
Research Article
Austin J Biotechnol Bioeng. 2021; 8(2): 1111.
Compartmentalization of Pectinase within Cellulose Hydrogel: An Efficient Technique to Enhance the Catalytic Properties of Pectinase for Industrial Applications
Rehman HU* and Majeed A
Department of Natural and Basic Sciences, University of Turbat, Turbat, Balochistan, Pakistan
*Corresponding author: Haneef Ur Rehman, Department of Natural and Basic Science, University of Turbat, Balochistan, Pakistan
Received: July 10, 2021; Accepted: July 24, 2021; Published: July 31, 2021
Abstract
Pectinase catalyze the breakdown of pectin polymer and widely has been used in different industrial preparations such as fruit juice preparation, liquification and scarification of plant biomass as well as coffee and tea fermentation. In this study, the pectinase from Bacillus licheniformis was compartmentalized within cellulose beads hydrogel using encapsulation technique to make it reusable with easy recovery from reaction mixture. The compartmentalization improved catalytic properties of pectinase and ensured its reusability for continuous uses. It was observed that 5.0% cellulose concentration was enough to form stable cellulose hydrogel beads with retention of high relative of activity of pectinase. The hydrogel compartmentalization didn’t change the optima pH and temperature for maximum relative activity and both the hydrogel compartmentalized pectinase and free pectinase maximum relative at same pH and temperature but the hydrogel compartmentalization increased the stability of pectinase against various temperatures and pH, and hydrogel compartmentalized pectinase showed higher relative activities against various temperature and pH as compared to free pectinase. The hydrogel compartmentalization slightly reduced the affinity of pectinase toward the substrate (pectin) and hydrogel compartmentalized pectinase showed little bit higher Km value as compared to soluble pectinase. Cellulose hydrogel compartmentalization retained the pectinase activity for reutilization in different reaction preparation and hydrogel compartmentalized pectinase showed more than 80% of its original activity after three times reusing.
Keywords: Cellulose; Hydrogel; Beads; Pectinase; Bacillus licheniformis
Introduction
Pectinase is an emerging enzyme in industrial sector due to its application in various industrial processes such as paper manufacture, production of fruit and vegetable juices, coffee and tea fermentation, textile preparations etc [1-3]. The low operational stability of pectinase in industrial preparation restricted its applications in industrial preparation. Numerous techniques are used such as immobilization, chemical modification, protein engineering and adding additives to upgrade enzyme properties for meeting industrial needs [4-8]. Immobilization is the most significant method that not only enhances the catalytic characteristics of enzymes but makes it reusable in multiple reactions. Immobilization manages the reaction process and the product can be recovered easily in immobilized enzyme reactions [9]. The immobilization techniques used to retain the enzyme by binding onto or within specific support and maintain its catalytic activity. The technique was initially utilized in the production of amino acids and catalysis of penicillin G [10,11]. The immobilized enzyme had great functional stability and a simple to run process [12,13]. It can perfectly be used for the production of essential substances and treatment of pollutants [14,15]. The Immobilization technique is classified into three main classes including binding of enzyme to specific support, crosslinking of enzyme into each other and compartmentalization of enzyme within polymer [16].
The compartmentalization of enzymes with polymer has less effect on structural and functional properties of enzymes, and the possibility of retention of enzymatic activity is higher as compared to others retain. The successful compartmentalization process depends on the type of polymer and protocol used for it. Immobilization protocols are trial and error methods to get fruitful results [17]. Polysaccharides are excellent, sustainable and biocompatible polymers with strong chemical and mechanical properties, and can be used as excellent support for the compartmentalization of enzymes for industrial preparations [18-20]. Cellulose is the most abundant polysaccharide and its hydrogel particles are spherical in nature, and can be used in various applications ranging from protein compartmentalization to drug delivery [21,22]. The cellulose hydrogel preparation is an easy process; start with dissolution and shaping of the cellulose solution, and sol-gel transition and solidification of solution into beads form [22]. In this study, cellulose beads were tried for the compartmentalization of pectinase to enhance its operational stability and make it reusable for continuous industrial processes as well as on demand utilization.
Materials and Methods
Pectinase production
Bacillus licheniformis was used for pectinase production [23]. The pectinase was partially purified from cell free filtrates using 50% ammonium sulphate and used for further studies.
