization of extracts is necessary, due to its numerous benefits to science and society. The information obtained, makes pharmacological studies possible. It leads to the possible synthesis of more potent drug with reduced toxicity. The mode of action of the plants producing effect can also be better investigated if the active ingredients are characterized [4-6]. The use of phytochemicals as natural antimicrobial agents, commonly called biocides is gaining popularity [7]. The most essential of these bioactive constituents of plants are alkaloids, tannins, flavonoids and phenolic compounds.
Vigna is well known for its detoxification activities and is used to refresh mentality, alleviate heat stroke, and reduce swelling in the summer [8-10]. It was recorded to be beneficial in the regulation of gastrointestinal upset and to moisturize the skin [11]. The seeds and sprouts of Vigna are also widely used as a fresh salad vegetable or common food in India and western countries. As a food, because they contain balanced nutrients, including protein and dietary fiber, and significant amounts of bioactive phytochemicals [6,12]. High levels of proteins, amino acids, oligosaccharides, and polyphenols in Vigna species are thought to be the main contributors to the antioxidant, antimicrobial, anti-inflammatory, and antitumor activities of this food and are involved in the regulation of lipid metabolism [13,14]. Flour from the seed is used as a substitute for soap; it makes the skin soft and smooth [15-25]. In traditional medicine, the seed is used for its suppurative, cooling and astringent properties, e.g. pounded and applied as a poultice on abscesses [5]. With increasing clinical evidence suggesting that plant-derived foods have various potential health benefits, their consumption has been growing at the rate of 5%-10% per year [26,27]. Moreover, many worldwide health organizations have recommended an increase in the intake of plantderived foods to improve health status and prevent chronic diseases [8].
Contribution of pulses to agriculture and daily life has been tremendous besides being one of the important constituents of our diet. An important feature of the Vigna species is it has the potential of producing higher yield [25]. Nutrient elements are needed in relatively very small quantities for adequate plant growth and production [9]. An important feature is its ability to establish a symbiotic partnership with specific bacteria, setting up the biological N2-fixation in root nodules that supply the plant’s needs for N2[10].
Hence the present study focuses to investigate on the presence of phytochemicals in ethanolic, methanolic and chloroform extracts of Vigna as therapeutic agents against some human pathogenic diseases.
Materials and Methods
Materials
5 varieties of Vigna seed sample (Vigna radiata, Vigna mungo, Vigna angularis, Vigna aconitifolia, Vigna unguiculata).
Chemicals
Methanol, ethanol, chloroform, conc. sulphuric acid, picric acid, strong base (sodium hydroxide), aqueous copper sulphate, alcoholic sodium hydroxide, Fehling’s solution A, Fehling solution B, 1% aqueous HCL, 10% ferric chloride solution, 10% NaOH, distilled water.
Other requirements
Eppendorfs tube, test tubes, dropper, beakers, motor pestle and other required glassware.
Preparation of the extract
All the 5 varieties of seeds were collected and then sterilized using twin-twenty, sodium hypochloride and mercuric chloride. They were then kept in hot air oven for 6 hrs and dried. They were crushed and then dipped in the solvents i.e., methanol, ethanol and chloroform in the ratio of 1:4. The extract was further used for phytochemical screening.
Tests done for analysis
Test for steroids: 1 ml of the extract was dissolved in 10 ml of the chloroform and equal volume of sulphuric acid was added by the sides of the test tube. If the upper layer turns red and sulphuric acid layer showed yellow with green fluorescence. This indicates the presence of steroids.
Test for alkaloids: 5 ml of extract was added to 2ml of HCL, to this acidic medium 1ml of Dragendroff’s reagent was added. An orange or red precipitate produced immediately indicates the presence of alkaloids.
Biuret test for protein: Aqueous sample treated with an equal volume of 1% strong base (sodium or potassium hydroxide) Followed by few drops of aqueous copper sulphate (II).
Test for coumarin: To 2 ml of test solution, a few drops of alcoholic sodium hydroxide were added. Appearance of yellow colour indicates the presence of coumarin.
