
Research Article
Austin J Biotechnol Bioeng. 2021; 8(1): 1109.
Evaluation of the Growth Performance of Microalgae Based on Fine pH Changes
Filali R1,Tian H2, Micheils E1 and Taidi B1,3*
¹LGPM, CentraleSupélec, Université Paris-Saclay, SFR Condorcet FR CNRS 3417, Centre Européen de Biotechnologie et de Bioéconomie (CEBB), 3 rue de Rouges Terres 51110, Pomacle, France
²ENCIACET, 4, allée Emile Monso - CS 4436231030 TOULOUSE Cedex 4, France
³University of Paris Saclay, Centralesupélec, 3 rue Joliot Curie, 91190Gif-sur-Yvette, France
*Corresponding author: Behnam Taidi, University of Paris Saclay, Centralesupelec, 3 Rue Joliot Curie, 91190Gif-sur-Yvette, France
Received: April 15, 2021; Accepted: June 01, 2021; Published: June 08, 2021
Abstract
Microalgae are photosynthetic microorganisms with many potential applications in the food, cosmetics, pharmaceutical and environmental industries. Currently, commercial microalgae production remains limited. Therefore, improving the growth and the culture density of the microalgae cultivation is one of the key enablers to open the way to mass production and commercialisation of these microorganisms. The effect of culture pH on the photoautotrophic growth of C. vulgaris over a large range of values has been investigated in pH-regulated cultures. For each microalgae culture, the specific growth rate, the cell density, the chlorophyll content, the intracellular carbon content and the nitrogen source consumption were monitored. Optimal growth and carbon incorporation have been observed at pH of 7.0. The fastest growth rate and highest biomass production of C. vulgaris were 0.074 h-1 and 0.896 g/L respectively. Under these conditions, a maximum carbon content of cells was 49 % (w/w).
Keywords: Microalgae; Culture pH; Specific growth rate; Chlorella vulgaris; Photosynthesis; Photobioreactor
Introduction
Microalgae are photosynthetic eukaryotic microorganisms with a high level of morphological and biochemical diversity. These microorganisms are capable of converting CO2 into organic carbon through photosynthesis by using light as energy source. The biomass and the high value compounds produced offer potential commercial products for various application fields such as cosmetic, pharmaceutical, food and aquaculture [1-4].
Microalgae culture optimization depends on the establishment of the optimum growth conditions that maximize algae productivity. Culture conditions can be categorised as physical and biological. The former consists of the hydrodynamics conditions of the system with mixing degree, retention time, mass transfer coefficients and local shear forces. These factors strongly depend on an ideal choice of the microalgal culture systems geometries. The latter biological factors encompass the cell wall ultrastructure, the cell size and the specific characteristics of the microalgae strain including its light requirements. The abiotic factors (i.e. environmental parameters) are those of temperature, light intensity, photoperiod, pH, growth medium nutrient content, medium salinity and carbon dioxide levels.
The efficiency of the algal biomass productivity depends on the pH level, which influences the algal growth and the cellular metabolism through photosynthetic activity, carbon fixation and allocation of carbon into different types of molecules [5]. The culture pH affects the solubility and the availability of carbon by regulating the distribution of the carbonate species present in the medium (Figure 1). Due to the Carbon Concentrating Mechanisms (CCMs), the uptake of the inorganic carbon source by microalgae strongly increases the pH of the medium [6]. At higher pH values, the carbon is present in form of carbonate [7], and therefore, C. vulgaris growth is limited by the availability of the readily assimilable CO2 form [8,9]. At lower pH, the algal growth is favoured by the presence of the CO2 species, but negatively affected by the alteration of the nutrient uptake [10] or by metal toxicity [11-13].
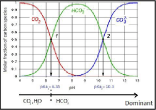
Figure 1: Molar fraction of carbon species with various pH and at 20°C [13].
The optimal pH of each algae species varies depending on the specific physiological reaction of the microalgae to the environmentalpH variation [14-16]. The pH can affect the enzymatic activities. In the same way, the variation of the pH affects the metabolism of microalgae. Indeed, by changing the external pH of the medium, a new pH gradient between the cell and the medium is formed and a new equilibrium is establish between the inside and the outside of the cell, potentially inducing an intracellular pH modification. The ability of the cell to survive this pH variation depends on its own ability to adapt the cell physiology to counteract the pH gradient [17], which may be energy dependent.