Compartmentalization of pectinase within cellulose hydrogel beads
The encapsulation of partially purified pectinase with cellulose beads was done by mixing equal amount of 5.0% cellulose with the enzyme solution and then the mixture was dropwise added in coagulation bath of ultra-pure distilled water with needle of fixed nozzle diameter (0.80 mm) [24]. The beads were washed with ethanol and dried at room temperature for further processes.
Enzyme activity
The enzymatic activity of both cellulose hydrogel compartmentalized pectinase and free pectinase was determined by measuring the concentration of galacturonic acid using the DNS method [25]. 0.5 g of compartmentalized cellulose beads hydrogel were added into 5.0 mL of 1.0% pectin solution and incubated at 45°C for 10 min. 1.0mL reaction mixture solution was taken and added into 1.0 DNS solution and boiled for 10 min. The solutions were cooled at room temperature and diluted up to 10 mL with deionized water. Finally, the galacturonic acid concentration was quantified using a spectrophotometer at 540nm.
“One unit of enzyme is defined as the amount required to produce 1 μM of galacturonic acid per min under standard enzyme assay conditions”.
Influence of cellulose concentration
The influence of cellulose concentration on the preparation of pectinase compartmentalized cellulose hydrogel beads was analysed by using various concentrations of cellulose (1.0 to 10%) during the encapsulation procedure.
Influence of pH
The influence of pH on catalytic activity of both pectinase compartmentalized cellulose hydrogel beads and free pectinase was investigated via measuring the enzyme activity in different pH ranges (5-10).
Influence of temperature
The influence of temperature on the enzymatic activity of cellulose beads hydrogel compartmentalized pectinase was analysed by performing the enzyme in various temperatures (30°C to 60°C).
Influence of reaction period
The influence of reaction period on the catalytic activity of compartmentalized pectinase was analysed by measuring the enzyme assay in various reaction periods ranging from 05-20 min with reference of free pectinase.
Influence of substrate concentration
The influence of substrate concentration on the enzymatic reaction of both cellulose hydrogel beads compartmentalized pectinase and free pectinase was determined by measuring the enzyme activity in various substrate concentrations (1% to 10% pectin).
Thermal stability
The thermal stability of cellulose beads compartmentalized pectinase and free pectinase was investigated by pre-incubation of the compartmentalized pectinase cellulose hydrogel and free pectinase in various temperature ranging from 30°C to 60°C for 24 h before enzyme assay.
Reusability
The reusability of compartmentalized pectinase cellulose hydrogel beads was measured by re-utilization of same compartmentalized pectinase cellulose hydrogel beads multiple times. The beads were washed with deionized water after each reaction cycle and fresh substrate was added for the next reaction.
Results and Discussion
Influence of cellulose concentration on the compartmentalization of pectinase
The concentration of polymer is important to design hydrogel compartmentalized enzyme with maximum biological activities and the concentration of cellulose on the compartmentalization of pectinase from B. licheniformis within cellulose hydrogel beads was optimized using various concentration of cellulose ranging from 1.0 to 10%. The relative activity of compartmentalized pectinase was increased with the increased of cellulose concentration and maximum relative was observed at 5.0% cellulose (Figure 1). The con-centration of cellulose is inversely proportional to the pore size. The pore size of cellulose hydrogel beads was decreased with the increased of cellulose concentration and 5.0% cellulose was enough to form pectinase compartmentalized cellulose hydrogel with retention of maximum relative activity. pectinase encapsulated cellulose bead bioreactor with the maximum immobilization yield. Higher concentration of cellulose reduced the relative activity of pectinase which may be due to hindrance effects of polymer pore size that limit the molecular interaction between enzyme and substrate. Lower concentration of cellulose formed fragile cellulose hydrogel beads with larger pore size and less stable to retained enzymes.
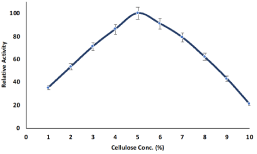
Figure 1: Influence of cellulose concentration on the encapsulation of
pectinase from Bacillus licheniformis KIBGE-IB21 within cellulose beads.
