
Research Article
Austin J Biotechnol Bioeng. 2021; 8(1): 1108.
Evaluation of Cell-Viability, Intracellular Lipid-Component and Efficiency of Lipid-Extraction of Chlamydomonas reinhardtii Cells Treated by UV-C Irradiation Aiming to Use Cell Directly
Nakanishi A1,2*, Ozawa N†1, Watanabe M2 and Sakihama Y3
¹Graduate School of Bionics, Tokyo University of Technology, Hachioji, Japan
²School of Bioscience and Biotechnology, Tokyo University of Technology, Hachioji, Japan
³Tokyo University of Technology, Hachioji, Japan
†Co-first author
*Corresponding author: Nakanishi A, Graduate School of Bionics, Tokyo University of Technology, 1404-1 Katakuramachi, Hachioji, Tokyo, Japan; School of Bioscience and Biotechnology, Tokyo University of Technology, 1404-1, Katakuramachi, Hachioji, Tokyo, Japan
Received: March 20, 2021; Accepted: April 20, 2021; Published: April 27, 2021
Abstract
Aim: We aimed finally to construct a system to utilize intracellular lipids of Chlamydomonas reinhardtii by direct cell-use. To realize the system, a system of simple cell-sterilization to avoid environmental contamination without degradation of intracellular lipids was required. Industrially, a simple collecting system of internal lipids was also required.
Methods and results: C. reinhardtii cultured in a photo bioreactor under autotrophic condition was irradiated by UV-C. After the irradiation with different time to cells under different culturing conditions, those cells were evaluated for cell-viability by staining with neutral red, intracellular cell components and efficiency of lipid-extraction with GC/FID, respectively. By UV-C irradiation for 10 min, C. reinhardtii cells after N-depletion were sterilized. Additionally, although the cells were morphologically crumbled under an optical microscope, the contents and the components of intracellular lipids showed few differences.
Conclusion: C. reinhardtii cells were efficiently sterilized by UV-C irradiation and few treated cells leaked the intracellular lipids, indicating that the lipids could not be simply collected by centrifuging direct cell collection.
Significance and impact of study:
In this study, the sterilized cells could gradually leak the intracellular contents, indicating the possibility of direct-use of the cells to utilize lipids produced by C. reinhardtii.
Keywords: Green alga; Algal lipids; UV-C treatment; Direct cell-use
Abbreviations
GC/FID: Gas chromatography/flame ionization detector; N-depletion: Nitrogen depletion; OD: Optical Density; PBR: Photo- Bioreactor; SEM: Scanning Electron Microscope; UV: Ultraviolet; vvm: Volume per Volume per Minute
Introduction
In recent years, bio-production by photosynthetic plants has attracted attention because of the effectiveness of plant-derived components such as vitamins and oils, carbon recyclability and low environmental burden [1,2]. Regarding bio-production by plants, the material productivity is calculated by the product of biomass productivity and content of intracellular material [3], resulting in enhanced biomaterial productivity with high biomass productivity. Relating to biomass productivity, several green algal strains show superior biomass productivity with higher carbon fixation efficiency more 10-50 times than popular terrestrial plants [4] for instance, Chlamydomonas sp. JSC4 and Dunaliella salina exhibited high biomass productivity as 915mgL-1d-1 [5] and 540mgL-1d-1 [6], respectively. Depending on the high biomass productivity, green algae Coccomyxa subellipsoidea and Haematococcus pluvialis demonstrated high material productivity of 232.37mgL-1d-1 of lipid [7] and 17.1mgL-1d-1 of astaxanthin [8]. Among the green algae, the unicellular green alga Chlamydomonas reinhardtii is especially paid attention in industrial use in recent years [9] because of accumulation of the information and technology of culturing condition [10], metabolic flow [11] and safety [12] regarding to this strain so far as a model green algae. Particularly, the proven safety of C. reinhardtii is an advantageous property for the use of its metabolites as value-added products in the industrial fields of cosmetics and foods. C. reinhardtii is able to accumulate approximate 10~20 % lipid (e.g., linoleic acid, lauric acid, stearic acid) as its cell component [13,14] so those lipids are expected to be used for moisturizing in the cosmetic field [15]. The use of lipids as the cell component, however, faces the problem of cost to extract lipids from the cells because of the robustness