Several studies have shown that the optimal pH for the maximal growth of microalgae is situated around pH (7.0–7.6) [8,18]. Some studies have shown a better efficiency of CO2 utilization for photosynthesis at pH values below neutral (7.0) [19]. These studies report that the affinity for dissolved CO2 becomes more and more significant [9,20] and the main metabolism of CCMs is governed by the passive movement of CO2 into the cell under acidic conditions [21,22]. On the other hand, in alkaline environments, where the predominant carbon species is HCO3-, the algae cells require a high supply of carbonates for their photosynthesis activity. An active transport of HCO3- overcomes the low efficiency of carbon accumulation under these conditions [5]. A low pH can inhibit microalgae growth in several ways: by denaturation of the enzymatic activity, especially proteolytic enzymes [16], by limiting the motility of algae cells [23] or by limiting photosynthesis by decreasing the total accumulated carbon and oxygen release [24].
To deal with extreme variations in external pH, microalgae have several mechanisms to adapt. Some microalgae are able to adapt the cell physiology by changing intracellular pH in response to the variation of the external pH [25,26]. This mechanism [25,26] of cellular metabolism modification that is specific to certain microalgae species consists in: i) accumulating glycerol in response to the osmotic imbalance [27], ii) accumulating storage lipids [28] or increasing saturated fatty acid content in order to inhibit high proton influx [28,29].
Several studies have focused on the effect of the pH on the growth of the C. vulgaris species [30-38]. Even if the optimal value of pH highly depends on the culture conditions, the reported optimum pH for C. vulgaris culture varies from one study to another and is not well defined in the literature (Table 1).
Medium
pH Control
T(°C)
Light
pH
References
Synthetic Sea Water and Freshwater Medium
1% CO2
20
Continuous light
7.9
[33]
Modified
Fitzgeral d MediumCO2
-
-
5.5-7.0
[34]
Biogas Slurry
MediumHCL-NaOH
25
Light/dark period
18h/6h6.5-7.0
[35]
Modified
CHU-10 MediumCO2
-
72h light
7.9
[36]
Mauro’s Modified Medium
-
25
Continuous light
6.0
[37]
Modified
Bristol MediumHCL-NaOH
19
Continuous light
7.0-7.5
[38]
BG 11 Medium
HCL-NaOH
25
Light/dark period
12h/12h10-10.5
[31]
BG 11 Medium
C8H5KO4 KH2PO4 Na2B4O7
10H2O25
No light
7.5-8.0
[30]
Table 1: Optimal pH of C. vulgaris culture.
The aim of this study is to investigate the effect of pH on the growth rate, biomass productivity, intracellular carbon content and chlorophyll content of the microalga C. vulgaris grown photoautotrophically. CO² injection was used to control the pH at a set value for each culture. The optimal value of pH for C. vulgaris growth based on the biological performance of algae species in terms of specific growth rate and biomass productivity is 7.0.
Materials and Methods
Microalga specie and growth conditions
The green unicellular microalga C. vulgaris CCAP211/e 11B (trebouxiophycae) was obtained from the Culture Collection of Algae and Protozoa, CCAP (Göttingen). Precultures were maintained at ambient temperature, under continuous light intensity of 20μmol m-2.s-1 and continuous orbital agitation, in Bristol 3 N medium with the following composition (in mg.l-1): NaNO3, 750; CaCl2, 2H2O, 25; MgSO4, 7H2O, 75; FeEDTA, 20; K2HPO4, 75; KH2PO4, 175 and NaCl, 20, supplemented with a solution of microelements containing (in μg.l-1): H3BO3, 2860; MnCl2., 4H2O,, 1810; ZnSO4., 7H2O, 220; CuSO4., 7H2O, 80; MoO3, 36 and CoSO4., 7H2O, 90. The pre-culture was maintained by inoculating (1% v/v) of one-week-old culture into fresh medium on a weekly basis.
Photobioreactor culture conditions
C. vulgaris was grown in a baffled bioreactor (GPC, France) with a working volume of 5 L (Figure 2) equipped with a 4-bladed segment impellers (500 rpm). The temperature was maintained at 25°C using a double jacket and was measured continuously in the culture with a Pt100 temperature. Continuous aeration (200 ml air.min-1) was achieved by passing compressed air across a gas filter (0.2μm Sartorius, Germany) and through a sparger under the impeller ax. The airflow was regulated by a mass flow meter (Bronkhorst). The constant external light source was composed of 3 X 3 vertically arranged LED bulbs (Ledare 130, 78 lumen, 2700 Kelvin, 27° dispersion angle, IKEA, France). The three lamps illuminated the culture from three directions at 120° angles to each other. The incident light intensity on the internal surface of the bioreactor, measured with a photometer (LI250A, LI-COR, USA), was 18744 μmol.m².s-¹ in the total form the 9 bulbs.
