
Research Article
Austin J Biotechnol Bioeng. 2018; 5(1): 1089.
Development of a Nerve Regeneration Electrode and its Application to the Rat Vagus
Zheng C*, Kawada T, Li M, Uemura K, Inagaki M and Sugimachi M
Department of Cardiovascular Dynamics, National Cerebral and Cardiovascular Center, Japan
*Corresponding author: Zheng C, Department of Cardiovascular Dynamics, National Cerebral and Cardiovascular Center, Osaka, Japan
Received: December 20, 2017; Accepted: February 02, 2018; Published: February 09, 2018
Abstract
Appling a silicon semiconductor process, we developed a nerve regenerationtype electrode for application to the rat vagus nerve. The diaphragm of the electrode was 12 μm thick and perforated with 30 to 81 through holes (square side length: 50–100 μm) for nerve regeneration. Around the edges of six to eight of these holes, we fabricated 10-μm-wide Au electrodes. In order to facilitate long-term implantation, the Au electrodes were protected from corrosion by coating with a silicon nitride film. Three months after implantation in the rat vagus, using cuff wire electrode which attached to proximal and distal of the sieve electrode under anesthesia, we were able to record evoked compound action potentials and stimulate the regenerated nerve through either the cuff electrodes or the sieve electrodes. We conclude that the regeneration electrode will serve as a potential neural interface for recording from and stimulation of the vagus nerve.
Keywords: Sieve electrode; Nerve regeneration; Biocompatibility; Vagus; Electric stimulation; Compound action potential
Abbreviations
CAP: Compound Action Potential; RE: Regeneration Electrode
Introduction
Because the sympathetic and vagal nervous systems regulate circulation, functional analysis of these nervous systems and their application to the treatment of circulatory diseases are important areas of research. Sympathetic over activity and vagal withdrawal contribute to aggravating chronic heart failure. In rats, electrical stimulation of the right vagus nerve has been demonstrated to improve the survival of chronic heart failure following myocardial infarction [1], suggesting that correction of autonomic balance using a neural interface can be used to treat certain cardiovascular diseases. However, long-term recording of vagal nerve activity has not been reported. In the evaluation of circulatory regulation, measurements under awake and non-restrained conditions are important, and thus the development of nerve electrodes capable of long-term stable recording is desirable. Recent developments in various artificial organs and assist devices will also require advanced neural interfaces that can integrate these artificial devices with the living body. Various electrodes have been developed to function as a neural interface, including the wire-type [2], cuff-type [3], needle array-type [4] and collagen-type [5] electrodes. Although these electrodes enable the recording of nerve activity or nerve stimulation under both anesthetized and conscious conditions, long-term and stable measurement of nerve signals have thus far not been achieved. Measuring action potentials from specific nerve fibers in a nerve bundle presents a further challenge to be resolved, particularly when the nerve bundle consists of nerve fiber populations with different biological functions. The nerve regeneration-type electrode (here after, RE: regeneration electrode) has the potential to be used as a permanent neural interface with the capability of recording action potentials from selected nerve fibers. Most previous studies on REs have focused on their application to motor and sensory nerves such as the peroneal nerve [6], sciatic nerve [7,8], chorda tympani nerve [9] and glossopharyngeal nerve [10]. Considering the importance of the vagus in cardiovascular control, the objective of the present study was to construct a neural interface with the rat vagus by implanting a RE that we designed utilizing Silicon (Si) semiconductor process, and to conduct basic evaluations of this electrode.
Materials and Methods
Concept of the regeneration electrode
In a regeneration electrode, a diaphragm with sieve holes is sandwiched between the two cut ends of a transected nerve. The nerve fibers are allowed to regenerate through the sieve holes, and the action potentials are measured after nerve regeneration. The merit of the RE is that the regenerated nerve fibers are anchored to the electrode holes; therefore, specific nerve fibers can be fixed stably to given electrodes. Furthermore, the RE allows multiple channel measurement of action potentials from different nerve fibers in a given nerve bundle. In the future, with optimization of the size of the electrode holes, it may become possible to connect single nerve fibers to individual electrodes.
