
Research Article
Austin J Biotechnol Bioeng. 2021; 8(1): 1106.
Water Solubility and Dissolution Enhancement of Fisetin by Spherical Amorphous Solid Dispersion in Polymer of Cyclodextrin
Skiba M*, Gasmi H, Milon N, Bounoure F and Lahiani-Skiba M
Normandy University, UNIROUEN, France
*Corresponding author: Skiba M, Normandy University, UNIROUEN, DC2N INSERM U1239-Galenic Pharmaceutical Team, UFR of Health, 22 boulevard Gambetta 76000 Rouen, France
Received: March 08, 2021; Accepted: March 29, 2021; Published: April 05, 2021
Abstract
Fisetin (3, 7, 3', 4'-tetrahydroxyflavone) is an active ingredient characterized by a large spectrum of biological activities with a wide range of nutraceutical properties, including neuroprotecting, antidiabetic and suppression or prevention of tumors. The only drawback limiting its use is its low water solubility. The present work was focused on the solubility improvement of fisetin using spray-drying solid dispersion method, using also cyclodextrin polymers and which is extensively employed as technique to enhance poorly-water soluble compounds. Fisetin solubility was evaluated in the presence of poly-aβ-CD, poly-aγ-CD, poly-aβγ-CD and poly-methyl-β-CD by Higuchi solubility study. Spherical Amorphous solid dispersion formulations were prepared using polymethyl- β-CD (choice of the polymer was based on the better results of solubility study) at different ratios drug: polymer (1:1) and (1:3). In addition, different formulations were investigated using several techniques such as: Fourier Transformed Infrared Spectroscopy (FT-IR), Differential Scanning Calorimetry (DSC), Thermogravimetric Analysis (TGA), NMR and Scanning Electron Microscopy (SEM). The in vitro dissolution was performed in water at 37°C. The results of dissolution studies showed a complete dissolution observed with the formulation ratio 1:3 while the formulation ratio 1:1 showed a maximum dissolution rate about 59%. This difference is due to the formation of watersoluble inclusion complex and on the other hand to the high hydrophilicity of the cyclodextrin polymer.
Keywords: Fisetin; Cyclodextrin-polymer; Spherical amorphous solid dispersion; Spray-drying
Introduction
Fisetin (3, 7, 3', 4'-tetrahydroxyflavone) is a flavonol that belongs to the flavonoid group of polyphenols which is present in some fruits and vegetables [1] including strawberries [2], apples [3] and grapes [4]. It is characterized by a large spectrum of biological activities, which include: anti-inflammatory [5], antioxidant [6], apoptotic and anti-proliferative [7,8] action. Recently, fisetin has shown anti-cancer activity, in studies performed in vitro and in vivo, against several cancer types such as: lung cancer [9], prostate cancer [10], colon cancer [11] and breast cancer [12]. Fisetin is Class II drug according to BCS classification, its low solubility (<1 mg/mL) [13] and its low bioavailability are the limiting factors of its administration in vivo. To improve the dissolution rate of poorly water soluble drugs, several techniques were used such as particle size reduction (formation of micro- and nano-systems), formation of water soluble complexes [14] and amorphous solid dispersion preparations [15].
The solid dispersion technique is one of the most strategies used to improve oral bioavailability of poorly-water soluble drugs. It offers several advantages for enhancing drug solubility such as: (i) reduced particle size and thus the surface area is improved and increased dissolution rate ; (ii) improved wettability and this parameter dependent on the characteristic of drug carrier; (iii) transferring the drug from crystalline to amorphous state [16]. Various solid dispersion preparations of hydrophilic carrier/poorly water soluble drugs demonstrating the improvement of its solubility have been reported in the literature. Borba et al. showed that sodium alginate was successfully used as carrier in solid dispersion and demonstrated to be able to improve the apparent solubility of telmisartan (up to 16.7 fold) [17]. Another study was performed by Yuvaraja et al. which consists of using native cyclodextrins in solid dispersion formulation to enhance the solubility of carvedilol [18].
In the case of fisetin, several studies have been made to improve its water solubility and therefore its bioavailability. Among the latter, the nanoemulsion formation [19], liposome formulation [20,21], complexation with dimer of cyclosophoroase [22], cocrystallization with another substance [23] and formation of inclusion complex with natural cyclodextrins [24,25].
