
Research Article
Austin J Biotechnol Bioeng. 2018; 5(2): 1095.
Abiotic Conditions on Growth of Pseudomonas fluorescens (DS17R) and Its Ability to Produce Secondary Metabolites (Including Phenazines) Against Phytophthora colocasiae, the Causal Agent of Taro Leaf Blight
Ntyam Mendo SA1,2, Kouitcheu Mabeku LB¹, Tounkara Lat S², Tchameni Nguemezi S³, Ngono Ngane RA³ and Sameza Modeste L³*
¹Department of Biochemistry, Faculty of Science, University of Dschang, Cameroon
²Biotechnology Unit, Institute of Food Technology (ITA), Senegal
³Department of Biochemistry, Faculty of Science, University of Douala, Cameroon
*Corresponding author: Sameza Modeste Lambert, Department of Biochemistry, Faculty of Science, University of Douala, P.O. Box 24157 Douala, Cameroon
Received: March 06, 2018; Accepted: April 27, 2018; Published: May 04, 2018
Abstract
Control of Taro Leaf Blight (TLB) by chemical pesticides remains ineffective in Cameroon. Alternative methods of control are required to fight against the causative agent of this epidemy. Fluorescent Pseudomonas is the main rhizobacteria widely used against plant pathogens because they produce a broad range of antimicrobial secondary metabolites (including phenazines) involved in the biocontrol mechanism. However many ecological factors could influence the production of compounds involved in biocontrol. In the present study, we evaluated the abiotic conditions of P. fluorescens DS17R growth, phenazine production and antimicrobial activity against Phytophthora colocasiae the causal agent of TLB. The abiotic factors were pH, temperature, carbon and nitrogen sources, and osmotic stress. The bacterium was grown on King B medium and monitored by spectrophotometer. The secondary metabolites were extracted with chloroform and the phenazines content estimated by the MnO2- based reduction assay. The antimicrobial activity of each extracts was evaluated by the well diffusion method. Results showed that the growth, phenazine production and the antimicrobial activity of secondary metabolites extracted from P. fluorescens DS17R was influenced by different abiotic conditions. Overall, glycerol was the best carbon source while peptone and NH4Cl were the best nitrogen sources. These best sources of growth promoted significant phenazine production in correlation with the antimicrobial activity observed. In addition the optimum pH and temperature for phenazine production was 6 and 28°C respectively. On other hand, the positive correlation between growth, antimicrobial activity and phenazines production of the extract was observed at 2.5% NaCl. These findings show that Pseudomonas fluorescens DS17R extract could be positively optimized by certain abiotic factors and has the potential to be further developed as natural antimicrobial agent against P. colocasiae.
Keywords: Pseudomonas fluorescens; Phenazine; Antimicrobial activity; Phytophthora colocasiae; Taro leaf blight
Introduction
Taro Leaf Blight (TLB) disease caused by Phytophthora colocasiae is the main constraint of Taro production worldwide. This disease can spread rapidly to other plant part and has resulted in yield loses of up to 80-100% in many countries [1-3]. Several methods have been used to solve this major problem in Cameroon. One of them is the use of systemic pesticides such as metalaxyl and mancozeb. Unfortunately, the resistance developed by the pathogen strains, accumulation of residues and environmental problems can occur when these chemicals are repeatly applied. Moreover the appearance of the disease during the rainy season makes pesticides spraying ineffective [4]. Therefore, alternative ecofriendly treatment is needed in order to control the disease. In this respect, rhizobacteria have received considerable attention as an alternative approach to control plant diseases. Many rhizobacteria can actively colonize roots [5,6] and suppress phytopathogens by the production of siderophores or secondary metabolites like phenazines antibiotics [7,8]. Among benefit rhizobacteria, fluorescent Pseudomonas is non-pathogenic microorganisms, which suppress the soil-borne pathogens through multiple mechanisms [9] including the production of secondary metabolites [10-12]. Among these secondary metabolites, phenazines are highlight of biological significance. Chemically, they belong to the alkaloid class of compounds, which contain a basic amino group in their structure. Phenazines are water-soluble and are secreted into media at concentrations as high as grams per liter of bacterial culture [13]. Most species synthesize two or more species-specific phenazines except Pseudomonas fluorescens, which, so far, is known to produce only Phenazine-1-Carboxylic Acid (PCA) [14]. Many ecological factors such as carbon and nitrogen source, temperature, pH, osmotic stressetc, could influence the production of this metabolite [15-18]. In the previous work Ntyam et al. [19] demonstrated the antimicrobial activity of Pseudomonas fluorescens DS17R chloroform extract against Phytophthora colocasiae, the causal agent of the TLB. They correlated the observed activities with the presence of some secondary metabolites like phenols and flavonoids. For successful use of Pseudomonas fluorescens DS17R as biocontrol agent, we need to understand which and how environmental factors affect the production of antimicrobial components. The identification of conditions that control secondary metabolite production by P. fluorescens DS17R could lead to a better understanding of their regulation and their exploitation. The present study aimed to investigate the effects of various abiotic conditions on phenazine production by Pseudomonas fluorescens isolate DS17R and their respective antimicrobial potential against Phytophthora colocasiae.
