
Research Article
Austin J Biotechnol Bioeng. 2021; 8(2): 1112.
Extraction and Analysis of Natural Rubber from the Latex of Ficus carica, Artocarpus heterophyllus and Polymer Analysis of Durio zibethinus
Wagner I* and Lackner M
University of Applied Sciences Technikum Wien, Höchstädtplatz 6, 1200 Wien, Austria
*Corresponding author: Isabella Wagner, University of Applied Sciences Technikum Wien, Höchstädtplatz 6, 1200 Wien, Austria
Received: July 10, 2021; Accepted: July 27, 2021; Published: August 03, 2021
Abstract
In tropical fruits such as durian (Durio zibethinus) and jackfruit (Artocarpus heterophyllus), only one quarter to one third of the fruit is edible. Finding more ways to industrially use the other components of the fruit can reduce the waste burned or dumped in landfills. Another fruit tree of interest that can also grow in Austria is the fig tree (Ficus carica). Currently, the fruits are the main product of that plant; however, components in the latex of the tree are of interest too. The latex is known to contain natural rubber, which could potentially be used for industrial applications. Jackfruit trees also produce latex, which contains natural rubber. In both cases, the natural rubber has different properties compared to the conventionally used rubber from the rubber tree (Hevea brasiliensis). This could provide new opportunities in various applications. Therefore, the purpose of this research is to analyze the properties of the natural rubber obtained from the jackfruit and fig trees. Additionally, durian fruit also produces a sticky liquid, so the same experiments were also carried out with durian samples.
The experimental procedure included extraction with acetone and cyclohexane as well as polymer length determination with gel permeation chromatography and polymer analysis with differential scanning calorimetry.
The results show that in both jackfruit and fig tree latex, there is natural rubber of similar polymer length. Durian pulp also contains a polymer; however, with these experiments, it could not be identified. Further research is required to identify the durian polymer and to confirm the results of this experiment.
Keywords: Ficus carica latex; Artocarpus heterophyllus latex; Durio zibethinus polymer; Natural rubber extraction; Natural rubber DSC; Natural rubber GPC
Abbreviations
d: Polydispersity Mw/Mn; DSC: Differential Scanning Calorimetry; FTIR: Fourier-Transform Infrared Spectroscopy; GC: Gas Chromatography; GC-MS: Gas Chromatography Coupled with Mass Spectrometry; GPC: Gel Permeation Chromatography; HPLC: High Performance Liquid Chromatography; IR: Infra-Red; M: Molecular Weight; Mn: Number Average Molecular Weight; Mw: Weight Average Molecular Weight; NMR: Nuclear Magnetic Resonance; R2: Coefficient of Determination; RI: Refractive Index; Tg: Glass Transition Temperature; TGA: Thermogravimetric Analysis; THF: Tetrahydrofuran; UV: Ultraviolet
Introduction
Natural rubber is an irreplaceable resource for tire production, medical products, and many other applications. Contrary to synthetic rubber, where the production is based on fossil resources such as oil, natural rubber is a renewable resource [1]. In 2019, 13.5 million tons of natural rubber have been produced, and the production figures have been continuously rising over the past 10 years [2]. The rubber is almost exclusively obtained from the rubber tree Hevea brasiliensis, mostly from South East Asian countries like Thailand, Indonesia, and Vietnam [3]. The main components of the natural rubber are isoprene monomers that are polymerized in a cis-1,4-configuration. Additionally, there are proteins bound to the polymer chain, which can cause allergic reactions. These proteins, other components and mainly the chain length give the natural rubber unique properties that cannot be synthesized [4]. Historically, there has been a lot of research conducted on replacing the rubber tree as the source of latex, especially in times where this resource was very rare. Nowadays, there are still great concerns about using only one source; the prices are not always stable, there are diseases that can significantly reduce the yield, there is high competition over production resources because of palm oil plantations and much more [4].
The latex contains a certain percentage of dry rubber content, which is specific for the plant type. The majority are non-rubber contents, which contain proteins, carbohydrates, lipids, and inorganic salts [1]. In order to obtain the rubber from the rubber tree Hevea brasiliensis, a procedure called rubber tapping is applied. For this, trees that are over 5 to 6 years old are cut with a knife at a downwards angle with the length of around half the circumference of the trunk [5]. Every two days, the trees are tapped, and the new incisions are placed slightly underneath the old ones. The obtained latex is a colloidal suspension, which is separated from the non-rubber content by coagulation with formic acid [1]. Then, the rubber is either airdried or smoked and can be shipped in this form. Additionally, for some applications like surgical gloves, the rubber can also be sold in a concentrated, liquid form [5].
Many plants contain latex; however, most of them cannot replace the latex of the rubber tree. The polymer chains are in general too short, the yield too low or the extraction and purification very complex. Some plants contain even more proteins that can promote latex allergies and therefore are not appropriate for medical use [4]. Guayule (Parthenium argentatum A. Gray) [5] and the Russian dandelion (Taraxacum koksaghyz) are seen as potential replacements for the rubber tree, with limited commercial success so far.