Symbols (means ± S.E., n=6).
Influence of pH on the activity of cellulose beads hydrogel compartmentalized pectinase
The ionic strength of solution changes the orientation of enzyme and substrate to interact with each other and pH is important to provide an accurate ionic environment to maintain the fittest orientation of both enzyme and substrate for catalytic reactions. The effect of pH on biological activity of cellulose hydrogel beads compartmentalized pectinase was determined by performing the enzyme assay of compartmentalized pectinase in different pH (5- 10) with the comparison of free pectinase. The relative activity of compartmentalized and soluble pectinase had significantly changed against different pH and maximum activity was observed at 7.0 (Figure 2). The compartmentalized pectinase showed higher relative activities against the acidic and basic pH as compared to free pectinase due to micro-environment of the polymeric network. The cellulose hydrogel compartmentalization didn’t affect fittest orientation of enzyme and substrate for reaction and pectinase compartmentalized cellulose hydrogel beads showed similar relative activity like soluble pectinase with greater stability.
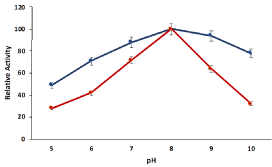
Figure 2: pH influence on the relative activity of pectinase encapsulated
cellulose beads with comparison of soluble pectinase. Symbols (means ±
S.E., n=6).
Influence of temperature on the catalytic activity of cellulose beads hydrogel compartmentalized pectinase
The cellulose beads hydrogel compartmentalization didn’t change the optimum temperature for maximum relative activity of pectinase and both the hydrogel compartmentalized and free pectinase showed maximum enzymatic activity at 45°C (Figure 3). However, the cellulose hydrogel compartmentalization enhanced the relative activity of pectinase against various temperature and hydrogel compartmentalized pectinase showed higher relative activities in various temperatures as compared free pectinase. The reduction of relative activity of soluble pectinase at higher temperature may be due to conformational changes of pectinase at higher temperature. The hydrogel compartmentalized pectinase faced less conformational changes due to the protective sheet of cellulose polymeric network and retained greater relative activity at higher temperature as compared to free pectinase.
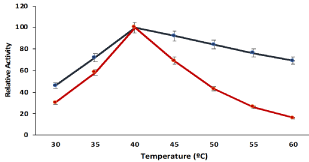
Figure 3: Effect of different temperatures on the activity of cellulose beads
encapsulated pectinase with comparison of soluble pectinase. Symbols
(means ± S.E., n=6).
Effect of reaction time on the relative activity of cellulose hydrogel compartmentalized pectinase
The effect of reaction time on the catalytic activity of cellulose beads hydrogel compartmentalized pectinase was determined by performing the enzyme assay for different reaction time (05-20 min) with the comparison of free pectinase. The cellulose hydrogel compartmentalization changed the reaction time of pectinase for maximum relative and hydrogel compartmentalized pectinase showed maximum relative activity after 10 min of reaction period as compared to soluble pectinase which gave maximum activity after 05 min of reaction period (Figure 4). The cellulose network may limit the diffusion of substrate to reach the active site of compartmentalized pectinase and therefore, the reaction time for maximum relative activity was increased to 10 min after hydrogel compartmentalization.
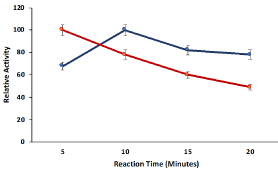
Figure 4: Reaction time influence on the relative activity of cellulose beads
encapsulated pectinase bioreactor and free pectinase. Symbols (means ±
S.E., n=6).
Thermal stability of cellulose hydrogel compartmentalized pectinase
Thermal stable enzymes are important for industrial applications because it can tolerate the harsh industrial reactions conditions. The rate of thermal deactivation of cellulose hydrogel compartmentalized pectinase was minor than the free pectinase at different temperature and compartmentalized pectinase maintained 100% of its original activity at 30°C and 40°C after 24 h. While, the free pectinase lost its more than 10 and 20% relative activity under same conditions. The free pectinase lost its complete enzymatic activity at 60°C after 24 hours but hydrogel compartmentalized pectinase showed more than 70% of its original activity at 60°C after 24 hours. The polymeric network of cellulose hydrogel increased the rigidity of pectinase, so as compared to soluble pectinase the three-dimensional structure of pectinase within the polymeric network was less affected by higher temperatures.