Test for reducing sugar: Mix 15ml of Fehling solution A with 15ml of Fehling solution B; add 2 ml of this solution to an empty test tube. Add 3 drops of this compound to be tested, place tube in a water bath at 60°c.
Test for terpenoids: 1ml of extract was placed into the test tube and added 0.4ml of chloroform, 0.6ml of concentrated sulphuric acid was poured gently into the tube at an inclined portion. A reddish brown coloration was indicative of the presence of terpenoids.
Test for phlobatannins: Deposition of a red precipitate when an aqueous extract of each plant sample was boiled with 1% aqueous hydrochloric acid was taken as evidence for the presence of phlobatannins.
Test for glycosides: 1ml of concentrated sulphuric acid gently poured on the walls of an incline test tubes containing 1ml of plant filtrate. Drop wise was added 10% ferric chloride solution and observe for a brown, violet or greenish ring.
Test for flavonoids: 1ml of 10% NaOH was mixed with 1ml of filtrates, shaken vigorously and observes for the development of yellow colouration.
Test for tannins: 1ml of filtrate was added to 1ml of distilled water in a test tube. A few drops of 10% ferric chloride was added and observed for brownish green or blue black colouration.
Results
A variety of phytochemical tests were performed using five different seeds of Vigna species namely Vigna radiata, Vigna mungo, Vigna unguiculata, Vigna aconitifolia and Vigna angularis. These tests revealed the presence of steroid, alkaloid, protein, coumarin, reducing sugar, terpenoids, phlobatannins, glycosides, flavonoids and tannins tests in all the three types of extracts namely ethanolic extract, methanolic extract and extract using chloroform.
In the ethanolic extract all the five varieties showed low presence of steroid, protein, coumarin, reducing sugar, phlobatannins and tannins. Vigna radiata showed maximum positive results in case of alkaloids, terpenoids, and flavonoids. Vigna mungo showed maximum positive results in case of alkaloids, terpenoids, and glycosides. Vigna angularis and Vigna aconitifolia showed maximum positive results in case of alkaloids (Table 1, Figure1).
Sl.No
Phytochemical
V. radiata
V. mungo
V. unguiculata
V. aconitifolia
V. angularis
1
Steroid
-
+
+
++
+
2
Alkaloid
+++
+++
++
+++
+++
3
Protein
+
+
+
+
+
4
Coumarin
++
+
--
-
--
5
Reducing sugar
-
-
-
-
-
6
Terpenoids
++
++
++
++
+
7
Phlobatannins
+
+
-
-
++
8
Glycosides
+
++
+
++
++
9
Flavonoid
++
+
--
--
-
10
Tanins
+
-
-
-
+
Table 1: Phytochemicals analysis in Vigna varieties (ethanol extract of seeds).
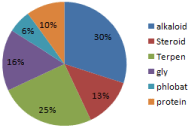
Figure 1: Ethanol extract.
In methanolic extract steroids, proteins, reducing sugar and phalobatannins were in low amount. Vigna radiata showed presence of alkaloids, terpenoids, and flavonoids in maximum amounts. Vigna aconitifolia showed maximum presence of alkaloids, terpenoids, and glycosides. Vigna mungo showed maximum presence of alkaloid, terpenoids and glycosides (Table 2, Figure 2).
Sl.No
Phytochemical
V. radiata
V. mungo
V. unguiculata
V. aconitifolia
V. angularis
1
Steroid
++
+
+
+
++
2
Alkaloid
++
+++
+++
+++
+++
3
Protein
+
+
+
++
+
4
Coumarin
+++
++
--
+++
++
5
Reducing sugar
-
-
-
-
-
6
Terpenoids
+++
+++
+++
+++
+++
7
Phlobatannins
-
-
-
+
+++
8
Glycosides
++
+
++
++
+++
9
Flavonoid
++
+
---
++
+
10
Tanins
++
+++
++
++
++
Table 2: Phytochemical analysis in Vigna varieties (methanol extract of seeds).
