
Review Article
Austin J Biotechnol Bioeng. 2017; 4(4): 1086.
Influence of Coating Developed from Oligomer Isolated from Lac Resin on Post-Harvest Quality and Shelf-Life of Peaches (Prunuspersica L.)
Bishnoi A1,2*, Chawla HM1 and Shah MP3
¹Department of Chemistry, Indian Institute of Technology, India
²Department of Polymer & Rubber Technology, Shrof S. R. Rotary Institute of Chemical Technology, India
³Enviro Technology Limited, India
*Corresponding author: Anjali Bishnoi, Department of Chemistry and Polymer & Rubber Technology, Indian Institute of Technology and Shrof S. R. Rotary Institute of Chemical Technology, HauzKhas, New Delhi and Bharuch, Gujarat, India
Received: July 07, 2017; Accepted: December 19, 2017; Published: December 26, 2017
Abstract
Large quantity of fresh fruits is produced that never reach the consumers due to heavy post-harvest losses, lack of storage, transportation care & less acceptable quality. These losses are not only concerned in terms of revenue but it also concern in terms of health, life style and environment. To full fill consumer demand and environmental, several methods are being utilized to reduce these losses of fruits. Fruit coatings are considered as one of the widely used methods. This work investigates the effect of fruit coating developed from oligomer (P-104), isolated from lac resin on quality of peach (Prunuspersica L.) when stored at room temperature (38±2°C) and at 4°C temperature. Fruit quality was evaluated by measuring physiological weight loss, color and textural changes as well as microbiological evaluation at a regular interval of four days. When kept at room temperature, uncoated peaches remained fresh and microbiologically safe for 4 days only while shelf life of coated peaches increased to 12 days. Again the shelf-life of coated peaches prolonged to 24 days when stored at 4°C. These results showed that combine effect of coating and low storage temperature could improve shelf life of peaches, indicating that coating and low temperature preservation could be a potential method for fruits preservation. Thus, this study suggests that fruits may be coated and preserved at 4°C to ensure the health, life style, economic and environment by extending the shelf life of peaches.
Keywords: Prunuspersica; Coating; Color; Firmness; Shelf-life; Microbiological evaluation\
Introduction
It is well known that fruits and vegetables play a vital role in human diet as sources of calories, vitamins, dietary fiber and special nutraceuticals. Many fruits develop away coat on their epidermis as they mature on the plant but this natural waxy coat is not adequate to offer protection against water loss and high respiration rate that follow when they are removed from the living tree, it leads to the spoilage of these fresh produce. Such post-harvest losses can be reduced to some extent by increasing the wax content on fruit surface [1,2], utilizing low temperature technologies [3], efficient packaging [4], use of coatings [5-10], nanotechnology [11,12] osmotic dehydration and irradiation [13,14] etc.
The peach fruit softens quickly after harvest and leads to huge losses in the marketing chain due to over-ripeness. Post-harvest decay due to rapid ripening in peaches is the major factor that limits their shelf-life which poses a serious constraint for efficient handling and transportation [15]. Application of low temperature techniques have been determined to provide delayed fruit degradation by reducing its biological and chemical activity but these methods require refrigerated transport and storage tanks [16-19]. Koukounaras et al. [20] have also investigated the effect of short-term heat treatment on quality of fresh-cut peach. Intermittent warming has been reported to increase the shelf-life of firm-mature and firm-breaker peaches by 1 and 2 weeks, respectively [21,22]. Likewise Zhang et al. [23] have reported that self-defense capability of peach fruit was improved by heat treatment. Recently High Pressure Processing (HPP) technique has been utilized to achieve enzyme inactivation for preserving texture and color of minimally processed peaches. This research showed that higher pressure levels were more effective to inactivate enzymes and to preserve color than longer times [24].