Native cyclodextrins a, β and γ are cyclic oligosaccharides derived primarily from starch by enzymatic degradation of this later and constituted by six, seven and eight glucopyranoside units; respectively. Cyclodextrins have a truncated conical shape and characterized by a hydrophilic outer surface and a hydrophobic internal cavity thus able to host other hydrophobic molecules forming inclusion complexes. The formation of the inclusion compounds greatly modifies the physical and chemical properties of the guest molecule, mostly in terms of water solubility. This is the reason why cyclodextrins have attracted much interest in many fields, especially pharmaceutical applications [26]. In the literature, some published papers have reported the interaction between fisetin and native cyclodextrins (mainly β and γ cyclodextrins) [24,27].
The aim of this work was to improve the solubility and the dissolution rate of fisetin by preparing amorphous solid dispersion using new polymers based on cyclodextrins (poly-aβ-CD, poly- aγ-CD, poly-aβγ-CD and poly-methyl-β-CD) as a hydrophilic carrier, which is characterized by higher water solubility and greater complexing property than nature cyclodextrins. The physicochemical characterizations were performed using different techniques such as DSC, TGA, FT-IR spectroscopy, NMR and SEM. The in vitro dissolution was performed in water at 37°C to evaluate the efficacy of the polymers on fisetin release. The Sink conditions were provided throughout the experiments.
Experimental Methods
Materials
Native cyclodextrins a, β and γ were ordered from Wacker (France). Citric acid and sodium phosphate dibasic were supplied by Sigma Aldrich (France). Fisetin was received from BiosynTec (China). Ethanol was obtained from VWR (France).
Preparation of co- and terpolymer Poly-aβ-CD, Poly-aγ- CD, poly-aβγ-CD and poly-methyl-β-CD
The copolymer Poly-aγ-CD was synthesized according to M.Skiba [28]. Briefly, a mixture of known amount (w : w) of cyclodextrin a, γ, citric acid and sodium phosphate dibasic was transferred into a reactor which was maintained at temperature ranging between 140- 150 °C for 15 to 30 min. the obtained solid form was dissolved in water and dialyzed using polyether sulfate membrane filter with molecular weight cut of 10000 Da. The dialysis was controlled by measuring the conductivity of the purified water at T0 and after 4 hours of dialysis, the resulted solution was spray-dried using Büchi Mini Sprayer Dryer B-290. Spray-dryer parameters were validated by preliminary works and were as follow: inlet temperature: 130°C; outlet temperature 80- 85 °C; aspiration 70%; pump: 10% and pressure: (-45 mbar). The same procedure was used to synthesis the poly-aβ-CD, poly-aβγ-CD and poly-permethyl-β-CD.
Phase solubility study
Phase solubility studies of fisetin were performed according to the method reported by Higuchi and Connors. Excess amounts of fisetin were added to amber glass flasks containing 5 mL of an aqueous solution of increased concentration of cyclodextrin copolymer: poly-aβ-CD, poly-aγ-CD, poly-aβγ-CD and poly-permethyl-β-CD, agitated in a thermostatically water bath maintained at 37±0.2 °C during 7 days. Samples were centrifuged, diluted and analyzed by UV-spectrophotometry (λ=360 nm; UV-1600 PC).
Solid dispersion preparation
A Büchi 290 nozzle type mini spray dryer (Flawil, Switzerland) was used for the preparation of fisetin-loaded solid dispersion using cosolvent method. Two solid dispersions were prepared with 1 g of fisetin and 1-3 g of poly-methyl-β-CD polymer in the weight ratio 1:1 and 1:3. Fisetin was dissolved in 230 mL of ethanol and the polymer was dissolved in 460 mL of water. The two solutions were mixed during 1h and delivered to the nozzle with 1.4 mm diameter, flow rate of pump at 15 % and spray-dried at 95±1 °C inlet temperature and 55±1 °C outlet temperature. The flow rate of the drying air was maintained at the aspirator setting of 80 % which indicated the pressure of the aspirator filter vessel as -45 mbar. The direction of air flow was the same as that of sprayed solution.
Preparation of physical mixture
The Physical Mixture (PM) was prepared through simple homogenization of fisetin and polymer powders with a mortar for 10 min in the ratio of 1:1 and 1:3 (w/w) until obtaining an apparent homogeneous powder and preserved in closed glass flasks.
Characterization of solid dispersions
Thermogravimetry analysis: The thermal stability of complexe fisetin/poly-methyl-β-CD, polymers (poly-methyl-β-CD) and also the physical mixtures were investigated using TGA 4000 thermogravimetric analyzer (TA instrument, PerkinElmer, USA). 1-3 mg of sample was heated at a rate of 10°C/min from 30°C to 400°C under dynamic nitrogen atmosphere. The upper limit of thermal stability of samples was taken as the initial sample weight loss.