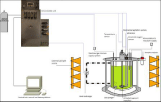
Figure 2: Representation of the C. vulgaris photobioreactor.
The bioreactor was equipped with a pH (EasyFerm Bio Arc 325, Hamilton, Swiss) probe and a dissolved oxygen sensor (VisiFerm DO Arc 325, Hamilton, Swiss). The pH probe was calibrated before autoclaving at pH 4.01 and 7.00 or 7.00 and 9.03 depending on the pH range of the experiment, and then recalibrated at a single point each week against a calibrated bench-top pH meter (PL-700 PC, GOnDO benchtop pH/mV/Temperature Meter).
The dissolved oxygen sensor was calibrated at two points: at 0% immediately after autoclaving and at 100% after reaching an equilibrium with air, following continuous air injection under operating conditions. During the experiments, the pH of the culture was adjusted automatically using injection of CO2 into the constant air flow (200 ml/min) at a maximum concentration 2 % (v/v) CO2. The supply of the carbon dioxide was regulated by a mass flow meter (Bronkhorst). For the lowest pH value, the adjustment of the pH was achieved by adding 1 mol/L H3PO4. benchtop pH/mV/Temperature Meter). The dissolved oxygen sensor was calibrated at two points: at 0% immediately after autoclaving and at 100% after reaching an equilibrium with air, following continuous air injection under operating conditions. During the experiments, the pH of the culture was adjusted automatically using injection of the tested pH values are 5.0, 5.5, 6.0, 6.5, 7.0, 7.5, 8.0, 9.0 and 10.0. The inoculation of the bioreactor with C. vulgaris was carried out with 1% of pre-culture giving 1-2 million cells.ml-1 at the beginning of the culture.
During growth, several parameters such as cellular concentration (Multisizer 4, Beckman), dry weight, absorbance at 800 nm (UV-1800, SHIMADZU), total CO2 supply, chlorophyll content (Porra method, [39]), internal carbon content, ionics analysis, pH and dissolved oxygen concentration were measured. The experiments were always started on a Monday and two sample were collected and analyzed every day. Samples were not taken in duplicate but several of the analyses were performed in replicated: Cell counts and size distribution by Coulter counter were performed in triplicate and the results presented as mean averages, CHNS analyses were performed in duplicate on single samples and the other off-line analyses were performed without repeats.
Analytical methods
Biomass quantification: The biomass concentration was estimated by three different methods: i) a spectrophotometric measurement of Optical Density (OD) at 800 nm; ii) the cell count and the distribution of the cell size were obtained using the Multisizer 4e (Beckman Coulter); Measurements were made in triplicate on single samples. iii) The dry weight of the biomass, DW in g.L-1, was determined by centrifugation (3200 g, 10 min) of 15 ml of microalgal sample (centrifuge 5840R). The pellet was re-suspended in the same volume of deionised water and was then subjected to a second centrifugation. The final pellet was frozen (-18°C) for at least 24 h, then the frozen pellet was dried for 24 h by lyophilisation (Alpha 1-2 LD Entry Freeze Dryer, MARTIN CHRIST).
Chlorophyll concentration: The concentration of chlorophyll a and b was obtained based on a modified extraction method of Porra [39]. Chlorophyll a and b were obtained according to the following equations after extracting the chlorophyll:
Chl a (mg/L) = 16.41A664 - 8.09A650 (1)
Chl b (mg/L) = 30.82A650- 12.57A664 (2)
Determination of mineral ions concentration: The sample supernatants obtained through centrifugation (3214 g, 10 min) were diluted appropriately with deionized (MilliQ) water, filtered through C18 and 0.2 μm filters in order to retain all proteins and macromolecules that could interfere with the analysis. The ions analysis was performed with ionic chromatography (DIONEX ICS 5000+ Thermo Scientific equipped with anionic column DIONEX IONPACTM AS11-HC 2x250 mm and cationic column DIONEX IONPACTM CS16 3x250 mm). This method allowed to determinate the concentrations of the major anions and cations, such as sodium, ammonium, potassium, calcium, magnesium, sulphate, orthophosphate and nitrate.
Elemental content analysis: The elemental intracellular content of microalgal cells was obtained by the CHNS-O Elemental Analysis (ThermoFisherScientific). This technic allows quantification of the percentage by weight of carbon, hydrogen, nitrogen, sulfur and oxygen contained in homogeneous organic materials.