Kinetic parameters
The Km and Vmax define the affinity between substrate and enzymes, and determine the efficacy of polymer and method used for the compartmentalization of enzymes. The Km and Vmax values of hydrogel compartmentalized pectinase was determined using lineweaver-Burk plot to investigate that how the hydrogel compartmentalization affects the affinity of enzyme and substrate. It was observed that the compartmentalization of pectinase within cellulose hydrogel slightly declined the Km and Vmax values of pectinase (Table 1). It has been indicated that the cellulose hydrogel due to its less steric obstruction effect on the active site of enzyme didn’t change the affinity of enzyme and substrate, and the enzyme didn’t lose its flexibility required to bind the substrate.
Enzyme
Km Value
Vmaxvalue
Soluble Pectinase
1.017
23800 IU
Encapsulated Pectinase
1.12
16920 IU
Table 1: Km and Vmax values of pectinase encapsulated cellulose beads and soluble.
Reusability of cellulose hydrogel compartmentalized pectinase
The recycling efficacy of cellulose hydrogel compartmentalized pectinase is important for its cost-effective applications in various industrial preparation. The reusability of hydrogel compartmentalized pectinase was analysed by measuring the relative activity of hydrogel compartmentalized pectinase in various batch reactions (Figure 5). The compartmentalized pectinase exhibited excellent reusability and retained more than 70 % of its initial activity in the third cycle. The decrease of enzymatic activity is due to leaching out of enzyme from the cellulose hydrogel by washing of beads after each cycle. The compartmentalized pectinase cellulose beads retained more than 30% of its relative activity after 10 time reusing.
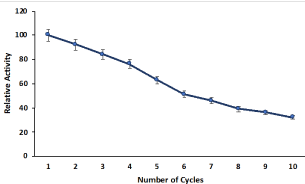
Figure 5: Recycling efficiency of pectinase encapsulated cellulose beads in
batch reactions. Symbols (means ± S.E., n=6).
Conclusions
Pectinase compartmentalized cellulose hydrogel beads was prepared by encapsulation of pectinase within cellulose polymer network to enhance its catalytic properties and ensure its reusability for continuous industrial processes. Maximum compartmentalization of pectinase was observed when 5.0% cellulose was used for hydrogel beads formation. The catalytic properties and kinetic parameters of compartmentalized pectinase were determined with the comparison of free pectinase. It was observed that both free and cellulose hydrogel beads compartmentalized pectinase showed maximum pectinolytic activity at 45°C and pH 10. The hydrogel compartmentalization slightly changed the Km and Vmax of pectinase and compartmentalized pectinase showed higher Km and lower Vmax value as compared to soluble pectinase. The hydrogel compartmentalized pectinase showed excellent thermal stability against different temperatures ranging from 30°C to 60°C as compared to free pectinase. The compartmentalization of pectinase within cellulose hydrogel enhanced the possibility of its reutilization and pectinase retained more than 80% of its initial activity even after reusing in three batches of reactions.
References
- Jayani RS, Saxena S, Gupta R. Microbial pectinolytic enzymes: a review. Process Biochem. 2005; 40; 2931-2944.
- Hoondal GS, Tiwari RP, Tiwari R, Dahiya N, Beg QK. Microbial alkaline pectinase and their industrial application. Appl. Microbiol. Biotechnol. 2002; 59: 409-418.
- Kashyap DR, Vohra PK, Chopra S, Tewari R. Applications of pectinases in the commercial sector: a review. Bioresour. Technol. 2001; 77: 215-227.
- Mishra A, Melo JS, Agrawal A, Kashyap Y, Sen D. Preparation and application of silica nanoparticles-Ocimum basilicum seeds bio-hybrid for the efficient immobilization of invertase enzyme. Colloids Surfaces. 2020; 188: 110796.
- Minshull J, Govindarajan S, Cox T, Ness JE, Gustafsson C. Engineered protein function by selective amino acid diversification. Methods. 2004; 32: 416-427.