Differential scanning calorimetry: Thermal analysis was conducted on PerkinElmer 6 DSC (USA). Samples (5-6 mg) were hermetically sealed in a flat-bottomed aluminum pan and heated from 30 to 400°C at a scanning rate of 10°C/min under a nitrogen gas flow. An empty aluminum pan was used as reference. The DSC cell was calibrated with indium (Tpeak 156°C) and zinc (Tpeak 419°C).
Fourier transformed infrared spectroscopy: The FT-IR spectra of fisetin, polymers, physical mixture and solid dispersion were acquired on a PerkinElmer SpectrumTM One Fourier-Transform Infrared (FT-IR) Spectrophotometer. Samples were scanned at room temperature from 4000 to 450 cm-1 and at a spectral resolution of 2 cm-1 and 10 scans were performed for each sample.
Scanning electron microscopy: The external morphology of solid dispersion formulations was studied using a FEI QUANTA 200 scanning electron microscope. Samples were fixed with a ribbon carbon double-sided adhesive and covered with a fine layer of carbon and analyzed.
NMR spectroscopy: 1H NMR spectra was used to determine the chemical shift changes of drug protons resulting of its interaction and insertion into the hydrophobic cavity of cyclodextrin. It was recorded at 22°C on a Bruker AVANCE III 400 NMR spectrometer using a presaturation of the water resonance, 32 K data points, a recycling delay of 2 s and a spectral width of 10 ppm. Samples were prepared by dissolving 1 to 2 mg of solid samples in Deuterated Water (D2O). Setting the water resonance at 4.75 ppm referenced the chemical shifts. 1H-NMR peak assignment was established from published data and by using basic correlation experiments.
In vitro dissolution
The dissolution study was performed in USP type II dissolution apparatus II (Vankel, VK7000). Briefly 22 mg of free fisetin or its equivalent of fisetin-loaded spray-dried dispersion formulations and physical mixtures were added to vessel containing 1 L of water at 37±0.2 °C with paddle speed of 100 rpm for 90 min. Two milliliters sample was withdrawn, replaced with fresh medium and then analyzed using UV-spectrophotometry (λ=360 nm; UV-1600 PC). Each experiment was conducted in triplicate and Sink conditions were provided throughout the experiments.
Results
Phase solubility studies
The phase solubility diagram at 37°C was obtained by plotting the equilibrium concentrations of fisetin against polymer cyclodextrins concentrations. Its solubility profile in various concentrations of cyclodextrin co- and ter-polymers was significantly increased as it was shown in Figure 1. A better result was observed with polymethyl- β-CD and poly-aγ-CD. According to Higuchi an Connors phase-solubility diagram classification [29], the solubility diagram of the complex fisetin/poly-methyl-β-CD and fisetin/ poly-aγ-CD can be classified as type An which is characterized by the formation of soluble inclusion complex between the guest and the host molecules and it was present in the aqueous solution [30]. The slope value was calculated from the linear plot of the phase solubility diagrams of poly-methyl-β-CD and poly-aγ-CD; it was equal to 0.015 and 0.013; respectively. Moreover, the slope values were lower than one which indicate that the inclusion complex was about 1:1 molar ratio between the fisetin and the cyclodextrin polymers, as it was reported by Doile et al. [31]. The fisetin solubility in water was increased to 3.71 mg/ mL and 2.96 mg/mL so 42-fold and 33-fold when poly-methyl-β-CD and poly-aγ-CD; respectively; were used in concentration of 800 mg/ mL compared to its initial solubility in water without CDs which is around 89 μg/mL. According to these results, poly-methyl-β-CD was chosen to be used for the preparation of solid dispersion formulations at different ratios.
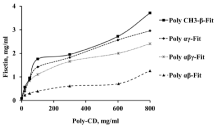
Figure 1: Representative phase solubility diagrams of fisetin in different
concentrations of poly-aβ-CD, poly-aγ-CD, poly-aβγ-CD and poly-rmethyl-β-
CD at 37°C.