Design of the diaphragm
The challenges faced when constructing a RE include designing a stable structure that can be implanted in the body long-term, that has a high mechanical strength and that achieves a high rate of nerve regeneration. We used silicon chips of 3mm × 6mm, each with a 1-mm2 thin diaphragm in the center. Through holes were constructed in the diaphragm in order to facilitate nerve regeneration. A Siliconon- Insulator (SOI) substrate was used and a process was developed for precise integration of the thin diaphragm with a surrounding rim. In order to ensure high mechanical strength and a high regeneration rate, the thickness of the diaphragm was set at 12mm, the thickness of the surrounding rim at 250mm and the percentage of total through hole area at 20% to 25%. Diaphragms with three different square hole sizes (side length, 50, 75 and 100mm) were prepared. Depending on the hole size, 30 to 81 through holes were fabricated in the form of a sieve. Gold (Au) electrodes of 10mm width were fabricated around six to eight of the through holes (Figure 1A).
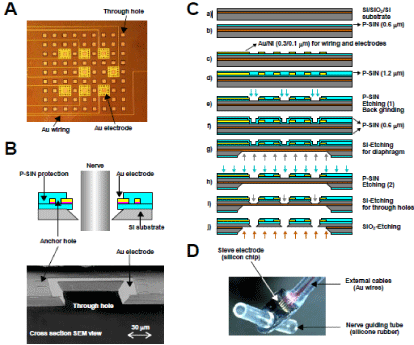
Figure 1: A: Diaphragm of a regeneration electrode. Through holes were constructed for nerve regeneration and Au electrodes were fabricated around several
through holes for action potential recording. The Au electrodes were connected to lead wires with Au wirings. B: Schema (top) and SEM (Scanning Electron
Microscope) view (bottom) of a cross section of a through hole surrounded by an Au electrode. The Au electrode was anchored by P-SiN layers. C: Process flow
of electrode fabrication (see main text for details). D: Assembly of a Si electrode chip with external lead cables and nerve guiding tubes.
Design of the metal electrodes
The metal electrodes used for the detection of nerve action potentials should satisfy not only the electrochemical characteristics such as polarization potential but should also be biocompatible (e.g. nontoxic) and compatible with the Si semiconductor process. In addition, once implanted in the body, the RE will be subject to constant erosion by body fluids. Whereas the body fluids are slightly alkaline (pH 7.35) under physiological conditions, they become acidic (with pH falling to 5.6–5.3) following tissue damage and inflammation. Depending on the configuration of the electrode material, corrosion of the electrode may occur, and consequently there exists the potential for the reliability of the electrode to become greatly reduced. In the RE we describe here, Au was used for the metal electrodes and wires on the silicon chip. The external lead cables were also fabricated from Au and were connected to the chip by ultrasonic bonding. These designs were intended to prevent the electrical field-induced corrosion associated with the use of dissimilar metals. Although Nickel (Ni) was used in the adhesive layer, a plasma chemical vapour deposition Silicon Nitride (P-SiN) film was deposited to prevent penetration of the electrolyte, thereby improving the resistance to corrosion. A cross section of a through hole surrounded by a metal electrode is shown in (Figure 1B).
Process flow
• The detailed electrode chip fabrication process is illustrated in (Figure 1C).
• Use an SOI substrate (top Si: 10mm/SiO2: 1.0mm/substrate Si: 525mm).
• Deposit P-SiN film (0.6mm) on the SOI substrate.
• Deposit P-SiN film (1.2mm) as a protective film on the patterned Au/Ni.
• Pattern the chip outline, electrode and through holes in the P-SiN film using photolithography and dry etching, and reduce the water to a thickness of 280mm using back grinding.
• Deposit P-SiN film on both surfaces of the substrate as a masking and protective film for substrate Si etching (process g).
• Conduct patterning of the P-SiN film on the back surface to expose Si. Etch the SOI substrate using KOH to form the diaphragm.
• Using dry etching, remove the P-SiN film deposited on the top surface (process f).
• Etch the top Si using KOH to form the through holes.
• Remove the SiO2 layer using HF solution to perforate the through holes.
Assembly
The completed Si chip was connected to nerve guide tubes fabricated from silicone rubber. Au wires (75mm in diameter) with urethane shielding were attached to the chip by ultrasound bonding as external lead cables. The appearance of the assembled electrode is shown in (Figure 1D).