Fruit coatings are used as carriers of antimicrobial compounds, color or aroma additives, anti-oxidants, or anti-ripening compounds [25]. Aloe arborescens and Aloe vera gels [26] carboxymethyl cellulose [27.28], 1-methylcyclopropene [29-31], sodium alginate [32], edible gum and calcium lactate [33] etc, have been used for extension of shelf-life of peaches. Specific fruit coatings for easily perishable fruits like peaches also provide improvements in arresting physiological weight loss, retardation of ripening, reduction of chilling and mechanical injury, reduced decay and plausible added shine or gloss to the fruits. However it has been observed that fruit coatings indirectly induce changes in flavor due to delayed ripening or as a result of anaerobic respiration with increased ethanol concentrations [34] and hence have to be carefully selected and used so that original texture, color and flavor of the fruits retained. The present study was conducted to assess the potential of a coating developed from a naturally occurring terpenoidal oligomer P-104. Experiments on evaluation of effect of coating have been conducted on storage at 4°C and at room temperatures in the month of June (38±2°C).
temperatures in the month of June (38±2°C). Experimental
Fruit source
Peaches (PrunuspersicaL.) used for the present study were purchased from authentic Agricultural Produce Marketing Cooperative. Uniform and non-damaged fruits were selected and they were washed with water and shade dried before treatment.
Preparation of coating and fruit treatment
Active ingredient P-104 was obtained from lac resin as per the patented process [35]. The purity of P-104 was analyzed by comparison of its FTIR, UV and NMR spectroscopy, molecular weight distribution and morphology of P-104 were investigated by Gel Permeation Chromatography (GPC), and SEM, AFM respectively. Coating (O/W type emulsion) was developed from P-104 by mechanical stirring of 10 g of P-104 and 2 ml of triethyl amine in 70 ml of 100 mg/l SDS and final volume was made upto 100 ml with double distilled water. Uniformity of formulation was ensured at different intervals by following recommended protocols [36]. Peaches were randomly distributed into two groups. In both the groups, half of fruits were kept uncoated while the other half was treated with the coating solution by dip coating (with 2-3 minutes of contact time) method. One group of fruits was stored at room temperature (38±2°C) while the fruits of other group were stored in the refrigerator (4°C) for progressive assessments. Reprehensive scheme is mentioned in (Figure 1).
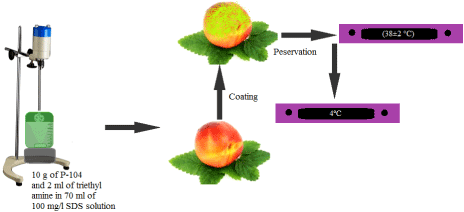
Figure 1: Schematic scheme of experiment.
Characterization Techniques
Scanning electron microscope
Glass slides coated with P-104 solution in isopropyl alcohol (by dip coating), was mounted on the stub and coated with silver paste to render a conducting surface to determine the surface topography of films formed by using LEO, 435 VP, scanning electron microscope operating at 20 kV and a working distance of 12.11 mm.
Atomic force microscopy: A glass slide was dipped in a solution of P-104 in isopropyl alcohol and taken out to dry subjected to analysis by Atomic force microscope (Digital instruments, Nanoscope III-A) to study the variation in surface topography. Since contact mode AFM operates by scanning a tip attached to the end of a cantilever across the sample surface it could monitor the change in surface which deflects the cantilever with a split photodiode detector.
Transmission electron microscopy: Philips CM-6 electron microscope operating at 120 kV was used for electron microscopy investigations. The morphological and the particle size in the nano particle samples and the Selected Area Electron Diffraction (SAED) patterns have been examined. For TEM measurements, P-104 particles were deposited on carbon coated copper grids by dispersed in hexane ultrasonically and then lifted onto a fine mesh of carbon coated copper grid. The grid was then mounted on the specimen holder and examined under the microscope.
Physiological weight loss
Six fruits of each treatment (the same fruit during all the storage time) were weighed at the beginning of the experiment and at every fourth day of storage. The results were expressed as percentage loss of initial weight.
Firmness
For each fruit sample, texture was determined using a 2 mm diameter probe coupled on a TA. XT plus Texture Analyzer (Stable Micro Systems, Surrey, UK) interfaced to a personal computer. Penetration rate was 2 mm sec-1 for 5 mm after contacting the flesh and results of firmness were expressed in N.