For the elemental determination, 2 mg of the dried microalgal sample, was transferred into an oven at 930°C filled with oxygen for very fast and complete dynamic combustion “flash”, using an elemental analyzer Flash 2000 (ThermoFisherScientific). The detection of carbon, hydrogen and sulphur is based on the thermal conductivity of combustion gases.
For the determination of oxygen, a complementary module allowed the analysis of this element by a pyrolysis process in an oven at a temperature of 1000°C.
Results
C. vulgaris growth
C. vulgaris grew at all the pH values (5.0-10.0) tested, however, at the extremes of the tested pH-values, the growth of this organism was severely retarded (lower μ). The lag phase duration was also influenced by the culture-pH with considerably longer lag phases encountered at the extremes of pH.
Growth of C. vulgaris under autotrophic conditions can be divided into three phases: a lag phase, a short exponential phase and a long linear growth phase [40]. C. vulgaris grew best (Figure 3) at neutral pH (μ=0.047 h-1 and μ=0.049 h-1 for pH 6.5 and 7, respectively). On either side of pH 7, the specific growth rate of C. vulgaris decreased to a value of approximately 0.03 h-1. The impact of pH variation around the neutral value on the biological growth of C. vulgaris could be, at least partially, caused by the energetic demand of cells in order to adapt their cell physiology to maintain intracellular stasis. Moreover, the photosynthetic activity of C. vulgaris is also affected by the external pH variations; specifically, at low pH, the total carbon accumulation and the nutrient uptakes rate are reduced. The lower external pH may cause undesirable effects on cell metabolism [41,42]. In alkaline environments the carbonate species are dominant inducing carbon limitation in its CO2 form.
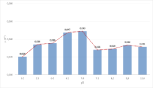
Figure 3: Growth rate of C.vulgaris culture at different pH values. The μ
values were determined at the beginning of the exponential phase based on
6 time points.
The biomass produced by the end of the exponential phase decreased on either side of neutral pH (Figure 4) reaching its lowest value at pH 10.0 (0.235 g.L-1). The highest value of final algal biomass at the end of exponential phase was obtained at pH 7.0, (0.824 g.L¹). This reflects that varying growth rate with pH. The variation of the external pH may have caused a modification of cell metabolism in order to counteract the unfavourable environmental pH by changing the internal cellular pH [25-26] or in order to maintain internal stasis. The data obtained leads to the conclusion that the optimum pH value of C. vulgaris growth is 7.0 (Table 2). At neutral pH, the distribution of the carbon species is dominated by CO2 and HCO3-, with the majority of the distribution tending towards the latter species. Carbon assimilation can be directly from free CO2 or by active transport of HCO3-. The modification of the cell metabolism is less drastic compared with acid or basic environments, and therefore, the cell requires less energy to adapt to a supposedly aggressive environment. The growth performance obtained at pH 6.5, is slightly lower than for pH 7.0. There may be an optimal carbon species (HCO3-/CO2) ratio for carbon assimilation by C. vulgaris.
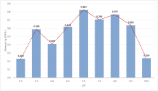
Figure 4: C. vulgaris biomass concentration at the end of the exponential
growth phase. (n=3, Sd generally less than 5% of the mean).
Parameter
Units
pH
5.0
6.0
6.5
7.0
8.0
9.0
10.0
Lag Phase
h
65
51
8
6
25
23
27
Growth Rate
h-1
0.02
0.036
0.047
0.049
0.029
0.033
0.031
Doubling Time
h
34
20
15
14
24
21
22
Biomass Productivity
g.L-1.day-1
0.034
0.058
0.1
0.12
0.11
0.095
0.035
Final Biomass Produced
g.L-1
0.229
0.407
0.619
0.824
0.767
0.636
0.235
Table 2: Biological parameters of C. vulgaris culture at different pH values.
The average C. vulgaris cell size varied from 3.177 μm to 2.972 μm for the pH values between 6.5 and 7.5 (Figure 5). The variation observed in cell size suggests that at pH values between 6.5 and 7.5 the cells were “younger” as on average they would not have time to grow in size due to the faster replication rate. Under acidic conditions, the cell size of C. vulgaris was larger (increase up to 19.6%) than at neutral pH values. The higher pH induces a larger cell size (increase up to 8 %), indicating a possible cell physical modification possibly due to an accumulation of storage lipids increasing fatty acid content (reserves). The modification of the cell size is perhaps related to the growth rate of the microalga.
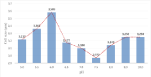
Figure 5: Average cell size of C .vulgaris at different pH values at the end
of exponential growth phase. (n=3, Sd generally less than 5% of the mean).