Materials and Methods
Microbial strains
The microbial strain used in this study (Pseudomonas fluorescens DS17R and Phytophthora colocasiae) came from the culture collection of the laboratory of Biochemistry, University of Douala (Cameroon). The data regarding the characterization of these isolates were given by Ntyam et al. and Sameza et al. [20]. Bacterium was routinely cultivated on King B medium and preserved in King B Broth 20% (v/v) glycerol at -70°C for long-term maintenance whereas Phytophthora colocasiae was maintained on Potato Dextrose Agar (PDA) slant at 4°C.
Various growth conditions of Pseudomonas fluorescens DS17R
Carbon and nitrogen source: For carbon source, basic medium (KMP) consisted of 1.5 g/l KH2PO4, 1,5 g/l K2HPO4, 2 g/l MgSO4, peptone 20 g/l. Various carbon sources (Glycerol, Glucose, Mannitol and Sucrose) were added at a rate of 10g /l. The basic medium of nitrogen was made of GKM (10 ml Glycerol, 1.5 g/l KH2PO4, 1,5 g/l K2HPO4, 2 g/l MgSO4).This medium was supplemented respectively with nitrogen sources at a rate of 20 g/l each. They were: Peptone, NH4Cl, (NH4)2SO4, (NH3)2SO4 and yeast extract. A volume of 200 ml of each medium was introduced in 250 ml conical flasks and the pH adjusted to 6.7 after sterilization. Two microliters of two days preculture of the bacteria suspension (1.5. 108 UFC/ml) were introduced into each flask. After 3 days of incubation, at 26°C, samples were collected and the cell growth monitored by measuring the optical density at 600 nm using UV spectroscopy (Biotech Ultrospec®3000).
Osmotic stress
The study of osmotic stress was made only with the best media conditions of C and N sources. Each medium was supplemented with different salt (NaCl, KCl and Na2SO4) at concentrations of 2.5; 5; 7.5 and 10% (w/v). Pseudomonas fluorescens DS17R inoculum was introduced in the media and growth monitoring was made as previously described.
pH and temperature
The effect of the pH and the temperature on the growth of P. fluorescens DS17R was performed as described by Petatán-Sagahón [21] with some modifications. Preculture of the rhizobacterium was grown at different pH conditions (4, 6, 8 and 10) and temperatures (20, 25, 28, 30, 35 and 40°C). The growth was monitored as previously described.
Secondary metabolites extraction
The crude extracts were prepared according to Liu et al. [22] and Ntyam et al. with some modifications. Briefly, the bacterium was grown in 200 ml of King B broth at 26±2°C for 72 hours and the pH value of this broth adjusted to 2.0 with 1 M HCl. After centrifugation at 28,000 rpm for 30 min, the supernatant was collected, and extracted four times with total of 120 ml of chloroform. Finally, the organic phase was evaporated under vacuum and residue dissolved in 500 μl of sterilized distilled water.