Color analysis
Color characteristics (L, a and b) were assessed using a Color Quest Hunter colorimeter (Hunter Lab, Hunter Associates Laboratories Inc., Virginia, USA to determine L value (lightness or brightness), a value (redness or greenness) and b value (yellowness or blueness) of peach samples. Twelve readings were obtained for each treatment from 6 replicates with 2 readings for each replicate by changing the position of the peach to get uniform color measurements. Measurements were taken at every fourth day upto 12 days for fruits stored at ambient temperature and upto 24 days for fruits stored at 4°C. A standard white plate (X =78.45, Y =83.16, Z =88.81) and a black plate were used to standardize the instruments. Color changes were converted to hue angle (θ) = tan-1 (b/a). Results were expressed as hue angle (θ) and L values.
Microbiological evaluation
Plate Count Agar medium was used for all the experiments. The pH of the medium was adjusted at 7.0 ± 0.2 and autoclaved at 15 lb/ inches2 pressure for 20 minutes. The medium was then poured into petriplates. Samples of 10 g of fruit pulp were blended and then added to 100 ml of 1% sterile peptone water at different dilutions (10- 1-10-8). Aerobic mesophilic microorganisms were counted by plating 1 ml of the corresponding dilution and the plates were incubated at 35°C for 48 h (Harrigan and McCance 1976). Experiments were done in triplicate and only counts of 30-300 Colony Forming Units (CFU) were considered. Microbial counts were determined by using standard procedure available in the literature.
Statistical analysis
Analysis of Variance (ANOVA) for each quality parameter with respect to coating and temperature of storage was carried out and presented in (Table 1). Differences among means were compared by L.S.D. test (P = 0.05) (Figure 1).
Source
DF
SS
MS
F
P
Weight loss
Treatment
5
274.36
54.7
75.26
0.0001
Temperature
1
63.11
63.11
86.56
0.0002
Error
5
3.64
0.72
-
-
Total
11
341.12
-
-
-
Firmness
Treatment
7
2.05
0.29
3.17
0.07
Temperature
1
0.04
0.04
0.46
0.51
Error
7
0.64
0.09
-
-
Total
15
2.74
-
-
--
L value
Treatment
7
76.4
10.91
2.27
0.15
Temperature
1
3.08
3.08
0.64
0.44
Error
7
33.59
4.78
-
-
Total
15
113.09
-
-
---
Hue angle
Treatment
7
345.2
49.32
3.39
0.06
Temperature
1
103.5
103.58
7.11
0.03
Error
7
101.89
14.55
-
-
Total
15
550.71
-
-
-
Microbial counts
Treatment
3
11.59
3.86
2.45
0.2
Temperature
1
15.1
15.12
9.60
0.05
Error
3
4.72
1.57
-
-
Total
7
31.4
-
-
-
Table 1: ANOVA for various quality parameters of peach.
Results and Discussion
Spectral data for the isolated P-104
Yield: 65%, decompose at 78-80°C; IR (νmax, KBr): 33438, 2925, 2853, 1714, 1640, 1454, 1375, 1252, 1158, 1032, 937, 521 cm–1; 1H NMR (300 MHz, DMSO, δ in ppm) δH (ppm): 9.66 (s, CHO), 6.41 (bs, olefinic-H), 5.77 (bs, allylic-H), 4.74 (bs, CH2OH); UV (λmax, methanol): 226 nm.
Morphology of P-104
The surface topography of P-104 has been evaluated by Scanning Electron Microscopy (SEM) and Atomic Force Microscopy (AFM) (Figures 2 and 3). It has been observed that P-104 particles were found to assemble uniformly throughout the deposited films on glass together with the spherical agglomerate formation.
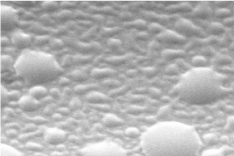
Figure 2: SEM image of P-104.
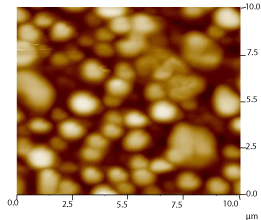
Figure 3: AFM image of P-104.