- Khajeh K, Ranjbar B, Naderi-Manesh H, Habibi AE, Nemat-Gorgani M. Chemical modification of bacterial a-amylases: changes in tertiary structures and the effect of additional calcium. Biochimica et Biophysica Acta. 2001; 1548: 229-237.
- Costa SA, Tzanov T, Carneiro AF, Paar A, Gubitz GM, Cavaco-Paulo A. Studies of stabilization of native catalase using additives. Enzym. Microb. Technol. 2002; 30: 387-391.
- Bílková Z, Mazurvá J, Churácek J, Horák D, Turková J. Oriented immobilization of chymotrypsin by use of suitable antibodies coupled to a nonporous solid support. J chromatogr A. 1999; 852: 141-149.
- Karim A, Bibi Z, Rehman HU, Aman A, Qader SAU, Rashid MH. Single step immobilization of CMCase within agarose gel matrix: Kinetics and thermodynamic studies. Colloids Surfaces. 2021; 200: 111583.
- Carleysmith SW, Lilly MD. Deacylation of benzylpenicillin by immobilized penicillin acylase in a continuous four-stage stirred-tank reactor. Biotechnol. Bioeng. 1979; 21: 1057-1073.
- Tosa T, Mori T, Fuse N, Chibata I. Studies on continuous enzyme reactions Part V. Kinetics and industrial application of aminoacylase column for continuous optical resolution of acyl-DL-amino acids. Agricul. Biolog. Chem. 1969; 33: 1047-1052.
- Moreira Filho RNF, Vasconcelos NF, Andrade FK, de Freitas Rosa M, Vieira RS. Papain immobilized on alginate membrane for wound dressing application. Colloids Surfaces. 2020; 194: 111222.
- Swaisgood HE. Immobilization of enzymes and some applications in the food industry. In: enzymes and immobilized cells in biotechnology. Laskin, A.I. (Ed), Benjamin Cummings, London. 1985: 1-24.
- Zhang Y, Zhu L, Wu G, Wang X, Jin Q, Qi X, Zhang H. Design of aminofunctionalized hollow mesoporous silica cube for enzyme immobilization and its application in synthesis of phosphatidylserine. Colloids Surfaces, 2021; 111668.
- Poznansky MJ. Enzyme-protein conjugates: new possibilities for enzyme therapy. Pharmacol. Therapeut. 1983; 21: 53-76.
- Sola-Rabada A, Sahare P, Hickman GJ, Vasquez M, Canham LT, Perry CC, et al. Biogenic porous silica and silicon sourced from Mexican Giant Horsetail (Equisetum myriochaetum) and their application as supports for enzyme immobilization. Colloids Surfaces. 2018; 166: 195-202.
- Rehman H, Baloch AH, Nawaz MA. Pectinase: Immobilization and Applications. A review. Trends Pept. Protein Sci. 2021; 6: 1-16.
- Huang LY, Yang MC, Tsou HM, Liu TY. Hemocompatibility and anti-fouling behavior of multilayer biopolymers immobilized on gold-thiolized drug-eluting cardiovascular stents. Colloids Surfaces, 2019; 173: 470-477.
- Klemm D, Heublein B, Fink HP, Bohn A. Cellulose: fascinating biopolymer and sustainable raw material. Angewandte chemie international edition. 2005; 44: 3358-3393.
- John,MJ, Thomas S. Biofibres and biocomposites. Carbohydr. Polym. 2008; 71: 343-364.
- Wang C, Wang L, Zhang Q, Cheng L, Yue H, Xia X, et al. Preparation and characterization of apoacynum venetum cellulose nanofibers reinforced chitosan-based composite hydrogels. Colloids Surfaces. 2021; 199: 111441.
- Gericke M, Trygg J, Fardim P. Functional cellulose beads: preparation, characterization, and applications. Chem. Rev. 2013; 113: 4812-4836.
- Rehman HU, Qader SAU, Aman A. Polygalacturonase: Production of pectin depolymerising enzyme from Bacillus licheniformis KIBGE IB-21. Carbohydr. Polym. 2012; 90: 387-391.
- Voon LK, Pang SC, Chin SF. Porous cellulose beads fabricated from regenerated cellulose as potential drug delivery carriers. J Chem. 2017; 2017.
- Miller GL. Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal Chem. 1959; 31: 426-428.