In vitro antimicrobial test
The antimicrobial assay of extracts against P. colocasiae was performed on Potato Dextrose Agar (PDA) plate using well diffusion method. A 5 mm agar plug of P. colocasiae was inoculated in the center of the plate and two wells (5 mm diameter) were created at the border of the plate equidistant from the center by punching the plate using sterile cork borer. One hundred microliter (100 μl) of chloroform extracts were added in each well while 100 μl of sterile distilled water were added in the control plates. The plates were then incubated at 26±2°C for 7 days and the radial growth of the pathogen measured. The inhibition of the radial growth was calculated according to the formula: %I=(Do-Dx)/Do X 100. Do is the growth of the pathogen on the control plate and Dx the growth in the test plate. All the tests were done in triplicate and the experiment repeated twice [21].
Quantification of Phenazines
Preliminary screening of the crude extracts for phenazines content was done by the reduction MnO2-based assay. Briefly, aqueous chloroform extracts (200 μl) obtained directly after extraction was mixed with 100 μl of MnO2 (3mM) in Eppendorf tube. The reaction of phenazine in the extract with MnO2 was detected after incubation at 30°C. The reduction of brown colored insoluble Mn (IV) to colorless Mn (II) was observed daily for 7 day. Phenazines were quantified on extracts from various nutritional conditions of growth (C and N source, pH and osmotic stress) by measuring the optical density at 367 nm using UV spectroscopy [23,24]. The total amount of phenazine in each extract was estimated using standard curve obtained by a range of concentrations (0.5, 1, 2, 4 and 16 μg/ mL) from a synthetic phenazine (Figure 1).
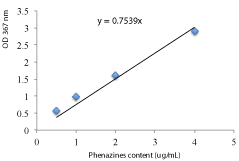
Figure 1: Standard curve related optical density (OD 367 nm) and pure
phenazine concentration (μg/mL).
Data analysis
Data obtained in different experiments were first subjected to Analysis of Variance (ANOVA) using SPSS for Mac Os statistical software version 22. The mean comparison of growth, antimicrobial test and quantification of phenazine was then performed using Duncan’s multiple range test. The correlation coefficient was determined to find a relationship between growth, C and N source, phenazine production and antagonism. Analysis by t-test confirms the relationship. Graph pad windows version 5.2 and SPSS were used at 5% (P < 0.05) level of significance.
Results
Effect of carbon and nitrogen sources on growth, extracts activity and phenazine production by DS17R
Table 1 shows the effects of two abiotic factors (C and N sources) on Pseudomonas fluorescens DS17R growth, antimicrobial activity and phenazine content of the extracts. These parameters varied significantly (p<0.05) with the two abiotic factors studied. Overall, the better growth rate occurred in the medium supplemented with glycerol as carbon source. Also, chloroform extract from this medium had the highest phenazine content (1.49 μg/mL) leading to a significant antimicrobial activity (82.50% of inhibition of mycelium). Moreover, peptone, yeast extract and ammonium chloride (NH4Cl) were the best nitrogen sources for the parameters studied i.e growth, antimicrobial activity and phenazine content. The antimicrobial activity was also characterized on agar plate by clear inhibition zones around the well containing chloroform extract (Figures 3). There was no correlation between Pseudomonas fluorescens DS17R growth, phenazine content and antimicrobial activity in the glycerol media supplemented with yeast extract. However, positives correlation were observed in the medium supplemented with peptone or NH4Cl as nitrogen sources (R2=0.6515 and R2=0.8996) (Figure 2).
Table 1: Growth, antimicrobial activity and Phenazine content of chloroform extracts under various C and N sources.
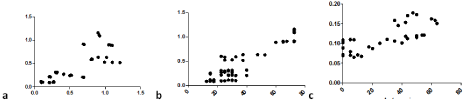
Figure 2: Correlation matrix between growth-phenazine content (a), growth-antimicrobial activity (b) and phenazine content-antimicrobial activity (c) of chloroform
extract at significant level a=0.05.
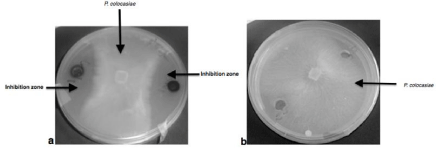
Figure 3: Growth inhibition of P. colocasiae by the chloroform extracts.