of the cell [16]. To extract lipids from the cell, C. reinhardtii needs strong crashing systems such as a bead-beater system [17]. Thus, research and development simply to use intracellular lipids could lead to an increase of the value of lipids derived from C. reinhardtii as a commercial use. Then, our study aimed to construct the system directly to use the cells for simple utilizing the intracellular lipids without the process of lipid purification based on non-toxicity [12]. The system, however, could be useful for industrial use; the direct use of living cells as the protector of intracellular metabolites could possibly cause contamination in the environment after releasing those cells to the environment [18], requesting a treatment simultaneously for supporting the efficient lipid-extraction and killing the cell. So far, the treatments using Ultraviolet (UV) irradiation have be considered [19], and the report revealed that the treatment using UV-B (280~320 nm) triggered the easy lipid-extraction because of necrosis and apoptosis of C. reinhardtii. However, the report focused on the extraction of intracellular lipids not aiming for complete cell death and does not evaluate the extraction efficiency relating nitrogen depletion in detail. Therefore, the treatment using UV irradiation needs more evaluations and improvements simply to use intracellular lipids by direct cell-use. On the other hand, although there are the reports relating the efficiency of sterilizing C. reinhardtii cells by the UV-C treatment, those did not refer to the efficiency of lipidextraction [20,21]. Therefore, the system-construction to use cells directly for the efficient use of intracellular lipids by UV treatment could be drastically meaningful on industrial use.
In this study, C. reinhardtii containing lipids was treated by UV-C irradiation, and the cell viability was evaluated by using the staining method with neutral red. Simultaneously, the production and composition of lipids in the treated cells were analyzed to evaluate the effect of UV-C treatment using GC/FID. The use of lipids from the UV-C treated C. reinhardtii cells could lead the progression of industrially green-algal use.
Materials and Methods
Microalgal strain and PBR operation
Chlamydomanas reinhardtii strain C-9: NIES-2235 was cultivated in a photobioreactor filled with Modified Bold 3 N medium (MB3N) as described in a previous study [22]. The photobioreactor was equipped with white fluorescent lamps (100μmol photons·m-2·s-1) and a bubbling system of 0.8% CO2 gas at an aeration rate of 0.05vvm and worked at room temperature (23°C).
Evaluation of culturing condition
Cell growth was evaluated as cell numbers using value of Optical Density (OD) of 750nm with a spectro-photometer U-2900 (Hitachi, Tokyo, Japan) via appropriate calibration curve for OD750 versus cell numbers. The pH of the broth was measured with pH meter FEP20 (Mettler Toledo, Tokyo, Japan) after centrifugation at 5000×g for 1min at 23°C. Nitrate concentration was measured using an optical method. The broth was centrifuged at 5,000×g for 1 min at 23°C, and the supernatant was collected and filtered with a 0.45μm filter (Millex®-LCR 13mm, Millipore, Carrigtwohill, Ireland). The flow through was diluted 50-fold with distilled water, and the absorbance of the diluted supernatant was measured at 220nm (i.e., Abs220) using a spectro-photometer U-2900. The residual nitrate content was evaluated using an appropriate calibration curve for Abs220 versus nitrate concentration.
Evaluation of cell viability after UV-C treatment
Cells (5.0×105 cells) were irradiated in 2mL of phosphate buffered saline (PBS) (pH=7.4) on a 33mm diameter glass share by using a UV irradiator Etbella (SEVEN BEAUTY Co., Ltd., Tokyo, Japan) equipped with a UV-C lamp (the ultraviolet radiation output: 3.49mWcm-2). One hundred eighty μL of the UV-treated suspension (4.5×104 cells) were mixed with 20μL of neutral red (red pigment) (Tokyo Kasei Co., Ltd., Tokyo, Japan) for 5 minutes [23]. The neutral red solution (1.5mgmL-1) was prepared as below, 1.5mg of neutral red was dissolved in 1ml of PBS (pH=7.4); the solution was filtered with 0.22μm filter (Nylon Syringe Filter, Membrane-Solutions). After the staining process, the cells were washed with PBS, and the viability was evaluated by observing the stain-treated cells on cell counter plates (Fukae Kasei Co., Ltd, Kobe, Japan). The life/death of the cells was decided with the criterion: staining/not staining with neutral red.