Typical TEM micrograph of powder is shown in (Figure 4). The dark contrast in gray background indicated P-104 particles. A distribution in particle size was observed in the powder. A histogram of particle size distribution of this sample showed that particle size distribution of this sample showed that particle size was roughly 97 nm (Figure 5).
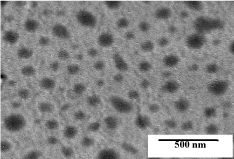
Figure 4: TEM image of P-104 powder.
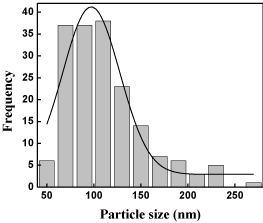
Figure 5: Histogram showing the particle size distribution of P-104.
Physiological weight loss
In our study, the highest weight loss was determined in control samples of peaches while coated samples suffered a comparatively insignificant loss for both the samples stored at room temperature and at 4°C. Coated fruits when stored at room temperature showed 12.24% weight loss after 12 days of storage while the loss was found 20.78% for uncoated fruits after 12 days. At the same time when the coated and uncoated peaches were stored in refrigerator, the fruits remained fresh for a longer period of time. Physiological weight loss reached 11.9% for coated fruits and 35.12% for uncoated fruits at the end of 24 days of storage (Figure 6). Many researchers have reported that most important problem, during storage of peaches and nectarines is weight loss [37,38]. The lower weight loss in coated fruits as compared to control is supposed to be originated from lower rate of water loss in coated fruits due to partial clogging of natural pores with coating developed from P-104.
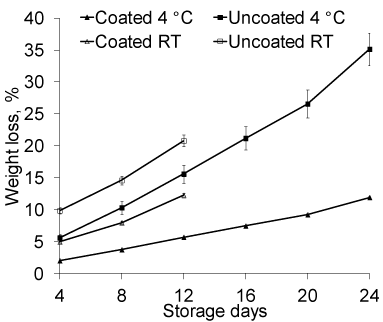
Figure 6: Changes in weight loss in coated and uncoated peach stored at
room temperature (38±2°C) and at 4°C (n=6).
Texture analysis
Firmness is an important quality attribute and the rate of firmness loss during ripening may influence not only the fruit quality but also its storage life. Reduction in fruit firmness on storage is believed to reflect solubilization of pectic substances with conversion of insoluble pectin to soluble pectin causing fruit softening and reduced resistance [39,40]. In our experiments it was determined that fruit firmness gets significantly reduced from 1st day of storage to the last day probably due to the conditions of fruit storage at room temperature. The reduction in firmness gets attenuated under refrigerated storage conditions. Figure 7 showed a decrease in firmness under both coated and uncoated peaches but the decrease was found to be less in coated peaches irrespective of storage conditions. Shewfelt et al. (1987) have identified maturity at harvest, ripening and temperature as most critical factors in the postharvest life of peaches while firmness as the main limiting quality attribute.
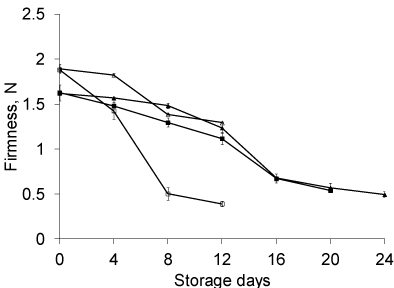
Figure 7: Changes in Firmness in coated and uncoated peach stored at room
temperature (38±2°C) and at 4°C (n=6).
Color analysis
Little changes in hue angle and lightness (L) values were observed when fruits were stored at room temperature (38±2°C) and at 4°C (Figure 8). These changes in Hunter L values were not significant. Changes associated with ripening include loss of green color and development of yellow, red and other color characteristics of the variety. Various studies suggested that normal ripening of peaches comprise of the softening process, changes in skin and flesh color (less green with a reduction in Hue angle values), and an increase in Total Soluble Solids (TSS), lower Titratable Acidity (TA) and a higher TSS/TA ratio [39-41] also emphasized that fruit firmness is an excellent indicator of maximum maturity but the combination of ground color and fruit firmness may be better than a single index to assay stone fruit maturity.