Effects of pH and temperature on the growth, antimicrobial activity and phenazine content of chloroform extracts
When Pseudomonas fluorescens DS17R was cultured on various pH and temperature, results showed that the growth rate, antimicrobial activity and phenazine content of the extract varied significantly with the abiotic factors. From the (Figures 4 and 5) we noticed that the optimum growth occurred around 28°C and pH 6 in both GN1 and GN2 media. There were a significant and positive correlation between growth and phenazine content (R2= 0.8092) as well as the activity of the extracts (R2= 0.716) at this temperature and pH. The same positive correlation was also observed at pH 8 in GN2 media. However, in GN1 medium a weak correlation was observed between growth, phenazine content and antimicrobial activity at pH 8 (R2= 0.3796).
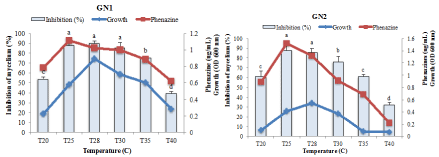
Figure 4: Effect of temperature on the growth of P. fluorescens (DS17R) into GN1 and GN2 carbon source and nitrogen after 48h of incubation.
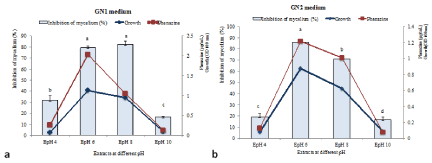
Figure 5: Effects of pH on growth, phenazine level and activity of DS17R under GN1 (a) and GN2 (b) sources of carbon and nitrogen after 48h of incubation period.
Osmotic stress effects on growth, phenazine content and antimicrobial activity of the extracts.
The effects of different salts concentration on growth, phenazine production and the activity of the extracts are shown in the (Figures 6 and 7). Overall, these parameters significantly (p<0.05) decrease with the osmotic stress. The best condition of growth and the extract activity occurred at 2.5% salt concentration in the tested media. At these concentrations, the activity of the extracts was always positively correlated with the growth as well as with phenazine production by Pseudomonas fluorescens DS17R
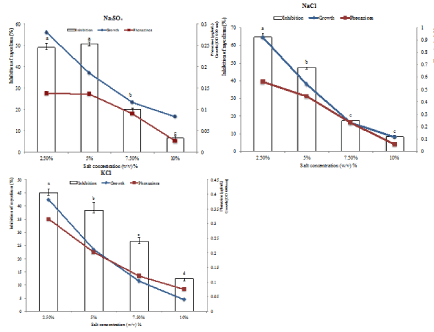
Figure 6: Effect of osmotic stress on antimicrobial activity of chloroform extracts under GN1 source of C and N.
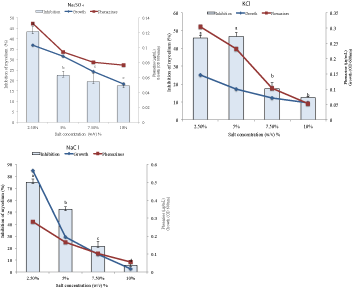
Figure 7: Effect of osmotic stress on antimicrobial activity of chloroform extracts under GN2.
Discussion
Pseudomonas fluorescens rhizobacteria can use a wide range of organic and inorganic compounds under diverse environmental conditions. In general, the productivity of microbial metabolites is closely related to the condition of culture used [25]. The changes of nutrients and their concentrations have different effects on the accumulation of different metabolites, which are controlled by intracellular effectors. Tjeerd et al. [26] showed that the carbon and nitrogen source can influence growth and antibiotic production by Pseudomonas sp. In this study we found that the growth, phenazine content ant activity of P. fluorescens DS17R extracts was influenced by different abiotic conditions including carbon, nitrogen, temperature, pH as well as osmotic stress. Tjeerd et al. demonstrated that the activity and the production of phenazine by PCL1391 fluorescent Pseudomonas was influenced by the pH of the culture medium. In some fluorescents Pseudomonas studied [27] the optimum pH for phenazine production varies with the microbial strain. Temperature has been reported to be a key factor influencing both growth of rhizobacteria and expression of bio-control mechanisms [28]. We found that under GN1 and GN2 media, the growth, phenazine content and antimicrobial activity vary between a range of temperature from 20 to 40°C. The activities of the extracts were correlated with the growth and phenazine production. This result could probably explain the role of phenazine as antimicrobial metabolite secondary metabolite produced by P. fluorescens DS17R. In recent study Waafa and Mostafa [29], showed that the growth inhibition of Botrytis by P. fluorescens was correlated with the production of secondary metabolites, including siderophores and phenazines in the culture media. Moreover, they found that the medium supplemented with glycerol as carbon source showed the best antimicrobial activity and siderophore production. In our study, glycerol gave similar result. When the pH of the media was adjusted to 8 we obtained similar phenomena. But the production of phenazine by the Pseudomonas fluorescens(DS17R) increases more when supplemented the medium at pH 6 by NH4Cl. This observation was also revealed by