Evaluation of intercellular contents of chlorophyll a/b after UV-C treatment
Cell-weights were evaluated for constant irradiation of UV-C to the cells. The cell-concentration in the broth was measured by using a calibration curve of OD750-weight, and then cells (0.1-0.5 mg) were collected from the broth by centrifugation at 5,000×g for 3min at 23°C. After discarding the supernatant, 1mL of methanol was added and vigorously stirred using putiburu MODEL 2330 (Waken B tech Co., Ltd, Kyoto, Japan) at 2,000rpm for 5min at 23°C. The solution containing chlorophyll a/b was collected by centrifugation at 5,000×g for 3min at 23°C. To evaluate the concentration of chlorophyll a/b, the values of A665 and A652 of the collected solution were measured after drawing a baseline with A750 using methanol with a spectrophotometer U-2900 and substituted in the formula as below, chlorophyll a (μgmL-1): 16.72 × A665 - 9.16 × A652; chlorophyll b (μgmL-1): 34.09 × A652 - 15.28 × A665 [24]. The contents of chlorophyll a/b in the cells were finally shown using the unit μgmg-1.
Preparation for analysis of lipid-extraction efficiency
One mg of cells was harvested from broth after 3 days of nitrogen depletion by centrifugation (5000×g, 5min, 23°C) and treated by following 3 steps of cell-breaking processes as below: 1) UVtreatment step, 2) glass beads-adding step, and 3) shaking step. In the 1) step, the cells were UV-irradiated for 60min on a 33mm diameter glass share with a UV irradiator Etbella (SEVEN BEAUTY Co., Ltd.). In the 2) step, the glass beads of 0.5mm were added into the cells. In the 3) step, the cells were vortexed at 23°C under the condition (2,500rpm, 60min) with putiburu MODEL 2330 (WakenBtech Co., Ltd, Kyoto, Japan). After those processes, the treated cell-components were dried with an evaporator and weighted in detail.
Evaluation of lipid-quantity and profile
The total lipids were esterified and methylated with fatty acid methylation kit (Nacalai Tesque, Kyoto, Japan). The fatty acid methyl esters were identified and quantified using a capillary gas chromatograph GC-2025 (Shimadzu, Kyoto, Japan) equipped with a DB-23 capillary column (60m, 0.25mm internal diameter, 0.15μm film thickness) (Agilent Technologies, CA, United States) with method previously described by Nakanishi et al. [13]. Heptadecanoic acid (Sigma-Aldrich Co., MO, US) was used as an internal standard, and rapeseed oil (Merck KGaA, Darmstadt, Germany) was used as a quantitative standard.
Results and Discussion
This study aimed to construct a practical system that could easily use intracellular lipids by directly using the cells of C. reinhardtii. First of all, the time-course profile of C. reinhardtii under each culture condition was evaluated (Figure 1). In this study, C. reinhardtii was cultured in autotrophic MBBM, resulting that the biomass production was 248 ± 20 mgL-1 at 96h (4d) of culture. So far, the abundant information of biomass production of C. reinhardtii cultured in various media has shown various data: around 700mgL- 1 in 4d of culture in a well-growing heterotrophic TAP medium; around 150mg in an autotrophic HSM medium [25], indicating that the autotrophic culturing system in this study works well. Although the nitrogen depletion was shown at 4d, the biomass production constantly increased and finally reached 1.8 ± 0.3gL-1 in 13d (9d of nitrogen depletion (9d N-depletion)). Additionally, the results also indicated that the cultural conditions at 4d (0 d N-depletion) and 13d (9 d N-depletion) showed initial and late log-phase in this study, respectively (i.e., log-phase: 4 ~ 13 d).
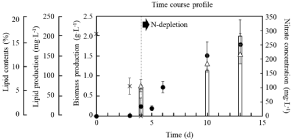
Figure 1: Time-course profiles of biomass production, lipid content, lipid
production and nitrate concentration during growth of C. reinhardtii on MB3N.