Characterization of solid dispersions and inclusion complex
The DSC thermograms of physical mixtures and solid dispersion formulations were shown in Figure 2 and also the thermograms of polymer (poly-methyl-β-CD), fisetin powder (as received) were shown for reasons of comparison. It was reported in the literature that the DSC analysis can be used to identify the inclusion complex formation between the guest (drug) and the host (cyclodextrin polymer). When the drug was embedded in cyclodextrin cavities, their melting peak generally could be disappeared [32,33]. The DSC curve of poly-methyl-β-CD showed several peaks between 200-230 °C while the DSC thermogram of fisetin powder showed an endothermic peak at 330°C with the enthalpy of fusion was 169.93 J/g; corresponding to the melting peak of crystalline drug but this latter disappeared completely in the solid dispersion formulations prepared by spraydrying with poly-methyl-β-CD as carrier and their corresponding physical mixtures as shown in Figure 2. On the other hand, the melting peak of poly-methyl-β-CD was represented by a broad peak with reduced intensity. However, the absence of fisetin peak in solid dispersion formulations was the consequence of complete drug amorphization resulting in complete drug miscibility in polymer matrix which is due to fisetin:poly-cyclodextrin interactions and/or suggested the formation of inclusion complex [34].
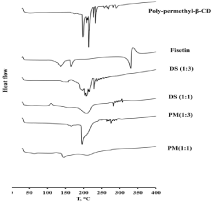
Figure 2: DSC thermograms of fisetin, poly-methyl-β-CD polymer, solid
dispersion formulations at ratio (1:1), (1:3) and their corresponding physical
mixtures.
The thermogravimetric analysis is performed to evaluate the thermal stability of poly-methyl-β-CD, fisetin, physical mixtures and also fisetin-cyclodextrin solid dispersion complex scanned between 30 to 400 °C at 10°C/min. Figure 3 shows a slight weight loss at about 5 % (w:w) observed for all samples at 60-110 °C and which present the absorbed water and water of crystallization. Fisetin showed a second important mass loss around 360 °C at about 68% (w:w) which can be probably due to the decomposition of the molecule. On the other hand, the poly-methyl-β-CD and their corresponding solid dispersion formulations and physical mixtures with different ratio presented the same profile with some difference in mass loss. Their second mass loss was observed approximately between 180 and 240 °C and it was varied of about 37% (w:w) for poly-methyl-β-CD. Spray-dried solid dispersions at ratios 1:1 and 1:3 and their corresponding physical mixtures presented a second mass loos ranged between 22-28 % and 18-21 % (w:w) for solid dispersions and physical mixtures at ratio 1:1 and 1:3; respectively. These can be explained by the modification in the cyclodextrin unit with the resulting loss of the crystalline nature of the cyclodextrin molecule [35,36]. Finally, the third important mass loss started at higher temperatures and it is the result of the decomposition of the cyclodextrin polymer. The difference in mass loss observed indicates that the formed complexes fisetin-cyclodextrins polymers were more stable than the polymer alone. In addition, we can note that the solid dispersion formulation in ratio 1:1 is more stable than ratio 1:3 when the poly-methyl-β-CD was used as carrier. The superposition of thermograms of solid dispersion formulations prepared by spray-drying and their corresponding physical mixtures gives information that the used preparation technique doesn’t alter the thermal stability of the obtained product.
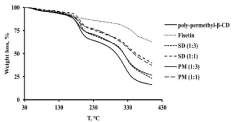
Figure 3: TGA of fisetin, poly-permethyl-β-CD and solid dispersion
formulations at ratio (1:1), (1:3) and their corresponding physical mixtures.
The variation of the shift and intensity of the IR absorption peaks of the guest or host can provide information for the inclusion complex formation. Figure 4 showed the FT-IR spectra of fisetin, cyclodextrin polymer (poly-methyl-β-CD) and also solid dispersion formulations and their corresponding physical mixtures at different guest: host ratio scanned between 4000 and 450 cm-1. IR spectrum of fisetin exhibited a characteristic peaks at 3446.54 cm-1 corresponding to O-H stretching; 1592.57 and 1463.04 cm-1 due to the aromaticring vibration and at 1112.03 cm-1 assigned to C-OH stretching vibration [37]. Interestingly, the FT-IR spectrum of solid dispersion formulations prepared with poly-methyl-β-CD at different ratio showed that the 3446.54 cm-1 and 1112.03 cm-1 FT-IR characteristic bands of fisetin disappeared completely. The intensity of 1463.04 cm-1 characteristic band appeared with reduced intensity and progressively disappeared by increasing polymer proportion which is accompanied by an increase of the intensity of polymer characteristic peak observed at 1724 cm-1 corresponding to the ester band which is resulting from the esterification reaction between the hydroxyl group of cyclodextrin and the carboxylic group of citric acid [35]. The fisetin band observed at 1592.57 cm-1 was shifted to 1607.2 cm-1 with reduced intensity. These results indicated substantial masking of the absorption peaks of fisetin by those of poly-methyl-β-CD and also confirm the formation of inclusion complex. On the other hand, the absence of new absorbance bands gives information that fisetin interacted with cyclodextrin polymers by noncovalent and weak intermolecular forces such as van der Waals [38] or hydrogen [13]. In contrast, the FT-IR spectra of physical mixtures at ratio 1:3 and 1:1 showed the presence of characteristic peaks of fisetin: 1112.03 cm-1 and 3446.54 cm-1 which is shifted to 3522.01 cm-1 and their intensity was reduced by increasing the proportion of cyclodextrin polymer. The slight shifting and disappearance of some absorption bands confirmed the presence of interaction between fisetin and cyclodextrins polymer.