The acclimatization of C. vulgaris toward pH variation could be observed from the duration of the lag phase of the cultures (Figure 6). The shortest lag phases were observed for near neutral pH values, with a minimum value (6 h) at an optimal pH 7.0. The variation of the external pH led to a prolongation of the lag phase, reaching 65 h under acidic environments and 27 h under alkaline environments. The variation of the external pH may induce metabolic adaptation to counteract the changes in conditions and overcome the high pH gradient [17]. This biological modification would have prolonged the lag phase, while a new equilibrium between the inside and the outside of the cell was established.
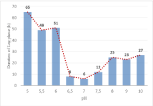
Figure 6: Duration of lag phase of C.vulgaris at different culture pH values.
Nitrogen consumption
Besides the distribution of the carbon species, another culture parameter that can affect the growth of C. vulgaris and that is affected by the pH is the availability of nitrogen species. Based on the previously obtained growth results (data not shown), the authors decided to no longer focus on the strongly alkaline pH (pH 9.0 and pH 10.0).
The specific nitrogen consumption rates of C. vulgaris at different pH values were determined (Figure 7). The best nitrogen utilization was observed at pH 7.0, while the lowest utilizations were observed in acidic environments. To explain these findings, it would be reasonable to suppose that the acidic and basic extreme pH environments significantly affect the microalgal enzymatic activity and thus reduce the assimilation capacity of nutrients in the medium, such as nitrogen source, therefore, inducing a starvation phenomenon of the nitrogen source.
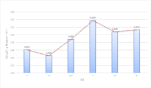
Figure 7: Specific nitrogen consumption rate of C. vulgaris at different pH
values measured at the end of the exponential growth phase (n=1).
Quantification of C:N ratio
The effect of the variation of the external pH on the carbon and nitrogen uptake can be estimated by the quantification of the cellular elemental content of carbon and nitrogen. The maximal intracellular contents of carbon and nitrogen were observed at a pH of 7.0. For acidic pH values, there was a decrease in the intracellular nitrogen content (26% approx. compared with pH 7.0) suggesting that the pH, under these conditions, alters the function of certain enzyme and thus, the assimilation capacity of nutrients such as nitrogen. Thus, the cellular carbon metabolism of C. vulgaris seems to change at different culture pH values. The major proportion of carbon in a cell is diverted towards lipid accumulation inside the cell. Acidic pH can inhibit the activity of nitrate reductase, involved in nitrate consumption, and therefore, for nitrogen uptake [32].
In alkaline environments, the intracellular carbon content is higher than in the acidic environments (45% and 48% for pH 5 and 8, respectively). As the culture pH was increased to a more neutral pH, it seems that C. vulgaris showed a better affinity to assimilate the HCO3-than the CO2. These results suggest that in an alkaline environment, when the primary metabolic pathway of MCC’s uses active transport of HCO3-, the assimilation of carbon is higher than that at lower pH. In the latter case, the carbon source is diverted towards protein synthesis.
One of the most important parameters that indicates the effect of the carbon and nitrogen limitation is the C:N ratio of the medium. When this ratio is low, carbon limitation occurs and the carbon source is privileged for protein synthesis. On the other hand, a low C:N ratio, tends to induce a lipid accumulation. For microalgae, the optimum C:N ratio is around 10 [43]. Another study [44] indicates that for fast growth, this ratio is approximately 6. The profile of C:N ratio of C. vulgaris at different external pH values (Figure 5) showed that the rise of the pH decreases the C:N ratio with a lower value for pH 7.0 (C:N ratio equal to 5.51). The increase in the C:N ratio and the decrease of the intracellular nitrogen content are possible consequences of nitrate starvation in the culture medium. These results are in accordance with other studies [45,46]. It could be that the C/N ratio should be taken into consideration in combination with μ. With fast μ, the effective carbon flux can be considered to be reduced. Industrially, this suggests that lipid accumulation could be more extensive at lower culture pH values.