Biomass production, lipid content, lipid production and nitrate concentration
were shown with circle, △, bar and cross. Error bars indicate standard
deviation of three replicated experiments.
Increasing lipid contents in the cells were generally triggered by depletions of medium-sources of nitrogen, phosphate and sulphate so a lot of trials of lipid-production by Chlamydomonas sp. was performed by N-depletion [26,27]. In this case, 6.2%, 10.5% and 12.4% of lipid contents were shown at N-depletions of 0 d, 6 d and 9 d, indicating that the trend increasing the contents could be derived from nitrogen starvation. Those lipid productions were slightly similar to ones in previous reports [19] so that the generality was certified for lipid-producing ability by C. reinhardtii and culturing system as PBR used in this study.
To construct a practical system to apply intracellular lipids by directly using cells, the user-friendly sterilizing system should be developed. In this study, UV-C irradiation, which is widely used for sterilizing microorganisms, was selected. For using the intracellular lipids, the cells after N-depletion should be required since the N-depletion strongly triggers to increase the lipids in the cells of C. reinhardtii [13,14]. Then, the cell-condition (e.g., viability and composition) should be different in each culture condition so that the effects of the sterilizing treatment by UV-C irradiation were evaluated on 0 d, 6 d and 9 d of N-depletion (Figure 2). As the results, the viabilities of cells before UV-C irradiation were 82.9%, 9.8% and 1.4% at 0 d, 6 d and 9 d of N-depletion, meaning that extending the culturing days after N-depletion led to decreasing the viabilities. The low cell-viabilities less than 10% at 6 d and 9 d of N-depletion and the effectively UV-irradiating affection to cells in log-phase rather than ones in stationary-phase [19] indicated the possibility effectively to sterilize the cells in N-depletion by using UV-C irradiation. The UV-C irradiation actually affected those cells as the sterilizer and the ten minutes irradiation completely sterilized cells at all conditions prepared in this study. However, five or less than five minutes irradiation should be required as a sterilizing treatment because of the possibility of adverse effects (e.g., lipid peroxidation) deriving from the high energy of UV-C. The UV-C treatment in 5 min showed 21.2%, less than 1% and 0% of cell-viabilities at 0 d, 6 d and 9 d of N-depletion. The result indicated that the UV-C treatment could lead to the complete death with progressively N-depleting cells. Especially, the one-minute treatment by UV-C was enough to reach 0% of the viability of cells at 9 d N-depletion, showing the effective sterilization. On the other aspect, UV treatment in this study was a useful system since the output of UV was 3.5mWcm-2, meaning that the energyrequirement was lower than 1.5Wcm-2 of the output of UV previously reported [19].
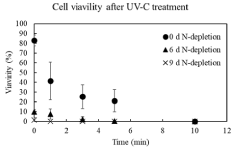
Figure 2: Cell-viability after UV irradiation for 10min. Time-course profiles
of cell-viabilities on 0 day, 6 day and 9 day after nitrogen depletion were
shown as circle, triangle and cross, respectively. Error bars indicate standard
deviation of three replicated experiments.