Animal preparation
Animal care was performed in accordance with Guideline Principles for the Care and Use of Animals in the Field of Physiological Sciences, which has been approved by the Physiological Society of Japan. All experimental protocols were reviewed and approved by the Animal Subjects Committee at the National Cardiovascular Center. Eight-week-old Sprague-Dawley rats were used in the implant experiment. Under halothane (1.5%) anesthesia, a midline incision was made in the neck region, and the right vagus was dissected. After transecting the vagus, the central and peripheral stump of the nerve were taken into the guide tubes and stitched (PROLENE, 9-0) close to the surface of RE. The lead cables were directed to a subcutaneous site of the posterior neck region, ready for exteriorization as necessary. After the implant surgery, the rats were reared under ambulatory conditions with free access to food and water. Two to three months after the implant surgery, the RE was evaluated under anesthesia.
Measurement of electrode impedance
The electrical characteristics of the RE were evaluated using the Alternating Current (AC) impedance method. Baseline interelectrode impedance was measured in Ringer’s solution before implantation and compared with the impedance measured at 3 months after implantation. Impedance was measured at 50 mV over a frequency range of 100 Hz to 100 kHz.
Recording of evoked CAPs
REs implanted for 90 days were used to measure evoked CAPs from the vagus under anesthesia. An incision was made in the neck region, and cuff electrodes fabricated from platinum wires (diameter: 0.1mm, distance: 1.5mm) were placed on the central and peripheral sides of the RE. Electrical stimulation was then conducted via the cuff electrodes, and the evoked action potential was recorded from the RE. Electrical stimulation to the cuff electrode was delivered in pulses of 300s duration and 2-Hz frequency, with the stimulating potential being increased from 1 V to 7.2 V (Stimulator: SEN-7203, NIHON KOHDEN, TOKYO, JAPAN). The evoked CAP was recorded as the differential amplification of the electrical potential between two randomly selected metal electrodes in the RE (Input box: JB-610J; Amplifier: MEG-6108, NIHON KOHDEN, TOKYO, JAPAN). Amplified analog signal were converted to digital signal (AD12- 8, CONTEC, OSAKA, JAPAN), and sampled at 5,000 Hz using a custom developed software. In order to reduce the noise level, the evoked CAP was averaged from 100 sweeps.
Nerve stimulation
We examined whether it was possible to stimulate the nerve fibers via the RE using the same configuration of two cuff electrodes placed on the central and peripheral sides of the RE. The vagus was stimulated via the RE, and the evoked action potential was recorded from the cuff electrodes. Data were sampled at 5,000 Hz. In order to reduce the noise level, the evoked CAP was averaged from 100 sweeps.
Results and Discussion
Nerve regeneration and biocompatibility
Table 1 summarizes the results of regeneration. According to a systematic study by Wallman et al. [7], a transparency factor (percentage of total through hole area) of 30% is superior to one of 20% for regeneration of the rat sciatic nerve. These authors also demonstrated that a hole size of 30mm was superior to one of 90mm with a transparency factor of either 20% or 30%. With a small transparency factor of 9.6%, however, nerve regeneration was not achieved with a hole size of 50mm, indicating the importance of the transparency factor (Figures 2B, C, D and E). Although we were unable to perform a systematic comparison in the present study due to the limited number of samples, the hole size (50–100mm) and transparency factor (20%–25%) were presumably in the requisite ranges for successful regeneration of the vagus nerve. Figure 2A shows an example of a regenerated vagus nerve penetrating through a RE 40 days after implantation. Although regeneration of nerve fibers was observed on the peripheral side of the RE (Figures 2D and E), the population was smaller compared to that on the central side (Figures 2B and C). In some rats, the diaphragm of the electrode was broken, possibly by mechanical stress, during nerve regeneration. There were more blood vessels in the distal guide tube than the proximal side (Figures 2C and D), and those broken diaphragm all shift to the proximal direction, therefore, it may exert pressure caused by revascularization propelling from the distal side. We observed that silicon diaphragm presented excellent biocompatibility after 3 months implantation; there were no dissolution or decomposition trace visible under microscope.
Hole size (μm)
100
75
50
Number of implantations
8
9
6
Time of examination (Days after implantation)
85-90
90-95
60-90
Number of regenerations
7
7
4
Regeneration rate (%)
88
78
67
Table 1: Identification of rat cervical vagal nerve regeneration through different hole size regeneration electrode.