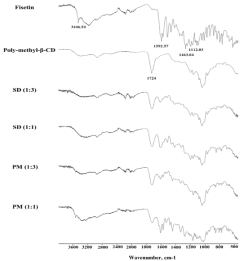
Figure 4: FTIR spectra of fisetin, poly- methyl-β-CD and solid dispersion
formulations at ratios (1:1), (1:3) and their corresponding physical mixtures.
Abstract
Figure 5 shows SEM pictures of spray-dried solid dispersions at several ratios of poly-methyl-β-CD. The different formulations were spray-dried at 55°C outlet temperature. As it can be seen, the spraydried particles are spherical in shape compared to those obtained by Boukhris et al. using high outlet temperatures varied between 80 and 90 °C. This can be explained by the lower internal pressure applied by the evaporation liquid at 55 °C which escape without deforming the solid shell, as it was reported by Maas et al. [39]. Moreover, the spray-dried formulation at ratio 1:3 showed more homogeneous surface with spherical morphology, whereas with lower proportion of polymer (ratio 1:1), slight concave depressions were observed. Note also the absence of drug particles adsorbed at the surface of the polymer matrix. These results demonstrated that fisetin was thoroughly blended in the poly-cyclodextrin matrix with the loss of its crystallinity (confirmed by DSC results). The SEM pictures give us as well an estimation of the particle size. The latter was ranged approximately between 1 and 4 μm.
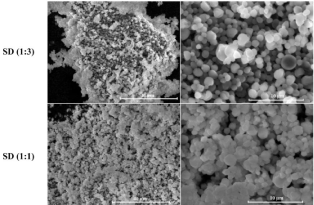
Figure 5: SEM pictures of spray-dried solid dispersions at ratios 1:3 and 1:1
of fisetin: poly-methyl-β-CD.
Furthermore, the interaction between fisetin and poly-methyl-β- CD was also evaluated by H1 NMR spectroscopy in D2O (Figure 6) in order to compare the chemical shift values of free and complexed fisetin. The comparison of the H1 NMR spectra of pure drug in H2O/DO2, its solid dispersion formulations and its corresponding physical mixtures with CD polymer showed that solid dispersion formulations provides a better solubilization of fisetin compared to their corresponding physical mixture. This result was based on the d chemical shift equivalent to 7.7 ppm with coupling constant J=30 Hz and an estimated proportion of the ratio H (7.7)/H1 beta = 8%.
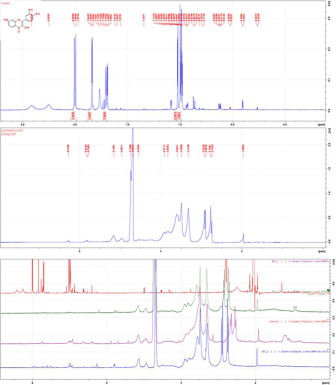
Figure 6: 1H NMR spectra (44 MHz), of Fisetin, Cd-Poly and C 1/1, MP 1/3,
MP 1/1 C3/1 the Top to Bottom.
The presence of interaction between fisetin and beta-CD was also reported in the literature by several studies. Guzzo et al. carried out a study to evaluate the complexation of fisetin with β-CD using several techniques in order to compare theoretical and experimental data and they reported that fisetin is able to complex with β-CD [13]. Another study performed by Nutho et al. using molecular dynamic simulation to investigate the complexation of fisetin with β-CD in aqueous solution and they concluded that there are four possible binding mode of fisetin in formed complex with β-CD [38].