The purpose in this study is to use intracellular lipids after UV-C treatment simply sterilizing C. reinhardtii. Therefore, the time-course shift of amounts of chlorophyll and the change of lipid composition related to UV-C irradiation were analyzed to evaluate the effect of UV-C in cells (Figure 3 and Table 1). The total amount of intracellular chlorophyll a and b at 0 d N-depletion showed a tendency to increase once at 5 minutes of UV-C irradiation and then decrease at 15 minutes. To date, Nassour et al. reported that short-term UV-C irradiation to C. reinhardtii increases the intracellular content of chlorophyll a and b [24]. In particular, UV-C irradiation was known to increase chlorophyll a in C. reinhardtii [24; 28] and chlorophyll b in C. reinhardtii [24] and Chlorella sp. [29]. On the other hand, several reports referred to chlorophyll degradation because of the collapse of chlorophyll on thylakoid membrane by UV-treatment [24,30-32]. The basic knowledge indicated the tendency in the cells at 0 d N-depletion as follows: the increasing the amount of chlorophyll a and b until 5min of UV-C irradiation, i.e., progressing cell-death as shown in Figure 2; the maintaining the amount of chlorophyll a and b to 10min of the irradiation; the decreasing the amount of chlorophyll a and b toward 15min of the irradiation. This study also revealed the higher amount of chlorophyll b than chlorophyll a and the increase of those amounts as response to the UV-C irradiation, which was remarkable in the cells at 0 d N-depletion. A previous report indicated that chlorophyll b could perform not only to transfer the light energy to the reaction centers in photosynthetic systems II and I but also to protect chlorophyll a from strong light such as UV [24], supporting the explanation of our results. In cells at 6 d N-depletion, the total amount of intracellular chlorophyll also showed a tendency of increase once at 5 minutes of UV-C irradiation time, and then of decrease at 15 minutes. Although the response of cells at 6 d N-depletion was weak rather than one at 0 d N-depletion, the tendency could be deeply related to the lower cell-viability (9.8%) at 6 d N-depletion than at 0 d N-depletion. In addition, in cells at 9 d N-depletion, the total amount of intracellular chlorophyll could tend to decrease throughout the irradiation time, also relating to the low viability (1.4%). Therefore, those results showed the correlation between the responsiveness of chlorophyll-amount and the viability, and the influence of UV-C toward the intracellular component. As the next step to analyze the UV-C effect to the intracellular, the effect was evaluated by comparing between the ratios of amounts of intracellular lipids before/ after UV-C irradiation (Table 1). After UV-C irradiation, the ratios of linoleic acid (C18:2) and linolenic acid (C18:3) tended to decrease in the cells at 0 d N-depletion; the ratios of unsaturated C18-lipids including oleic acid (C18:1) trendy decreased in the cells at 6 d N-depletion. Although the previous report used UV-B to analyze the effects of UV towards the intracellular of C. reinhardtii cells, the report revealed the lipid degradation including C18-lipids [19], corresponding to the results in this study. The UV-C irradiation normally prompts lipid peroxidation [33-35], so that C18-lipids could possibly protect the intracellular from the adverse effects derived by UV-C. On the other hands, after UV-C irradiation, the ratios of the lipid amounts showed a few differences in the cells at 9 d N-depletion. The viability of cells might relate to the UV-C effects toward lipids (e.g., metabolic flow) because of low cell-viability (1.4%) at 9 d N-depletion even before UV-C irradiation.
0 d-N depletion
6 d-N depletion
9 d-N depletion
Lipid Composition (w/w)
Lauric acid (C12:0)
1.08 ± 0.14
1.01 ± 0.01
1.04 ± 0.06
Myristic acid (C14:0)
1.07 ± 0.16
1.03 ± 0.09
1.10 ± 0.21
Palmitic acid (C16:0)
1.05 ± 0.17
0.95 ± 0.03
1.07 ± 0.09
Stearic acid (C18:0)
1.08 ± 0.20
0.98 ± 0.04
1.10 ± 0.14
Oleic acid (C18:1)
1.15 ± 0.66
0.86 ± 0.02
1.08 ± 0.08
Linoleic acid (C18:2)
0.76 ± 0.34
0.74 ± 0.07
1.00 ± 0.12
Linoleic acid (C18:3)
0.80 ± 0.44
0.59 ± 0.08
0.96 ± 0.17
Table 1: Ratios of change of lipid-compositions by UV-irradiation. The amount of each intracellular lipid after UV-C irradiation was divided with the one before UV-C irradiation. The results were collected from three replicated experiments.
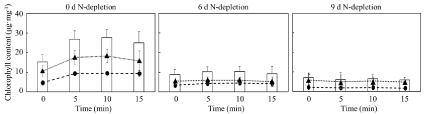
Figure 3: Contents of chlorophyll a, chlorophyll b and chlorophyll a/b in C. reinhardtii on 0 d, 6 d and 9 d N-depletion before/after UV irradiation for 5, 10 and 15
min. Contents of chlorophyll a, chlorophyll b and chlorophyll a/b were shown with circle, triangle and bar. Error bars indicate standard deviation of three replicated
experiments.