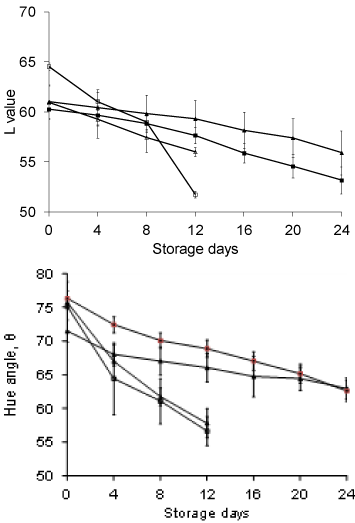
Figure 8: Changes in Lightness (a) and Hue Angle (b) in coated and uncoated
peach stored at room temperature(38±2°C) and at 4°C (n=6).
Microbiological evaluation
Coating developed from P-104 was found to be effective in reducing microbial Colony Forming Units (CFU) on PCA medium in peaches. International Commission on Microbiological Specifications for Food (ICMSF 1974) set a maximum limit of 7 log cfu/g of total aerobic mesophilic bacteria. In the present experiments it was determined that the room storage temperature seemed to favor the microbial growth (Figure 9). Total Plate Count (TPC) in uncoated peaches stored at ambient temperature increased drastically from 4.23 ± 0.02 to 8.35 ± 0.02 log cfu g-1 from 4 to 8 days of storage. Coated peaches stored at room temperature were found to have the TPC value of 9.67 ± 0.06 over a period of 16 days. Coated and uncoated peaches stored at ambient temperature were determined to have the shelf life of 12 and 4 days respectively which is in concurrence with earlier findings. It has been determined that 4°C temperature significantly (P < 0.05) lowered the rate of microbial growth. Our findings showed that uncoated peaches stored in refrigerator crossed the microbial limit of log 7cfug-1 after 12 days but the fruits were not in marketable conditions after 8 days of storage. On the other hand the coated peaches remain microbiologically safe even after 24 days when stored at 4°C.
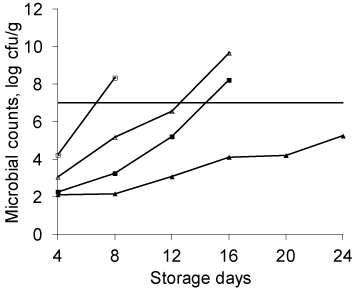
Figure 9: Changes in aerobic mesophilic microbial counts in coated and
uncoated apples during storage (37±2°C) (n=3). The straight horizontal line
indicates the maximum microbial limit for total aerobic growth (7 log cfu g-1).
Conclusion
This research provides an initial specific characterization of the impact of coating treatment on various quality parameters, for a longer period of time as compared to untreated peaches. It has been determined that the coating alone or in combination with refrigerated storage has positive effects on enhancement of shelf life of peaches and can be worked out further for commercial applications to significantly reduce post harvest losses of peaches. Based on the data reported in this paper, the predicted storage life of coated peaches was about 12day at 38±2°C and 24 days at 4°C.
Acknowledgement
Authors acknowledge the financial assistance received from the Department of Science and Technology (Govt. of India), Ministry of Science and Technology (Govt. of India), Ministry of Food Processing Industry (Govt. of India), Ministry of Rural Development (Govt. of India) and Ministry of Environment and Forests (Govt. of India).
References
- Ahmed I, Mabood Qazi I, Jamal S. Developments in osmotic dehydration technique for the preservation of fruits and vegetables. Inno Food Sci & Emerg Techno. 2016; 34: 29-43.
- Asghar A, Alamzeb, Farooq, Qazi IM, Ahmad S, Sohail M, et al. Effect Of Edible Gum Coating, Glycerin And Calcium Lactate Treatment On The Post Harvest Quality Of Peach Fruit. Food Science and Quality Management. 2014; 30: 40-47.
- Anthony BR, Phillips DJ, Badr S, Aharoni Y. Decay control and quality maintenance after moist air heat treatment of individually plastic-wrapped nectarines. J Am Soc Hort Sci. 1994; 114: 946-949.