Slininger et al. [30] where the synthesis of phenazine by P. fluorescens increased at pH 6 and was correlated to the antimicrobial activity. Although the significant growth of P. fluorescens DS17R occured, there were no correlation with phenazine production and the extract activity. These findings suggest that, although the antimicrobial activity of phenazine is well known [31-33], other secondary metabolites like phenols and flavonoids could be present. In fact, previous work of Ntyam et al. revealed that the chloroform extract of Pseudomonas fluorescens DS17R contain secondary metabolites including phenol and flavonoid. They suggested that, the antimicrobial activity of these extract could be related to these compounds. The osmotic stress can also influence the antimicrobial potential of Pseudomonas [34]. P. fluorescens (DS17R) isolate tolerate NaCl and KCl at 2.5%. In contrast, Chaubey’s works showed that a concentration less than 2.5% of KCl and NaCl reduced the production of phenazine by Pseudomonas fluorescensPCL1391 strain. This could probably imply that a mechanism for salt concentration sensing is integrated into the regulation of phenazine production [35]. Besides, this production depends on salt concentration as well as Pseudomonas strains. In this regard we found that Pseudomonas fluorescens DS17R resists to osmotic stress compared to PCL1391 strain, which is sensitive to increasing NaCl, KCl, Na2SO4 stress resulting in a decrease of phenazine levels. Moreover, Pseudomonas fluorescens strain GP72 showed resistance to 5% NaCl solution, whereas strain 30–84 can survive only at a concentration of 4% [36]. The two strains also differ in their ability to use different substrates. From previous results, this work showed that Pseudomonas fluorescens DS17R could produce an increasing amount of phenazine at 2.5% salt concentration correlated to a great antimicrobial activity. These abiotic factors are important under practical plant production conditions and influence phenazine production severely. For successful biocontrol by Pseudomonads, one needs to understand which and how environmental factors affect the production of other antimicrobial secondary metabolite in potential biocontrol products. Environmental regulation seems to differ between strains which have to be selected for different field conditions for achieving successful biocontrol.
Conclusion
Pseudomonas fluorescens (DS17R) produces secondary antimicrobial metabolites including phenazines against Phytophthora colocasiae. The activity of the extracts is sometimes correlated with P.fluorescens DS17R growth and phenazine production. We found that the growth, phenazine production and the antimicrobial activity of the extracts was influenced by different abiotic factors including carbon and nitrogen source, temperature, pH as well as osmotic stress. Therefore selection of abiotic factors that match with certain field conditions could be a strong tool in achieving successful biocontrol by Pseudomonas fluorescens (DS17R). Further detailed investigations to evaluate the effect of Pseudomonas fluorescens (DS17R) on Phytophthora colocasiae in vivo should be undertaken.
Acknowledgment
Authors thank the United Nation University and the Institute of Natural Resources of Africa (UNU-INRA) for financing this research through UNU-INRA Ph. D internships and the Operating Unit (Institute of Food Technology ITA-Dakar, Biotechnology, Chemistry and Microbiology research units).
References
- MINADER (Ministère de l’Agriculture et du Développement Rural). Les faits marquants du secteur agricole au Cameroun. Note de conjuncture, 1er Semestre. 2010.
- FAOSTAT FAO Economic and Social Department. The Statistics Division. Major Food and Agricultural Commodities and Producers. 2011.
- Food and Agriculture Organization of the United Nations. Production de taro, Base de données. 2014.
- Misra RS. Management of Phytophthora leaf blight disease of taro. Tropical tuber crops: food security and nutrition. Oxford and IBH, New Delhi, India. 2010; 460–469.
- De La Fuente L, Thomashow LS, Weller DM, Bajsa N, Quagliotto L, Chernin L, et al. Pseudomonas fluorescens UP61 isolated from birdsfoot trefoil rhizosphere produces multiple antibiotics and exerts a broad spectrum of biocontrol activity. European Journal of Plant Pathology. 2004; 110: 671-681.