In vitro dissolution study
The dissolution studies of fisetin (powder as received) and solid dispersion formulations prepared with poly-methyl-β-CD at two different ratios 1:1 and 1:3 (for reason of comparison, also their corresponding physical mixtures) were carried out in distilled water at 37°C. The fisetin powder showed a low dissolution rate in water at about 32 % during 90 min (as it can be seen in Figure 7) because of its hydrophobicity which caused the powder float on the surface of the dissolution medium. On the other hand, the dissolution rate of fisetin in physical mixtures was slightly increased to 36%, which can be explained by the solubilization effect of cyclodextrin polymer. The solid dispersion formulations with different host: guest ratio exhibited a faster dissolution rate of the molecular inclusion complex obtained compared to their corresponding physical mixtures and the pure drug. It increased from 59 to 97 % with increasing the proportion of poly-permethyl-β-CD. It was found that formulation with ratio 1:3 of fisetin: poly-permethyl-β-CD showed a maximum dissolution rate about 97% which is due to the formation of water-soluble inclusion complexes with cyclodextrin polymer and the entire amount of drug was released in 20 min.
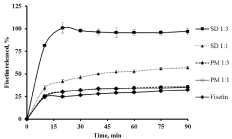
Figure 7: In vitro dissolution profile of fisetin (powder as received), Solid
Dispersion (SD) formulations ratios (1:1), (1:3) and their corresponding
physical mixtures in water at 37°C (n=3).
Moreover, the dissolution rate enhancing of fisetin in solid dispersion samples can be due to the amorphous state of drug (confirmed by DSC analysis). Sareen et al. reported in the literature that poorly-water soluble crystalline drugs in amorphous state generally present higher solubility. This improvement is related to the lack energy required to break crystal lattice in amorphous state during dissolution [40]. On the other hand; in solid dispersion formulations, the fisetin amorphization can be due to its interaction with the cyclodextrin polymer as it was confirmed by 1HNMR analysis, which showed a better solubilization of fisetin in solid dispersion formulations than in physical mixtures. The increase of the dissolution rate of fisetin in solid dispersion formulations can be explained also by the high hydrophylicity of poly-methyl-β- CD (aqueous solubility >1g/mL); this polymer property enhances the drug solubility by reducing the interfacial tension between the solid particles of drug and dissolution medium, thus, increasing its wettability [41]. In addition, the poly-methyl-β-CD is represented as a polymeric network in which the drug can be entrapped between the meshes and therefore an increase in its dissolution rate. Also, the reduction size of the obtained particles by solid dispersion formulations (confirmed by SEM analysis) plays an important role in the improvement of fisetin dissolution.
Conclusion
The solid dispersion technique based on poly-methyl-β-CD as carrier can be employed to improve the dissolution rate of fisetin. Dissolution studies indicated a better solubilizing effect of CD solid dispersion formulations than their corresponding physical mixture. The dissolution rate of fisetin from solid dispersion formulations depended on the proportion of the carrier and which is increased with an increase in carrier ratio. DSC, FTIR and NMR spectroscopy showed the presence of interaction between fisetin and poly-methyl- β-CD. The dissolution rates of solid dispersion formulations were higher than those of physical mixtures and pure drug and which can be explained by the increase of drug wettability. The solid dispersion technique using poly-methyl-β-CD as a carrier presents a promising tool to enhance the dissolution rate of fisetin. On the other hand, the combination of advantages of spray drying technique and CD polymer properties can provides a promising strategy to overcome the solubility problem of poorly water-soluble substances.
References
- Fiorani M, Accorsi A. Dietary flavonoids as intracellular substrates for an erythrocyte trans-plasma membrane oxidoreductase activity. Br J Nutr. 2005; 94: 338-345.
- Maher P, Dargusch R, Ehren JL, Okada S, Sharma K, Schubert D. Fisetin lowers methylglyoxal dependent protein glycation and limits the complications of diabetes. PLoS ONE. 2011; 6: e21226.
- Arai Y, Watanabe S, Kimira M, Shimoi K, Mochizuki R, Kinae N. Dietary intakes of flavonols, flavones and isoflavones by Japanese women and the inverse correlation between quercetin intake and plasma LDL cholesterol concentration. J Nutr. 2000; 130: 2243-2250.