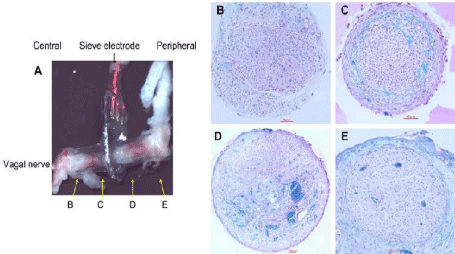
Figure 2: A: Regenerated vagus nerve growing through a regeneration electrode and show the position of tissue section. B and C: Microscopic view of the tissue
in the central guiding tube. D and E: Microscopic view of the tissue in the peripheral guiding tube. Solid horizontal bars represent 50 μm. The tissue sections were
stained by luxol fast blue and Hematoxylin Eosin.
Electrode impedance
Figure 3A shows the typical results of inter-electrode impedance of metal electrodes under in vitro conditions. The impedance values decreased with increasing test frequencies. As expected, the impedance at 1 kHz was lower for the 100-mm electrode (0.86 M) than for the 50- mm electrode (2.2 M). Figure 3B illustrates the mean and SD values of the inter-electrode impedance between randomly selected metal electrodes under in vivo conditions. The impedance was significantly lower for the 100-mm electrode (2.6 ± 2.0 Mm) than for the 50-mm electrode (32 ± 16 M, n = 4, P < 0.05). The impedance under in vivo conditions was higher than the corresponding impedance under in vitro conditions, suggesting that high-impedance tissues such as nerve fibers had regenerated. The extent of nerve regeneration may have contributed to the difference in the relative increase in the impedance between the 50-mm and 100-mm electrodes. The measured impedance values in the present study were considerably higher than those reported by Ramachandran et al, (Z = 5.7 k at 1 kHz) [8]. Nevertheless, in order to improve the signal-to-noise ratio when recording action potentials, it will be necessary to reduce interselectrode impedance.
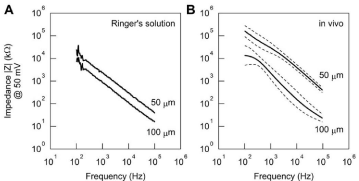
Figure 3: A: Inter-electrode impedance between two metal electrodes on a single sieve electrode measured in Ringer’s solution. Impedance was lower for the
100-μm electrode than for the 50-μm electrode. B: Inter-electrode impedance between two metal electrodes on a single sieve electrode measured 120 days after
implantation in vivo. Impedance was higher than that measured in vitro.
Recording of evoked CAP
Evoked CAPs were recorded from the RE in response to electrical stimulation applied to the central cuff electrode (Figure 4). The left panels of (Figure 4) shows single sweeps and the right panels show the data averaged from 100 sweeps. A stimulation artifact was noted between 0 and 5ms. When the stimulation amplitude was 1V, no definitive evoked CAP was observed. At 2.2-V stimulation, a small deflection was noted after the stimulation artifact. With stimulations of 3.2 to 7.2 V, evoked action potentials were recorded with a latency of approximately 12 ms, and no significant change in the amplitudes of the recorded potentials was observed, indicating that all of the nerve fibers in the nerve bundle had been activated. If the recordings had been artifacts transmitted outside the nerve fibers, the recorded deflections would have increased in proportion to the increase in stimulation amplitude. For these recordings, the distance between the stimulation electrode and the RE was approximately 2 cm, and the conduction velocity of the major component was calculated as approximately 1.3 m/s, which may be classified as unmyelinated C fiber.
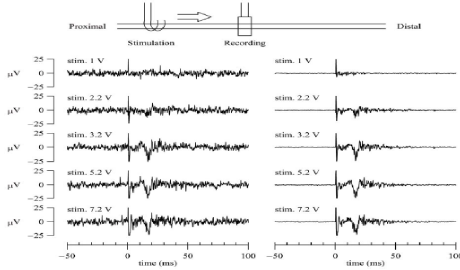
Figure 4: Threshold characteristics of evoked CAPs. The CAPs were measured from the regeneration electrode while varying the amplitude of the stimulation
voltage. The stimulation was applied from the central cuff electrode at time zero (duration: 0.2 ms, frequency: 1 Hz). The left panels show typical single sweeps and
the right panels show the averaged data from 100 sweeps. At a stimulation voltage of 1 V, no definitive action potential was observed. At a stimulation voltage of
2.2 V, a small deflection was noted. The amplitude of the CAP was increased at a stimulation voltage of 3.2 V. However, further increase in the stimulation voltage
did not increase the amplitude of the CAP.