- Haas D, Défago G. Biological control of soil-borne pathogens by fluorescent pseudomonads. Nature Review of Microbial. 2005; 3: 307-319.
- Haas D, Keel C. Regulation of antibiotic production in root-colonizing Pseudomonas spp. and relevance for biological control of plant diseases. Annual Review of Phytopathology. 2003; 41: 117–153.
- Mazurier S, Corber T, Lemanceau P, Raaijmakers JM. Phenazine antibiotics produced by fluorescent pseudomonads contribute to natural soil suppressiveness to Fusariumwil. ISME J. 2009; 3: 977–991.
- Devaraj I, Nishanth LA, Paramasivan P, Manoharan S, Jeyaprakash R. Proteolytic enzyme mediated antagonistic potential of Pseudomonas aeruginosa against Macrophominaphaseolina. Indian Journal of Experimental Biology. 2013; 51: 1024-1031.
- Raaijmakers JM, de Bruijn I, de Kock MJD. Cyclic lipopeptide production by plant-associated Pseudomonas spp. diversity, activity, biosynthesis, and regulation. Molecular Plant Microbe Interaction. 2006; 19: 699-710.
- Dubuis C, Keel C, Haas D. Dialogues of root-colonizing biocontrol pseudomonads. European Journal of Plant Pathology. 2007; 119: 311-328.
- De Vleesschauwer D, Höfte M. Rhizobacteria-induced systemic resistance. Advance Bot Response. 2009; 51: 223-281.
- Kerr B, Riley MA, Feldman M, Bohannan B. Local dispersal and interaction promote coexistence in a real life game of rock-paper- scissors. Nature. 2002; 418: 171-174.
- Parejko JA. Phenazine-1-carboxylic acid-producing Pseudomonas spp. of the inland pacific northwest. doctor of philosophy in microbiology washington state university school of molecular biosciences. 2012; 223-226.
- Shanahan P, O’Sullivan DJ, Simpson P, Glennon JD, O’Gara F. Isolation of 2,4- diacetylphloroglucinol from a fluorescent pseudomonad and investigation of physiological parameters influencing its production. Applied Environmental Microbiology. 1992; 58: 353-358.
- Duffy BK, Defago G. Environmental factors modulating antibiotic and siderophore biosynthesis by Pseudomonas fluorescensbiocontrol strains. Applied Environmental Microbiology. 1999; 65: 2429-2438.
- Notz R, Maurhofer M, Schnider-Keel U, Duffy B, Haas D. Biotic factors affecting expression of the 2,4-diacetylphloroglucinol biosynthesis gene phlAin Pseudomonas fluorescensstrain CHA0 in the rhizosphere. Phytopathology. 2001; 91: 873-881.
- Baehler E, Bottiglieri M, Pechy-Tarr M, Maurhofer M, Keel C. Use of green fluorescent protein-based reporters to monitor balanced production of antifungal compounds in the biocontrol agent Pseudomonas fluorescensCHA0. Journal of Applied Microbiology. 2005; 99: 24-38.
- Ntyam MSA, Kouitcheu MLB, Souk LT, Tchameni NS, Sameza ML. Potential Use of Pseudomonas fluorescens Chloroform Extracts against Phytophthora colocasiae, the Causative agent of the Taro Leaf Blight (TLB). European Journal of Scientific Research. 2017; 146: 224-233.
- Sameza ML, Bedine BM, Tchameni NS, Nguemnang MLC, Jazet D PM. Potential use of Eucalyptus globulus essential oil against Phytophthora colocasiae the causal agent of taro leaf blight. European Journal of Plant Pathology. 2014; 140: 243–250.
- Petatán-Sagahón I, Anducho-Reyes MA, Silva-Rojas HV, Arana-Cuenca A, Tellez-Jurado A. Isolation of bacteria with antifungal activity against the phytopathogenic fungi Stenocarpella may this and Stenocarpellamacrospora. International Journal of Molecular Science. 2002; 12: 5522-5537.