Chlorophyll content
For cultures at the optimum pH of 7.0 (Figure 10), the chlorophyll content of the microalgae cells peaks at 72 hours of growth corresponding to the end of the exponential phase (3.1 ng/g biomass). Lower contents were observed at the other pH values used (0.60-0.42 ng/g biomass). C. vulgaris exhibited the best performance in terms of photosynthetic activity at pH 7.0. The effect of pH on chlorophyll production in C. vulgaris is illustrated by the assimilation of nitrogen and carbon. The maximum specific yield coefficient (mg/g dry biomass) of chlorophyll correlated with the carbon and hydrogen content of the cells, at pH 7.0. When the pH was lower than 7.0, the chlorophyll accumulation was less. This might suggest that, at acidic culture conditions, denaturation of enzymatic activity and/or the decreasing of total accumulated carbon limit the photosynthesis activity of C. vulgaris. In fact, lower pH affects the uptake of nitrogen and the cellular metabolism changes by using the fixed carbon for lipid accumulation [28]. Nitrogen deficiency can decrease chlorophyll production [47]. A peak in chlorophyll production was observed after 24 hours of culture, under all operating conditions. This may suggest an “optimal” cell concentration that can be considered to have the maximal photosynthetic activity with respect to the culture conditions applied. This could be the case particularly in relation to the phenomenon of cellular auto-shading. The optimum photosynthetic activity coincided with the point when the cell concentration was approximately 20 million cells/ml. This of course is dependent on the geometry of the photobioreactor. The primary objective of this study was to identify the optimal growth pH for C. vulgaris growth in pHcontrolled cultures where the pH was continuously adjusted by CO2 addition. The results showed the best growth at pH 7.0. Under these operating conditions of light and temperature, C. vulgaris showed the maximal growth rate, productivity, specific nitrogen consumption and chlorophyll content over a pH range of 7.0 to 10.0. In non-pH adjusted batch cultures, the final pH of the culture can approach 10.0.
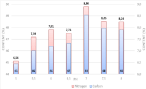
Figure 8: Elemental cellular content of C. vulgaris at different culture pH
values. (n=2, Sd generally less than 5% of the mean).
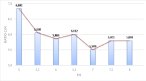
Figure 9: C:N ratio of C. vulgaris biomass at different pH values. (n=2, Sd
generally less than 5% of the mean).
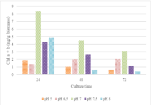
Figure 10: Changes in chlorophyll content of C. vulgaris culture in different
pH values (n=1).
In terms of intracellular elemental content, the microalgae cells had the highest carbon and nitrogen content and the lowest C:N ratio when grown at pH 7.0. The effect of pH variation can be explained by the modification of C. vulgaris metabolism to counteract the unfavourable pH gradient between the inside and the outside cell. This postulated metabolic modification could cause enzyme denaturation, interfere with the uptake of assimilable nitrogen and diminish photosynthetic activity. Nitrogen source limitation can induce the microalgae to switch their metabolism from protein synthesis to lipid accumulation.
The culture pH has a directly impact on the distribution of carbonate species in the medium. During this study, the affinity of C. vulgaris for carbon dioxide and bicarbonate species was considered. It seems that C. vulgaris has a better affinity to assimilate the HCO3- than the CO2, not surprising in the context of MCC’s biological phenomena.
Microalgae culture pH-control can be optimized by regulating the mineral acids or base content of the medium in combination with automated CO2 supply. Further work will involve confronting the various hypotheses developed during this study; the effect of pH on fatty acid accumulation. And photosynthetic activity Moreover, the affinity of C. vulgaris toward CO2 and HCO3- may be investigated by using different media with different carbon sources (CO2 gas or bicarbonate salts) under the same operating conditions. Another interesting perspective concerns the identification of the optimal C:N ratio of balanced photoautotrophic C. vulgaris culture in order to identify the transition point between carbon and nitrogen starvation.
Acknowledgement
We are grateful to Pr. PERRE Patrick, director of the Chair of Biotechnology of CentraleSupélec for founding all the work presented in this article.
Declaration of Authors Contributions
Rayen FILALI and Behnam TAIDI conceived and designed the study, performed the analysis and the interpretation of data, drafting the article and critical revising. Rayen FILALI and Hao TIAN have principally preformed the experiments with a contribution from Emilie MICHIELS. Rayen FILALI and Behnam TAIDI contributed to the manuscript preparation and revisions and all authors approved the final version.
References
- O Pulz, W Gross. Valuable products from biotechnology of microalgae, Journal of Applied Microbiology and Biotechnology. 2004; 65: 635–648..
- R Ranjbar, R Inoue, T Katsuda, H Yamaji, S Katoh. High efficiency production of astaxanthin in an airlift photobioreactor. Journal of Bioscience and Bioengineering. 2008; 106: 204–207..
- M Olaizola. Commercial development of microalgal biotechnology: from test tube to the marketplace, Biomolecular Engineering. 2003; 20: 459–466..
- GE Molina, Mass culture methods. Editors. In: Flickinger MC, Drew SW. Encyclopedia of Bioprocess Technology: Fermentation, Biocatalysis and Bioseparation. 1999; 3: 1753–1769..