The disruption of outer cell-structure by UV-C irradiation led to lipid leakage from cells before lipid collection, resulting in loss of lipids. Previous studies reported that C. reinhardtii causes necrosis and apoptosis by UV-B irradiation and then the cells collapse [19]; therefore, the observation of outer cell-structure after treatment of UV-C with an optical microscope was significantly important (Figure 4). After 5 minutes of UV-C irradiation, the cells under the N-depletion formed a small grain-like structure in the cells and showed a necrosislike response. Appearing the grain-like structure in the cells indicated that the UV-C treatment could effectively work for sterilizing cells. In fact, the C. reinhardtii necrosis caused by a protease inhibitor showed the multiple small grain-like structures in the cells [36]. In addition, the outline of the cells at 6 d and 9 d N-depletion became unclear after 15min and 5min of UV-C irradiation, respectively. Generally, the cell-outline of C. reinhardtii could be unclear after UV irradiation [37], and the knowledge corresponds to our results. In the case that the unclear outline might mean disruption of cells, the cell components could be possibly leaked from the cell. Although there could not be drastic differences in lipid contents in the cells before or after UV-B treatment [19], no research reveals the effect of lipid contents in the cells after UV-C treatment. Thus, the leakage was evaluated by analyzing lipid contents in the cells before and after UV-C irradiation.
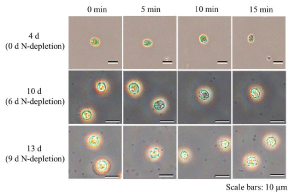
Figure 4: Optical micrographs of C. reinhardtii on 0 d, 6 d and 9 d N-depletion before/after UV irradiation for 5 and 10 min.
In order to clarify the effect of UV-C irradiation on the leakage of lipids from cells and the change in the efficiency of lipid-extraction, the lipid-extraction efficiency was evaluated by analyzing the lipid contents in the cells under the conditions of before/after UV-C treatment and with/without glass beads (Figure 5). First of all, the effects derived from UV-C irradiation were evaluated toward the leakage of intracellular lipids from cells. Under the extraction process with glass beads as a normal process, the lipid contents did not show significant differences even with UV-C irradiation. In this study, the lipid was extracted from UV-C irradiated cells after excluding liquid-phase by centrifugation. Thus, no significant differences of the lipid contents at N-depletion of 0 d, 6 d and 9 d indicated that the UV-C treated cells could not leak the intracellular lipids to liquidphase. Secondly, the change in the efficiency of lipid-extraction by UV-C irradiation was evaluated by comparing the lipid-contents extracted with/without the glass-beads treating process, resulting in no significant differences. The results indicated that the UV-C irradiated cells could not show the leakage of intracellular lipids, meaning that the lipids could be simply collected by centrifugation. In our research, the UV-C irradiated cells could not leak the lipids from the cells; however, the sterilized cells might gradually promote to leak the intracellular components depending on the progressive cell-collapse. The direct-use of cells could possibly show the simple utilization of intracellular lipids.
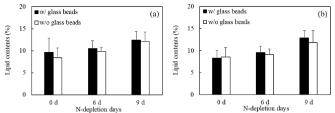
Figure 5: Evaluation of lipid-extraction methods with/without glass beads for cells treated with/without UV-C after nitrogen depletion of 0 d, 6 d and 9 d. Black/white
bars meant the evaluation with/without glass beads; (a) and (b) showed before and after UV-C treatment for 10min. Error bars indicate standard deviation of three
replicated experiments.
Conclusion
This research ultimately aimed to utilize the intracellular lipids simply by the direct use of the green algal cells without environmental contamination. The experimental results revealed that the cells after N-depletion were sterilized by the UV-C irradiation for 10 min and few differences of lipid contents after UV-C irradiation. Although the UV-C irradiated cells could not show the leak of the lipids from the cells, the sterilized cells might promote the leak of the intracellular lipids by the progressive cell-collapse, indicating the possibility of the direct-use of cells to utilize the intracellular lipids.
Acknowledgment
The authors appreciated Nihon Morita Yakusyo Co., Ltd. for research funding.
References
- Korac RR, Khambholja KM. Potential of herbs in skin protection from ultraviolet radiation. Pharmacogn Rev. 2011; 5: 164-173.
- Prommaban A, Utama-ang N, Chaikitwattana A, Uthaipibull C, Srichairatanakool S. Linoleic acid-rich guava seed oil: Safety and bioactivity. Phytotherapy Res. 2019; 33: 2749-2764.