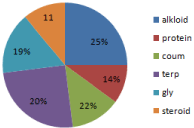
Figure 2: Methanol extract.
But the present study reveals that the extract which was made using the chloroform was more potent as compared to the other two. It showed the maximum phytochemicals in high and very high amounts. All the species showed maximum concentrations of extracted phytochemicals (Table 3, Figure 3).
Sl.No
Phytochemical
V. radiata
V. mungo
V. unguiculata
V. aconitifolia
V. angularis
1
Steroid
+++
+++
+++
+++
+++
2
Alkaloid
+++
+++
+++
+++
+++
3
Protein
++
++
++
++
++
4
Coumarin
+
+
+
+
+
5
Reducing sugar
+
++
++
++
++
6
Terpenoids
+
+
++
+
++
7
Phlobatannins
+
+
-
+
++
8
Glycosides
+
+
+++
+++
+++
9
Flavonoid
+
++
+
+
-
10
Tanins
+++
+++
++
++
+++
Table 3: Phytochemical analysis in Vigna varieties (chloroform extract of seeds).
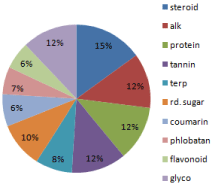
Figure 3: Chloroform extract.
Conclusion
Various species of Vigna are tested for presence or absence of certain phytochemicals, hence a qualitative testing is done and the best results are observed in case of Vigna mungo and Vigna aconitifolia, in contrast to other varieties. They have been tested for tannins, flavonoids, terpenes, steroids, proteins, glycosides and other such compounds. Solvents or extracting agents like ethanol, methanol, and chloroform are used in order to check for the efficiency of extraction. If the used solvents are taken into account, maximum positive results are seen in case when chloroform is taken as solvent. The findings can further be used in drug development, cosmetics, food industry and for other analytical purposes.
The potency of the extract that was made by using chloroform as the extracting agent is compared to the results that of ethanol and methanol extract. According to the pie chart, in ethanol extract the maximum result is observed for alkaloid and further in the terpenoids whereas others are in the fewer amounts. In methanol extract alkaloid, coumarin, terpenoids and glycosides are in good amount but protein is seen in slightly less amount. On the other hand it is seen in the chloroform extract that all steroid, alkaloid, protein, tannin, and terpenoids are in very high amount.
The phytochemicals that are obtained can further be used to make drugs so as to cure the diseases or supplement the deficiency of any nutrient in the body, and find wide range of applications in cosmetics industry.
References
- Anjum NA, Umar S, Iqbal M, Khan NA. Cadmium causes oxidative stress in mung bean by affecting the antioxidant enzyme system and ascorbateglutathione cycle metabolism. Russian J Plant Physiol. 2011; 58: 92-99
- Abd El-Monem AA, Saleh MM, Mostafa EM. Minimizing the quantity of mineral nitrogen fertilizers on grape vine by using humic acid, organic and biofertilizers. Res J Agr Biol Sci. 2008; 4: 46-50
- Abou El-Nour EA. Can supplemented potassium foliar feeding reduce the recommended soil potassium? Pakistan J Biol Sci. 2002; 5: 259-262.
- Babaeian M, Piri I, Tavassoli A, Esmaeilian Y, Gholami H. Effect of water stress and micronutrients (Fe, Zn and Mn) on chlorophyll fluorescence, leaf chlorophyll content and sunflower nutrient uptake in Sistan region. Afr J Agric Res. 2011; 6: 3526-3531.
- Bradl HB. Adsorption of heavy metal ions on soils and soils constituents. J Colloid Interface Sci. 2004; 277: 1-18.
- El-Adawy T, Rahma E, El-Bedawey AA, El-Beltagy AE. Nutritional potential and functional properties of germinated mung bean, pea and lentil seeds. Plant Foods Human Nutrition. 2003; 87: 247-253.