- A Juneja, R Ceballos, GS Murthy. Effects of Environmental Factors and Nutrient Availability on the Biochemical Composition of Algae for Biofuels Production: A Review. Energies. 2013; 6 :4607-4638..
- PJ Hansen. Effect of high pH on the growth and survival of marine phytoplankton: Implications for species succession. Aquat. Microb. Ecol. 2002; 28: 279–288..
- ES Nielsen. Marine Photosynthesis: With Special Emphasis on the Ecological Aspects; Elsevier: Amsterdam. The Netherlands. 1975; 13..
- CY Chen, EG Durbin. Effects of pH on the growth and carbon uptake of marine phytoplankton. Mar Ecol.-Prog Ser. 1994 ; 109: 83–94..
- Y Azov. Effect of pH on inorganic carbon uptake in algal cultures. Appl.Environ. Microbiol. 1982; 43: 1300–1306.
- RW Gensemer, REH Smith, HC Duthie. Comparative effects of pH and aluminum on silica limited growth and nutrient uptake in Asterionella ralfsii var. Americana (Bacillariophyceae). J Phycol. 1993; 29: 36–44..
- W Sunda. The Relationship between Cupric Ion Activity and the Toxicity of Copper to Phytoplankton. Ph.D. Thesis, Massachusetts Institute of Technology, Cambridge, MA, USA. 1975..
- DM Anderson, FMM Morel. Copper sensitivity of Gonyaulax tamarensis. Limnol. Oceanogr. 1978; 23: 283–295..
- M Thiery. Modélisation de la carbonatation atmosphérique des bétons–Prise en compte des effets cinétiques et de l’état hydrique. Thèse de Doctorat, Ecole Nationale des Ponts et Chaussées. France. 2005..
- O Necchi, M Ribeiro Zucchi. Photosynthetic performance of freshwater rhodophyta in response to temperature, irradiance, pH and diurnal rhythm. Phycological Researc. 2001; 49: 305–318..
- M Olaizola. Microalgal removal of CO2 from flue gases: Changes in medium pH and flue gas composition do not appear to affect the photochemical yield of microalgal cultures. Biotechnology and Bioprocess Engineering. 2003; 8: 360–367..
- A Gerloff-Elias, E Spijkerman, T Pröschold. Effect of external pH on the growth, photosynthesis and photosynthetic electron transport of Chlamydomonas acidophila negoro, isolated from an extremely acidic lake (pH 2.6). Plant, Cell and Environment. 2005; 28: 1218–1229..
- Clément-Larosière B. Etude de la croissance de Chlorella vulgaris en photobioréacteur batch et continu, en présence de concentrations élevées de CO2, Ph.D. Thesis, Ecole Centrale Paris, Châtenay-Malabry, France. 2012..
- I Visviki, D Santikul. The pH tolerance of Chlamydomonas applanata (Volvocales, Chlorophyta). Arch. Environ. Contam. Toxicol. 2000; 38: 147– 151..
- JV Moroney, NE Tolbert. Inorganic carbon uptake by Chlamydomonas reinhardtii. Plant Physiol. 1985; 77: 253–258..
- C Rotator, B Colman. The acquisition and accumulation of inorganic carbon by the unicellular green alga Chlorella ellipsoidea. Plant Cell Environ. 1991; 14: 377–382..
- B Colman, IE Huertas, S Bhatti, JS Dason. The diversity of inorganic carbon acquisition mechanisms in eukaryotic microalgae. Funct Plant Biol. 2002; 29: 261–270..
- R Ramanan, N Vinayagamoorthy, SD Sivanesan, K Kannan, T Chakrabarti. Influence of CO2 concentration on carbon concentrating mechanisms in cyanobacteria and green algae: A proteomic approach. Algae. 2012; 27: 295–301..
- J Hargreaves, B Whitton. Effect of pH on growth of acid stream algae. Eur J Phycol. 1976; 11: 215–223..
- JR Coleman, B Colman. Inorganic carbon accumulation and photosynthesis in a blue-green alga as a function of external pH. Plant Physiol. 1981; 67: 917–921..
- KA Gehl, B Colman. Effect of external pH on the internal pH of Chlorella saccharophila. Plant Physiol. 1985; 77: 917–921..
- AE Lane, JE Burris. Effects of environmental pH on the internal pH of Chlorella pyrenoidosa, Scenedesmus quadricauda and Euglena mutabilis. Plant Physiol. 1981; 68: 439–442..
- A Fuggi, G Pinto, A Pollio, R Taddei. The role of glycerol in osmoregulation of the acidophilic alga Dunaliella acidophila (Volvocales, Chlorophyta): Effect of solute stress on photosynthesis, respiration and glycerol synthesis. Phycologia. 1988; 27: 439–446..