- Tanadul O, Noochanong W, Jirakranwong P, Chanprame S. EMS-induced mutation followed by quizalofop-screening increased lipid productivity in Chlorella sp. Bioprocess Biosyst Eng. 2018; 41: 613-619.
- Wang B, Li Y, Wu N, Lan CQ. CO2 bio-mitigation using microalgae. Appl Microbiol Biotechnol. 2008; 79: 707-718.
- Ho SH, Nakanishi A, Kato Y, Yamasaki H, Chang JS, Misawa N, et al. Dynamic metabolic profiling together with transcription analysis reveals salinity-induced starch-to-lipid biosynthesis in alga Chlamydomonas sp. JSC4. Sci Rep. 2017; 7: 45471.
- Kim W, Park JM, Gim GH, Jeong SH, Kang CM, Kim DJ, et al. Optimization of culture conditions and comparison of biomass productivity of three green algae. Bioprocess Biosyst Eng. 2012; 35: 19-27.
- Wang C, Wang Z, Luo F, Li Y. The augmented lipid productivity in an emerging oleaginous model alga Coccomyxa subellipsoidea by nitrogen manipulation strategy. World J Microbiol Biotechnol. 2017; 33: 160.
- Wang JF, Han DX, Sommerfeld MR, Lu CM, Hu Q. Effect of initial biomass density on growth and astaxanthin production of Haematococcus pluvialis in an outdoor photobioreactor. J Appl Phycol. 2013; 25: 253-260.
- Scaife MA, Nguyen GTDT, Rico J, Lambert D, Helliwell KE, Smith AG. Establishing Chlamydomonas reinhardtii as an industrial biotechnology host. Plant J. 2015; 82: 532-546.
- Kropat J, Hong-Hermesdorf A, Casero D, Ent P, Castruita M, Pellegrini M, et al. A revised mineral nutrient supplement increases biomass and growth rate in Chlamydomonas reinhardtii. Plant J. 2011; 66: 770-780.
- Chapman SP, Paget CM, Johnson GN, Schwartz JM. Flux balance analysis reveals acetate metabolism modulates cyclic electron flow and alternative glycolytic pathways in Chlamydomonas reinhardtii. Front Plant Sci. 2015; 6: 474.
- Murbach TS, Glávits R, Endres JR, Hirka G, Vértesi A, Béres E, et al. A Toxicological evaluation of Chlamydomonas reinhardtii, a green algae. Int J Toxicol. 2018; 37: 53-62.
- Nakanishi A, Iritani K, Sakihama Y, Ozawa N, Mochizuki A, Watanabe M. Construction of cell-plastics as neo-plastics consisted of cell-layer provided green alga Chlamydomonas reinhardtii covered by two-dimensional polymer. AMB Expr. 2020; 10: 112.
- Li-Beisson Y, Beisson F, Riekhof W. Metabolism of acyl-lipids in Chlamydomonas reinhardtii. Plant J. 2015; 82: 504-522.
- Lautenschläger H. Essential fatty acids - cosmetic from inside and outside. Beauty forum. 2003; 4: 54-56.
- Yoo G, Park WK, Kim CW, Choi YE, Yang JW. Direct lipid extraction from wet Chlamydomonas reinhardtii biomass using osmotic shock. Bioresour Technol. 2012; 123: 717-722.
- Hounslow E, Kapoore RV, Vaidyanathan S, Gilmour DJ, Wright PC. The Search for a Lipid Trigger: The effect of salt stress on the lipid profile of the model microalgal species Chlamydomonas reinhardtii for biofuels production. Curr Biotechnol. 2016; 5: 305-313.
- Markou G, Wang L, Ye J, Unc A. Using agro-industrial wastes for the cultivation of microalgae and duckweeds: Contamination risks and biomass safety concerns. Biotechnol Adv. 2018; 36: 1238-1254.
- Sydney T, Marshall-Thompson JA, Kapoore RV, Vaidyanathan S, Pandhal J, Fairclough JPA. The effect of high-intensity ultraviolet light to elicit microalgal cell lysis and enhance lipid extraction. Metabolites. 2018; 8: 65.