One of advantages of the RE over the cuff-type electrode is that action potentials can be obtained from the different populations of nerve fibers in a nerve bundle. Shimatani et al. demonstrated that simultaneous recordings from different taste units were possible using a RE implanted in the rat chorda tympani nerve [9]. Simultaneous recordings of evoked action potentials were also possible from two different pairs of metal electrodes in a single RE in the vagus nerve (Figure 5). Under the present experimental conditions, because the whole nerve bundle was stimulated simultaneously from the central cuff electrode, the two evoked action potentials exhibited similar latency. Nevertheless, the profiles of the action potentials obtained from the two recordings did not match exactly, suggesting that, in the future, it may be possible to detect fiber- specific information from the vagus nerve. When the vagus nerve was stimulated from the central cuff electrode, an evoked CAP was recorded in both the RE and the peripheral cuff electrode (Figure 6A). Although the initial deflection was not so clear in the action potential recorded from the peripheral cuff electrode, the time to the first negative deflection (depicted as a small circle) was longer for the peripheral cuff electrode than for the sieve electrode, reflecting the greater distance from the central cuff electrode. When the vagus nerve was stimulated from the peripheral cuff electrode, an evoked CAP was recorded in both the RE and the central cuff electrode (Figure 6B). The action potential recorded from the central cuff electrode exhibited a more complex pattern than that recorded from the RE. The duration of the action potential was longer in the central cuff electrode than in the RE, suggesting that different populations of nerve fibers contributed to the generation of CAPs in the central cuff electrode. It is likely that information from the selected nerve fibers could be obtained from the RE but not from the cuff-type electrode.
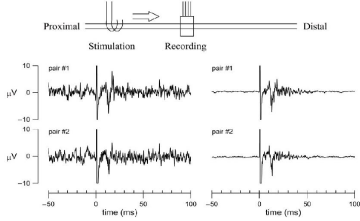
Figure 5: Simultaneous recordings of evoked action potentials measured from two different pairs of metal electrodes in a single regeneration electrode. The
stimulation was applied from the central cuff electrode at time zero (duration: 0.2 ms, frequency: 1 Hz, voltage: 3V). The left panels show typical single sweeps and
the right panels show the averaged data from 100 sweeps.
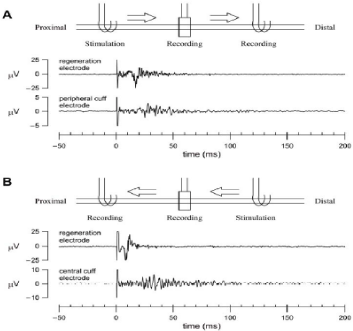
Figure 6: Simultaneous recordings of evoked CAPs (averaged data from 100 sweeps) measured from the regeneration and peripheral cuff electrodes. The
stimulation was applied from the central cuff electrode at time zero. Small circles indicate the first negative deflection of the evoked action potentials. B: Simultaneous
recordings of evoked CAPs (averaged data from 100 sweeps) measured from the regeneration and central cuff electrodes (duration: 0.2 ms, frequency: 1 Hz,
voltage: 3V). The stimulation was applied from the peripheral cuff electrode at time zero (duration: 0.2 ms, frequency: 1 Hz, voltage: 3V).
Nerve stimulation
We were able to stimulate the vagus from the RE (Figure 7). The left panels of (Figure 7) show single sweeps and the right panels show the data averaged from 100 sweeps. A long-lasting evoked CAP (approximately 50 ms) was recorded from the central cuff electrode, with a latency of approximately 10 ms. On the other hand, a relatively short-lasting evoked CAP (approximately 10 ms) was recorded from the peripheral cuff electrode, with a latency of less than 10 ms. The difference in latency may be partly explained by the fact that the distance between the RE and the cuff electrode was not completely consistent on the central and peripheral sides. The observation that evoked action potentials were short lasting on the peripheral side may suggest that the nerve fiber component was smaller on the peripheral side than on the central side; this is consistent with the histological observations (Figures 2B, C, D and E).