- H Tatsuzawa, E Takizawa, M Wada, Y Yamamoto. Fatty acid and lipid composition of the acidophilic green alga Chlamydomonas sp. 1. J Phycol. 1996; 32: 598–601..
- J Poerschmann, E Spijkerman, U Langer. Fatty acid patterns in Chlamydomonas sp. as a marker for nutritional regimes and temperature under extremely acidic conditions. Microb Ecol. 2004; 48: 78–89..
- M Sakarika, M Kornaros. Effect of pH on growth and lipid accumulation kinetics of the microalga Chlorella vulgaris grown heterotrophically under sulfur limitation. Bioresour Technol. 2016; 219: 694-701..
- Q Gong, Y Feng, L Kang, et al. Effects of Light and pH on Cell Density of Chlorella vulgaris. Energy Procedia. 2014; 61: 2012-2015..
- S Daliry, A Hallajisani, J Mohammadi Roshandeh, H Nouri, A Golzary. Investigation of optimal condition for Chlorella vulgaris microalgae growth, review paper. Global J Environ Sci Manage. 2017; 3: 217-230..
- JC Goldman, Y Azov, CB Riley, et al. The effect of pH in intensive microalgal cultures. I Biomass regulation. Journal of experimental marine biology and ecology. 1982; 57: 1-13..
- A Widjaja, CC Chien, YH Ju. Study of increasing lipid production from fresh water microalgae Chlorella vulgaris. Journal of the Taiwan Institute of Chemical Engineers. 2009; 40: 13-20..
- C Wang, H Li, Q Wang, et al. Effect of pH on growth and lipid content of Chlorella vulgaris cultured in biogas slurry. Sheng wu gong cheng xue bao, Chinese journal of biotechnology. 2010; 26: 1074-1079..
- PK Rai, N Mallick, LC Rai. Effect of Cu and Ni on growth, mineral uptake, photosynthesis and enzyme activities of Chlorella vulgaris at different pH values. Biomedical and environmental sciences: BES. 1994; 7: 56-67..
- B Lustigman, LH Lee, A Khalil. Effects of nickel and pH on the growth of Chlorella vulgaris. Bulletin of environmental contamination and toxicology. 1995; 55: 73-80..
- JW Rachlin, A Grosso. The effects of pH on the growth of Chlorella vulgaris and its interactions with cadmium toxicity. Archives of environmental contamination and toxicology. 1991; 20: 505-508..
- RJ Porra, WA Thompson, PE Kriedemann. Determination of accurate extinction coefficients and simultaneous equations for assaying chlorophylls a and b extracted with four different solvents: verification of the concentration of chlorophyll standards by atomic absorption spectrometry. Biochim Biophys Acta. 1989; 975: 384-394..
- H Ben Amor-Ben Ayed, B Taidi, H Ayadi, D Pareau, S Moncef. Magnesium uptake by the green microalga Chlorella vulgaris in batch cultures. J. Microbiol. Biotechnol. 2016; 26: 503–510..
- A Kumar, S Ergas, X Yuan, A Sahu, Q Zhang, J Dewulf, et al. Enhanced CO2 fixation and biofuel production via microalgae: recent developments and future directions. Trends Biotech. 2010; 28: 371-380..
- C Yan, Zhang, L Xingzhang, Z Zheng. Effects of various LED light wavelengths and intensities on the performance of purifying synthetic domestic sewage by microalgae at different influent C/N ratios. Ecol Eng. 2013; 51: 24–32..
- H Tijani, N Abdullah, A Yuzir. Integration of microalgae biomass in biomethanation systems. Renew. Sustain. Energy. Rev. 2015; 52: 1610- 1622..
- WJ Oswald. Fundamental factors in stabilization pond design. Int. J. Air Water Pollut. 1961; 5: 357-393..
- DH Turpin. Effects of inorganic n availability on algal photosynthesis and carbon metabolism. Journal of Phycology. 1991; 27: 14–20..
- A Sciandra, J Gostan, Y Collos, C DescolasGros, C Leboulanger, V Martin- Jézéquel, et al. Growth compensating phenomena in continuous cultures of Dunaliella tertiolecta limited simultaneously by light and nitrate. Limnology Oceanography. 1997; 42: 1325–1339..
- VS Ferreira, CS Anna. Impact of culture conditions on the chlorophyll content of microalgae for biotechnological applications. World J Microbiol Biotechnol. 2007; 33: 20..