- Almeida ACM, Quilty B. The response of aggregated Pseudomonas putida CP1 cells to UV-C and UV-A/B disinfection. World J Microbiol Biotechnol. 2016; 32: 185.
- Watanabe M, Masaki H, Mori T, Tsuchiya T, Konuma H, Hara-Kudo Y, et al. Inactivation effects of UV irradiation and ozone treatment on the yeast and the mold in mineral water. J Food Prot. 2010; 73: 1537-1542.
- Ho SH, Nakanishi A, Ye X, Chang JS, Chen CY, Hasunuma T, et al. Dynamic metabolic profiling of the marine microalga Chlamydomonas sp. JSC4 and enhancing its oil production by optimizing light intensity. Biotechnol Biofuels. 2015; 8: 48.
- Crippen RW, Perrier JL. The use of neutral red and evans blue for live-dead determinations of marine plankton (with comments on the use of rotenone for inhibition of grazing). Stain Technol. 1974; 49: 97-104.
- Nassour R, Ayash A, Mohamad I. The effect of ultraviolet radiation on chlorophyll in Chlamydomonas reinhardtii. Int J Agric Env Sci. 2017; 4: 22-26.
- Zhou Y, Schideman LC, Park DS, Stirbet A, Govindjee, Rupassara SI, et al. Characterization of a Chlamydomonas reinhardtii mutant strain with improved biomass production under low light and mixotrophic conditions. Algal Res. 2015; 11: 134-147.
- Yang L, Chen J, Qin S, Zeng M, Jiang Y, Hu L, et al. Growth and lipid accumulation by different nutrients in the microalga Chlamydomonas reinhardtii. Biotechnol Biofuels. 2018; 11: 40.
- Nakanishi A, Aikawa S, Ho SH, Chen CY, Chang JS, Hasunuma T, et al. Development of lipid productivities under different CO2 conditions of marine microalgae Chlamydomonas sp. JSC4. Bioresour Technol. 2014; 152: 247- 252.
- Gao Y, Cui Y, Xiong W, Li X, Wu Q. Effect of UV-C on algal evolution and differences in growth rate, pigmentation and photosynthesis between prokaryotic and eukaryotic algae, photochemistry and photobiology. Photochem. Photobiol. 2009; 85: 774-782.
- Borderie F, Alaoui-Sehmer L, Bousta F, Alaoui-Sosse B. Cellular and molecular damage caused by high UV-C irradiation of the cave-harvested green alga Chlorella minutissima: Implications for cave management. Int Biodeterior Biodegrad. 2014; 93: 118-130.
- Ganapathy K, Chidambaram K, Janarthanan R, Ramasamy R. Effect of UV-B radiation on growth, photosynthetic activity and metabolic activities of Chlorella vulgaris. J Microbiol Biotechnol. 2017; 6: 53-60.
- Marwood CA, Greenberg BM. Effect of supplementary UVB radiation on chlorophyll synthesis and accumulation of photosystems during chloroplast development in Spirodela oligorrhiza. Photochem Photobiol. 1996; 64: 664- 670.
- Agrawal SB. Effects of supplemental UV-B radiation on photosynthetic pigment, protein and glutathione contents in green algae. Environ Exp Bot. 1992; 32: 137-143.
- Hollosy F. Effects of ultraviolet radiation on plant cell. Micron. 2002; 33: 179- 197.
- Pessoa MF. Harmful effects of UV radiation in Algae and aquatic macrophytes - A review. Emir J Food Agric. 2012; 24: 510-526.
- Pessarakli M. Major environmental stresses and photosynthetic response. In: Handbook of photosynthesis, 2nd edition. Pessarakli M, editors. CRC press- Taylor & Francis Group, Tucson. Arizona. 2005; 735.
- Voigt J, Woestemeyer J. Protease inhibitors cause necrotic cell death in Chlamydomonas reinhardtii by inducing the generation of reactive oxygen species. J Eukaryot Microbiol. 2015; 62: 711-721.
- Hu Q, Sommerfeld M, Jarvis E, Ghirardi M, Posewitz M, Seibert M, et al. Microalgal triacylglycerols as feedstocks for biofuel production: perspectives and advances. Plant J. 2008; 54: 621-639.