- Baldwin EA, Nisperos-Carriedo M, Shaw PE, Burns JK. Effect of Coatings and Prolonged Storage Conditions on Fresh Orange Flavor Volatiles, Degree Brix, and Ascorbic Acid Levels. J. Agric. Food Chem. 1995; 43: 1321-1331.
- Baldwin EA, Burns JK, Kazokas W, Brecht JK, Hagenmaier RD, Bender RJ, et al. Effect of two edible coating with different permeability characteristics on Mango (Mangifera indica L.) ripening during storage. Postharvest Biology Technology. 1999; 17: 215-226.
- Bishnoi A, Chawla HM. Extending Storage Life of Pears Using a Formulation Developed from a Terpenoidal Oligomer from Lac. WIR ‘16 Proceedings of the ACM Symposium on Women in Research 2016. 2016; 103-108.
- Bishnoi A, Chawla HM, Rani G. Extension of Shelf Life of Plum by Formulation Developed from Oligomer Isolated From Lac. Int’l Journal of Advances in Chemical Engg & Biological Sciences. 2014; 1: 85-88.
- Bishnoi A, Chawla HM, Rani G, Vishnubhatla S. Effect of formulation derived from terpenoidal oligomer on shelf-life of apples without refrigeration. Journal of food science and technology. 2008; 45: 412-415.
- Bishnoi A, Chawla HM, Rani G, Saxena R. Effect of formulation derived from terpenoidal oligomer from lac on quality retention of of sweet lime. J Food sci Technol. 2009; 46: 588-590.
- Bonghi C, Ramina A, Ruperti B, Vidrih R, Tonutti P. Peach fruit ripening and quality in relation to picking time, and hypoxic and high CO2 short-term postharvest treatments. Postharvest Biology and Technology. 1999; 16: 213- 322.
- Chawla HM. A process for preparation of a component for coating fruits, vegetables, plants and hair. 1999
- Chawla HM. A formulation for fruit shine. Indian Patent 248173. 2011.
- Crisosto CH. Stone fruit maturity indices: a descriptive review. Postharvest News and Information. 1994; 5: 65-68.
- De León-Zapata MA, Sáenz-Galindo A, Rojas-Molina R, Rodríguez-Herrera R, Jasso-Cantú D, Aguilar CN. Edible candelilla wax coating with fermented extract of tarbush improves the shelf life and quality of apples. Food Packaging and Shelf Life. 2015; 3: 70-75.
- Denoya GI, Polenta GA, Apóstolo NM, Budde CO, Sancho AM, Vaudagna SR. Optimization of high hydrostatic pressure processing for the preservation of minimally processed peach pieces. Innovative Food Science & Emerging Technologies. 2016; 33: 84-93.
- Donglu F, Wenjian Y, Kimatu BM, Mariga AM, Liyan Z, Xinxin An, et al. Effect of nanocomposite-based packaging on storage stability of mushrooms (Flammulina velutipes). Innovative Food Sci & Emerging Technologies. 2016; 33: 489-497.
- Fernández-Trujillo JP, Artés F. Keeping quality of cold stored peaches using intermittent warming. Food Research International. 1997; 30: 441-4
- Fernández-Trujillo JP, Artés F. Chilling injuries in peaches during conventional and intermittent warming storage. International Journal of Refrigeration. 1998; 21: 265-272.
- Fernández-Trujillo JP, Artés F. Modified atmosphere packaging affects the incidence of cold storage disorders and keeps ‘flat’ peach quality. Food Research International. 1998; 31: 571-579.
- Guerreiro AC, Gago CML, Faleiro ML, Miguel MGC, Antunes MDC. The effect of edible coatings on the nutritional quality of ‘Bravo de Esmolfe’ freshcut apple through shelf-life. LWT - Food Science and Technology. 2017; 75: 210-219.
- Guilléna F, Díaz-Mulaa HM, Zapataa PJ, Valero D, Serranob M, Castilloa S. Aloe arborescens and Aloe vera gels as coatings in delaying postharvest ripening in peach and plum fruit. Postharvest Biology and Technology. 2013; 83: 54-57.
- Harrigan W. Laboratory Methods in Food Microbiology. 1998.