- Edwards CA, Arancon NQ, Greytak S. Effects of vermicompost teas on plant growth and disease. Biocycle. 2006; 47: 28-31.
- Jin WL. Appraisal of quality characters for seeds of Chinese adzuki bean local varieties. J Chin Cereals Oils Assoc. 2006; 21: 50-59.
- Kaya M, Atak M, Khawar KM, Ciftci CY, Ozcan S. Effect of pre-sowing seed treatment with zink and foliar spray humic acids on field of common bean (Phaseolus vulgaris L.). J Agric Biol. 2005; 7: 875-878.
- Lambrides CJGI. Mung bean. Gen Mapp Mol Breed Plants. 2007; 3: 69-90.
- Mahajan A, Gupta RD. Integrated Nutrient Management (INM) in a sustainable rice-wheat cropping system. Springer Netherland. 2009.
- Manners DJ. Enzymic synthesis and degradation of starch and glycogen. Adv Carbohydr Chem. 1963; 17: 371-430.
- Naeem M, Iqbal J, Ahmad M, Haji A, Bakhsh MA. Comparative study of inorganic fertilizers and organic manures on yield and yield components of mung bean (Vignaradiata L.). J Agric Soc Sci. 2006; 2: 227-22
- Ohdan T. Francisco PB, Sawada T, Hirose T, Terao T, Satoh H, et al. Expression profiling of genes involved in starch synthesis in sink and source organs of rice. J Exp Bot. 2005; 56: 3229-3244.
- Pradeep MD, Elamathi S. Effect of foliar application of DAP, micronutrients and NAA on growth and yield of green gram (Vignaradiata L.). Legume Res. 2007; 30: 305-307.
- Piccolo A, Celano G, Pietramellara G. Effects of fractions of coal-derived humic substances on seed germination and growth of seedlings (Lactuca sativa and Lycopersicum esculentum). Biol Fertil Soils. 1993; 16: 11-15.
- Randhir R, Lin YT, Shetty K. Stimulation of phenolics, antioxidant and antimicrobial activities in dark germinated mung bean sprouts in response to peptide and phytochemical elicitors. Process Biochem. 2004; 39: 637-646.
- Rangel A, Domont GB, Pedrosa C, Ferreir ST. Functional properties of purified vicilins from cowpea (Vignaunguiculata) and pea (Pisumsativum) and cowpea protein isolate. J Agric Food Chem. 2003; 51: 5792-5797.
- Roy F, Boye JI, Simpson BK. Bioactive proteins and peptides in pulse crops: pea, chickpea and lentil. Food Research International. 2010; 43: 432-442.
- Stancheva I, Mitova I, Petkova Z. Effects of different nitrogen fertilizer sources on the yield, nitrate content and other physiological parameters in garden beans. Environment Exp Bot. 2004; 52: 277-282.
- Tiwari R, Kumar A. Starch phosphorylase: Biochemical and biotechnological perspectives. Biotechnol Mol Bio Rev. 20125; 7: 69-83.
- Tomooka N. Two new species, new species combinations and sectional designations in Vigna subgenus Ceratotropis (Piper) Verdcourt (Leguminosae, Phaseoleae). Kew Bull. 2002; 57: 613-624.
- Ullah H, Khalil IH, Iltafullah I, Rahman HU, Amin I. Genotype × environment interaction, heritability and selection response for yield and yield contributing traits in mungbean. Afr J Biotechnol. 2011; 10: 475-483.
- Vanamala J, Reddivari L, Yoo KS, Pike LM, Patil BS. Variation in the content of bioactive flavonoids in different brands of orange and grapefruit juices. J Food Comp Anal. 2006; 19: 157-166.
- Kalia VC. Quorum sensing inhibitors: an overview. Biotechnol Adv. 2013; 31: 224-245.
- Smid EJ, Gorris LGM, Rahman MS. New D. Natural antimicrobials for food preservation. 1999.
- Patil US, Deshmukh OS, Ganorkar RP. European journal of pharmaceutical and medical research. EJPMR. 2015; 2: 171-176.