- Vinas P, Martinez-Castillo N, Campillo N, Hernandez-Cordoba M. Directly suspended droplet microextraction with in injection-port derivatization coupled to gas chromatography-mass spectrometry for the analysis of polyphenols in herbal infusions, fruits and functional foods. J Chromatogr A. 2011; 1218: 639-646.
- Park H-H, Lee S, Oh J-M, Lee M-S, Yoon K-H, Park BH, et al. Antiinflammatory activity of fisetin in human mast cells (HMC-1). Pharmacological Research. 2007; 55: 31-37.
- Woodman OL, Chan EC. Vascular and anti-oxidant actions of flavonols and flavones. Clin Exp Pharmacol Physiol. 2004; 31: 786-790.
- Jang KY, Jeong S-J, Kim S-H, Jung JH, Kim J-H, Koh W, et al. Activation of reactive oxygen species/AMP activated protein kinase signaling mediates fisetin-induced apoptosis in multiple myeloma U266 cells. Cancer Lett. 2012; 319: 197-202.
- Sinha R, Srivastava S, Joshi A, Joshi UJ, Govil G. In-vitro anti-proliferative and anti-oxidant activity of galangin, fisetin and quercetin: role of localization and intermolecular interaction in model membrane. Eur J Med Chem. 2014; 79: 102-109.
- Ravichandran N, Suresh G, Ramesh B, Siva GV. Fisetin, a novel flavonol attenuates benzo(a)pyrene-induced lung carcinogenesis in Swiss albino mice. Food Chem. Toxicol. 2011; 49: 1141-1147.
- Khan N, Afaq F, Syed DN, Mukhtar H. Fisetin, a novel dietary flavonoid, causes apoptosis and cell cycle arrest in human prostate cancer LNCaP cells. Carcinogenesis 2008; 29: 1049-1056.
- Suh Y, Afaq F, Johnson JJ, Mukhtar H. A plant flavonoid fisetin induces apoptosis in colon cancer cells by inhibition of COX2 and Wnt/EGFR/NF-κBsignaling pathways. Carcinogenesis. 2009; 30: 300-3007.
- Yang P-M, Tseng H-H, Peng C-W, Chen W-S, Chiu S-J. Dietary flavonoid fisetin targets caspase-3-deficient human breast cancer MCF-7 cells by induction of caspase-7-associated apoptosis and inhibition of autophagy. Int J Oncol. 2012; 40: 469-478.
- Guzzo MR, Uemi M, Donate PM, Nikolaou S, Machado AEH, Okano LT. Study of the Complexation of Fisetin with Cyclodextrins. J Phys Chem A. 2006; 110:10545-10551.
- Kawabata Y, Wada K, Nakatani M, Yamada S, Onoue S. Formulation design for poorly water-soluble drugs based on biopharmaceutics classification system: basic approaches and practical applications. Int J Pharm 2011; 420: 1-10.
- Huang Y, Dai W-G. Fundamental aspects of solid dispersion technology for poorly soluble drugs. Acta Pharm Sin B. 2014; 4: 18-25.
- Vasconcelos T, Sarmento B, Costa P. Solid dispersions as strategy to improve oral bioavailability of poor water soluble drugs. Drug Discovery Today. 2007; 12: 1068-1075.
- Borba PAA, Pinotti M, de Campos CEM, Pezzini BR, Stulzer HK. Sodium alginate as a potential carrier in solid dispersion formulations to enhance dissolution rate and apparent water solubility of BCS II drugs. Carbohydrate Polymers. 2016; 137: 350-359.
- Yuvaraja K, Khanam J. Enhancement of carvedilol solubility by solid dispersion technique using cyclodextrins, water soluble polymers and hydroxyl acid. Journal of Pharmaceutical and Biomedical Analysis. 2014; 96: 10-20.
- Ragelle H, Crauste-Manciet S, Seguin J, Brossard D, Scherman D, Arnaud P, et al. Nanoemulsion formulation of fisetin improves bioavailability and antitumour activity in mice. International Journal of Pharmaceutics. 2012; 427: 452-459.
- Seguin J, Brullé L, Boyer R, Lu YM, Romano MR, Touil YS, et al. Liposomal encapsulation of the natural flavonoid fisetin improves bioavailability and antitumor efficacy. International Journal of Pharmaceutics 2013; 444:146- 154.
- Mignet N, Seguin J, Ramos Romano M, Brulle L, Touil YS, Scherman D, et al. Development of a liposomal formulation of the natural flavonoid fisetin. International Journal of Pharmaceutics. 2012; 423: 69-76.