- Microorganisms in foods 2 Sampling for microbiological analysis: Principles and specific applications. International Commission on Microbiological specifications for Foods (ICMSF). 1974.
- Jacxsens L, Devlieghere F, Debevere J. Temperature dependence of shelflife as affected by microbial proliferation and sensory quality of equilibrium modified atmosphere packaged fresh produce. Postharvest Biology and Technology. 2002; 26: 59-73.
- Kader AA, Heintz CM, Chordas A. Postharvest Quality of Fresh and Canned Clingstone Peaches as Influenced by Genotypes and Maturity at Harvest. J. Amer. Soc. Hort. Sci. 1982; 107: 947-951.
- Khalil AHPS, Davoudpour Y, Saurabh CK, Hossain MdS, Adnan AS, Dungani R, et al. A review on nanocellulosic fibres as new material for sustainable packaging: Process and applications. Renewable and Sustainable Energy Reviews. 2016; 64: 823-836.
- Koukounaras A, Diamantidis G, Sfakiotakis E. The effect of heat treatment on quality retention of fresh-cut peach. Postharvest Biology and Technology. 2008; 48:30-36.
- Li P, Hu H, Luo S, Zhang L, Gao J. Shelf-life extension of fresh lotus pods and seeds (Nelumbo nucifera Gaertn.) in response to treatments with 1-MCP and lacquer wax. Postharvest Biol and Technol. 2017; 125: 140-149.
- Liguori G, Weksler A, Zutahi Y, Lurie S, Kosto I. Effect of 1-methylcyclopropene on ripening of melting flesh peaches and nectarines. Postharvest Biology and Technology. 2004; 31: 263-268.
- Lill RE, O’Donoghue EM, King GA. Postharvest physiology of peaches and nectarines. Horticultural Reviews. 2011; 11: 413-452.
- Maftoonazad N, Ramaswamy HS, Marcotte M. Shelf-life extension of peaches through sodium alginate and methyl cellulose edible coatings. International Journal of Food Science and Technology. 2008; 43: 951-957.
- McGuire RG, Hallman BJ. Coating guavas with cellulose- or carnauba-based emulsions interferes with postharvest ripening. Hort Science. 1995; 30: 294- 295.
- Mohebi E, Shahbazi Y. Application of chitosan and gelatin based active packaging films for peeled shrimp preservation: A novel functional wrapping design. LWT - Food Science and Technology. 2017; 76: 108-116.
- Najafabadi NS, Sahari MA, Barzegar M, Esfahani ZH. Effect of gamma irradiation on some physicochemical properties and bioactive compounds of jujube (Ziziphus jujuba varvulgaris) fruit, Radiat Phys Chem. 2017; 130: 62-68.
- Ortiz A, Graell J, López ML, Echeverría G, Lara I. Volatile ester-synthesising capacity in ‘Tardibelle’ peach fruit in response to controlled atmosphere and 1-MCP treatment. Food Chemistry. 2010; 123: 698-704.
- Rieger MM. The theory and practice of industrial pharmacy. 1970; 59: 1531.
- Roe B, Bruemmer JH. Changes in pectin substances and enzymes during ripening and storange of “Keitt” mangos. J Food Sci. 1981; 46: 186-189.
- Shewfelt RL, Myers SC, Resurreccion AV. Effect of physiological maturity at harvest on peach quality during low temperature storage. Journal of Food Quality. 1987; 10: 9-20.
- Tavassoli-Kafrani E, Shekarchizadeh H, Masoudpour-Behabadi M. Development of edible films and coatings from alginates and carrageenans. Carbohydr Polym. 2016; 137: 360-374.
- Togrul H, Arslan N. Extending shelf-life of peach and pear by using CMC from sugar beet pulp cellulose as a hydrophilic polymer in emulsions. Food Hydrocolloids. 2004; 18: 215-226.
- Wiley RC. Minimally Processed Refrigerated Fruits and Vegetables. Chapman & Hall. 1994.
- Zhang L, Yu Z, Jiang L, Jiang J, Luo H, Fu L. Effect of post-harvest heat treatment on proteome change of peach fruit during ripening. J Proteomics. 2011; 74: 1135-1149.