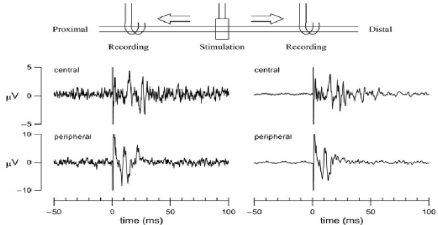
Figure 7: Simultaneous recordings of evoked CAPs measured from the central and peripheral cuff electrodes. The stimulation was applied from the regeneration
electrode at time zero (duration: 0.2 ms, frequency: 1 Hz, voltage: 4V). The left panels show typical single sweeps and the right panels show the averaged data
from 100 sweeps.
Limitation and future work
This preliminary study has conducted to verify the feasibility of application a silicon chip based sieve electrode to rat vagus nerve. In anesthetized rat, usually, spontaneous nerve activity of lung stretch afferent fiber is easy detectable by cuff electrode. The basic function as a peripheral nerve interface has been proved by evoked CAP recorded from either the RE or cuff electrode, however, we could not record spontaneous vagal nerve activity. The sensitivity of RE may be determined by electrode structure or number of regenerated nerve fiber, and the regeneration period might be not long enough for establishing lung –brain connection. Histological examination indicated that the number of regenerated fibers were far less than normal vagus nerve. Increasing transparency factor may be a solution, such as recently reported macro-sieve electrode [11]. Future studies may focus to understand pathophysiology of vagus regeneration and to improve the regeneration electrode structure.
Conclusion
The silicon chip based sieve electrode showed excellent biocompatibility that vagus nerve regenerated successfully via those through holes after three months implantation, and may be a feasible neural interface for recording and stimulating the regenerated nerve. Future studies are required to understanding vagal regeneration properties, and to improve the sieve electrode to achieve the goals of long term stimulating or recording the spontaneous nerve activity.
Acknowledgement
This study was supported by a Health and Labour Sciences Research Grant for Research on Advanced Medical Technology; a Health and Labour Sciences Research Grant for Research on Medical Devices for Analyzing, Supporting and Substituting the Function of the Human Body; and a Health and Labour Sciences Research Grant from the Ministry of Health, Labour and Welfare of Japan.
References
- Li M, Zheng C, Sato T, Kawada T, Sugimachi M, and Sunagawa K. Vagal nerve stimulation markedly improves long-term survival after chronic heart failure in rats. Circulation. 2004; 109: 120-124.
- Miki K, Kosho A, Hayashida Y. Method for continuous measurements of renal sympathetic nerve activity and cardiovascular function during exercise in rats. Exp Physiol. 2002; 87: 33-39.
- Loeb GE, Peck RA. Cuff electrodes for chronic stimulation and recording of peripheral nerve activity. J Neurosci Methods. 1996; 64: 95-103.
- Branner A, Stein RB, Normann RA. Selective stimulation of cat sciatic nerve using an array of varying-length microelectrodes. J Neurophysiol. 2001; 85: 1585-1594.
- Ninomiya I, Yonezawa Y, Wilson MF. Implantable electrode for recording nerve signals in awake animals. J Appl Physiol. 1976; 41: 111-114.
- Kovacs GT, Storment CW, Rosen JM. Regeneration microelectrode array for peripheral nerve recording and stimulation. IEEE Trans Biomed Eng. 1992; 39: 893- 902.
- Wallman L, Zhang Y, Laurell T, Danielsen N. The geometric design of micro machined silicon sieve electrodes influences functional nerve regeneration. Biomaterials. 2001; 22: 1187-1193.
- Ramachandran A, Schuettler M, Lago N, Doerge T, Koch KP, Navarro X, et al. Design, in vitro and in vivo assessment of a multi-channel sieve electrode with integrated multiplexer. J Neural Eng. 2006; 3: 114-124.
- Shimatani Y, Nikles SA, Najafi K, Bradley RM. Long-term recordings from afferent taste fibers. Physiol Behav. 2003; 80: 309-315.
- Bradley RM, Cao X, Akin T, Najafi K. Long term chronic recordings from peripheral sensory fibers using a sieve electrode array. J Neurosci Methods. 1997; 73: 177-186.
- Birenbaum NK, MacEwan MR, Ray WZ. Interfacing peripheral nerve with macro-sieve electrodes following spinal cord injury. Neural regeneration research. 2017; 12: 906-909.