- Liu HM, Dong DX, Peng HS, Zhang XH, Xu YQ. Genetic diversity of phenazine- and pyoluteorin-producing pseudomonads isolated from green pepper rhizosphere. Archives Microbiology. 2006; 185: 91–98.
- Whistler CA, Pierson LS. Repression of phenazine antibiotic production in Pseudomonas aureofaciensstrain 30–84 by RpeA. Journal of Bacteriology. 2003; 185: 3718–3725.
- Chaubey S. Studies on the antifungal metabolite production and biocontrol traits of Vignaradiata-growth-promoting strains of fluorescent pseudomonads. Thesis Doctor of Philosophy in microbiology. Faculty of Science, Department of microbiology, Maharaja Sayajirao University of Baroda, Vadodara – 390002, Gujarat, India. 2002; 210-220.
- Seuk C, Paulita T, Baker R. “Attributes Associate with Increased Bio-Control Activity of Fluorescent Pseudomonads”. Journal of Plant Pathology. 1998; 4: 218-225.
- Tjeerd E, Wesselink M, Thomas FC, Chin-A-Woeng, Guido V. Influence of Environmental Conditions on the Production of Phenazine-1-Carboxamide by Pseudomonas chlororaphisPCL1391. American Phytopathology Society. 2004; 17: 557–566.
- Ownley BH, Duffy BK, Weller DM. Identification and manipulation of soil properties to improve the biological control performance of phenazineproducing Pseudomonas fluorescens. Applied Environmental Microbiology. 69: 3333-3343.
- Ownley BH, Weller DM, Thomashow LS. Influence of in situ and in vitro pH on suppression of Gaeumannomyces graminis var tritici by Pseudomonas fluorescens2-79. Phytopathology. 1992; 82: 178-184.
- Wafaa M, Haggag MA. Production and Optimization of Pseudomonas fluorescensBiomass and Metabolites for Biocontrol of Strawberry Grey Mould. American Journal of Plant Science. 2002; 3: 836-845.
- Slininger PJ, Shea-Wilbu MA. Liquid-culture pH, temperature, and carbon (not nitrogen) source regulate phenazine productivity of the take-all biocontrol agent Pseudomonas fluorescens2-79. Applied Microbiology Biotechnology. 1999; 43: 794-800.
- Wood DW, Gong F, Daykin M, Williams P, Pierson LS. N-Acylhomoserine lactone-mediated regulation of phenazine gene expression by Pseudomonas aureofaciens30-84 in the wheat rhizosphere. Journal of Bacteriology. 1997; 179: 7663-7670.
- Mavrodi DV, Bonsall RF, Delaney SM, Soule MJ, Phillips G. Functional analysis of genes for biosynthesis of pyocyanin and phenazine-1- carboxamide from Pseudomonas aeruginosa PAO1. Journal of Bacteriology. 2001; 183: 6454- 6465.
- Zhang Z, Pierson LS. A second quorum sensing system regulates cell surface properties but not phenazine antibiotic production in Pseudomonas aureofaciens. Applied Environmental Microbiology. 2001; 67: 4305-4315.
- Sajben E, Manczinger L, Nagy A, Kredics L, Vágvölgyi C. Characterization of pseudomonads isolated from decaying sporocarps of oyster mushroom. Microbiology Response. 2007; 166: 255-267.
- Chin-A-Woeng TFC, van den Broek D, de Voer G, van der Drift KM, Tuinman S. Phenazine-1-carboxamide production in the biocontrol strain Pseudomonas chlororaphisPCL1391 is regulated by multiple factors secreted into the growth medium. Molecular Plant-Microbe Interaction. 2001; 14: 969- 979.
- Pierson LS, Thomashow LS. Cloning and heterologous expression of the phenazine biosynthetic locus from Pseudomonas aureofaciens30-84. Molecular Plant-Microbe Interaction. 1992; 5: 330-339.
Citation: Samuel Arsène NM, Laure Brigitte KM, Tounkara Lat S, Nguemezi Séverin T, Rosalie Anne NN and Modeste Lambert S. Abiotic Conditions on Growth of Pseudomonas fluorescens (DS17R) and Its Ability to Produce Secondary Metabolites (Including Phenazines) Against Phytophthora colocasiae, the Causal Agent of Taro Leaf Blight. Austin J Biotechnol Bioeng. 2018; 5(2): 1095.