- Jeong D, Choi JM, Choi Y, Jeong K, Cho E, Jung S. Complexation of fisetin with novel cyclosophoroase dimer to improve solubility and bioavailability. Carbohydrate Polymers. 2013; 97: 196-202.
- Sowa M, Slepokura K, Matczak-Jon E. Improving solubility of fisetin by cocrystallization. Cryst Eng Comm. 2014; 16: 10592-10601.
- Zhang J, Jiang K, An K, Ren S-H, Xie X, Jin Y, et al. Novel water-soluble fisetin/cyclodextrins inclusion complexes: Preparation, characterization, molecular docking and bioavailability. Carbohydrate Research. 2015; 418: 20-28.
- Pahari B, Sengupta B, Chakraborty S, Thomas B, McGowan D, Sengupta PK. Contrasting binding of fisetin and daidzein in γ-cyclodextrin nanocavity. Journal of Photochemistry and Photobiology B: Biology. 2013; 118: 33-41.
- Thatiparti TR, Shoffstall AJ, von Recum HA. Cyclodextrin-based device coatings for affinity-based release of antibiotics. Biomaterials 2010; 31: 2335- 2347.
- Banerjee A, Sengupta PK. Encapsulation of 3-hydroxyflavone and fisetin in β-cyclodextrins: Excited state proton transfer fluorescence and molecular mechanics studies. Chemical Physics Letters. 2006; 424: 379-386.
- Brevet WO2011080422A1-Procede de synthese de copolymeres, terpolymeres et tetrapolymeres de calixarenes et/ou cyclodextrines et leurs utilisations. 2016.
- Higuchi T, Connors K. Phase-solubility techniques. Adv Anal Chem Instr. 1965; 4: 117-212.
- Uekama K, Hirayama F, Irie T. Cyclodextrin Drug Carrier Systems. Chem Rev. 1998; 98: 2045-2076.
- Doile MM, Fortunato KA, Schmücker IC, Schucko SK, Silva MAS, Rodrigues PO. Physicochemical properties and dissolution studies of dexamethasone acetate-beta-cyclodextrin inclusion complexes produced by different methods. AAPS PharmSciTech. 2008; 9: 314-321.
- George S, Vasudevan D. Studies on the Preparation, Characterization, and Solubility of 2-HP-β-Cyclodextrin-Meclizine HCl Inclusion Complexes. J Young Pharm. 2012; 4: 220-227.
- Dawoud AA, Al-Rawashdeh N. Spectrofluorometric, thermal, and molecular mechanics studies of the inclusion complexation of selected imidazolinederived drugs with β-cyclodextrin in aqueous media. J Incl Phenom Macrocycl Chem. 2007; 60: 293-301.
- Pravin N, Babasaheb A, Neha D, Vilasrao K, Rajashree H. Solid state characterization of the inclusion complex of valsartan with methyl β-cyclodextrin. J Incl Phenom Macrocycl Chem. 2009; 65: 377-383.
- Skiba M, Lahiani-Skiba M. Novel method for preparation of cyclodextrin polymers: physico-chemical characterization and cytotoxicity. J Incl Phenom Macrocycl Chem. 2012; 75: 341-349.
- Shown I, Murthy CN. Grafting of cotton fiber by water-soluble cyclodextrinbased polymer. J Appl Polym Sci. 2009; 111: 2056-2061.
- Heneczkowski M, Kopacz M, Nowak D, Kuzniar A. Infrared spectrum analysis of some flavonoids. Acta Pol Pharm. 2001; 58: 415-420.
- Nutho B, Khuntawee W, Rungnim C, Pongsawasdi P, Wolschann P, Karpfen A, et al. Binding mode and free energy prediction of fisetin/β-cyclodextrin inclusion complexes. Beilstein J Org Chem. 2014; 10: 2789-2799.
- Maas SG, Schaldach G, Littringer EM, Mescher A, Griesser UJ, Braun DE, et al. The impact of spray drying outlet temperature on the particle morphology of mannitol. Powder Technology. 2011; 213: 27-35.
- Sareen S, Mathew G, Joseph L. Improvement in solubility of poor watersoluble drugs by solid dispersion. Int J Pharm Investig. 2012; 2: 12-17.
- Guyot M, Fawaz F, Bildet J, Bonini F, Lagueny A-M. Physicochemical characterization and dissolution of norfloxacin/cyclodextrin inclusion compounds and PEG solid dispersions. International Journal of Pharmaceutics. 1995; 123: 53-63.