Ficus carica
The genus Ficus contains several species of which the latex has been studied on whether it is applicable for industrial use. Ficus elastica (rubber fig) and Ficus benghalensis (Banyan tree) have a relatively high content of isoprene, although only half as much as the rubber tree latex [4]. The latex of Ficus elastica has been historically used to produce natural rubber.
The quality of the rubber is mainly determined by the molecular weight of the polymers. The latex of Ficus benghalensis and Hevea brasiliensis contain natural rubber of equal molecular mass [6]. Ficus carica, the fig tree, has around 6% of rubber in the latex, with a comparatively low molecular mass [7]. The properties of this rubber differ from the rubber of Hevea brasiliensis, which could be an advantage for an industrial, potentially new, application. Additionally, Ficus carica is commercially grown all over the world. Every year, over 1 million tons of figures are produced. The main producer is Turkey, followed by Egypt, Morocco, and Algeria [8]. Some species can also be grown in countries with a moderate climate. If there was another way of commercially using fig trees, without causing any reduction in fruit production, there would be an additional source of income for farmers and Ficus carica might be more popular to be grown in Europe. One way could be by using the latex in the branches that are commonly cut off in early spring to increase fruit production.
The natural rubber of Ficus carica has already been successfully extracted by many research groups, its molecular weight, and the FTIR (Fourier-Transform Infrared Spectroscopy) and NMR (Nuclear Magnetic Resonance) spectrograms have been analyzed by [7]. However, further research has focused on the biosynthesis of the rubber [7] or other (medically beneficial) properties of the latex, like collagenase activity [9], peroxidase enzymes [10] or anticonvulsant properties [11]. Another component of interest in the latex is ficin, a proteolytic enzyme. Ficin is responsible to coagulate the latex of the fig tree in case of an injury [12]. This enzyme can be used in the food industry as a meat tenderizer, for cheese production as well as in laboratory procedures [13]. Ficin further promotes blood coagulation [12] and can be used to identify antibodies in blood to reduce the risk of viral transmission in transfusions [14].
Because of a different focus of current research, the physical properties of the natural rubber in the latex have not been analyzed yet and it has not been determined whether the latex of fig trees can be used for industrial purposes. However, compared with Hevea brasiliensis, the molecular weight of the rubber is much smaller [4]. Indicates that a molecular weight over 1000kDa provides sufficient quality, e.g. as raw material for tires, whereas the rubber of Ficus carica is around 180-190 kDa [7]. The molecular weight of the polymer determines the processability [15], however, this disadvantage of the fig tree latex can be reduced by creating composites with synthetic rubber [16]. There are various sources of natural rubber, however, in many; the rubber content of the latex is even lower than in the fig tree [4]. The molecular weight of some substitutes is comparable with Hevea brasiliensis, however, most plants only grow in a tropical climate. The only plant that grows in a moderate climate and is used for rubber production, is Parthenium argentatum (also called guayule) [15].
For extracting the natural rubber of Ficus carica in the lab [7], used the method described in [17], where the rubber was extracted with acetone and benzene. First, the latex of the Ficus carica was obtained by shredding branches into small pieces. To dry the latex, it was spread out and then either air-dried at ambient conditions, or preferably dried at 70°C for a day [7].
They also mentioned the method of [18], who extracted the rubber of Parthenium argentatum with acetone and cyclohexane. Because of the toxicity of benzene, this method seems preferable.
First, to 2g of the sample, 67mL acetone was added. After homogenization for 30 seconds, the sample was centrifuged at 6000rpm for 10 minutes. The supernatant was removed, and acetone was added to the pellet. After homogenization, the sample was centrifuged again and then 67mL cyclohexane was added to the pellet. Then, after another homogenization and centrifugation step, the supernatant was removed and kept for further processing. Another extraction step with cyclohexane was carried out and afterwards, the cyclohexane was fully evaporated in a forced-air oven at 105°C [7,18]. Evaporated the solvent in a rotary vacuum evaporator. By weighing the residues, the percentage of rubber in the plant was determined [7]. According to [7], the latex of the fig tree contains the most natural rubber, compared to the fruit, leaves, or bark.
In [9], the latex was obtained by a mechanical incision every 15 days in a period of 4 months, with 10mL of latex collected each time. Afterwards, the latex was stored at -20°C. In [7], the latex was extracted by cutting a branch into small segments and drying them in a forced-air oven at 70°C for one to two days. They discussed that the percentage of rubber content in Ficus carica is much lower than that of Hevea brasiliensis and a bit lower than that of the traditional rubber tree Ficus elastica [7].
To determine the average molecular weight, GPC (Gel Permeation Chromatography) was used in all cases. The natural rubber can be dissolved in THF (Tetrahydrofuran), which is commonly used as a mobile phase in GPC [7]. Depending on the setup, different flow rates and columns are used.
The average molecular weight Mw of the fig tree natural rubber is around 180-190 kDa [6,7].
Artocarpus heterophyllus
Another fruit tree that could be used for natural rubber extraction is Artocarpus heterophyllus (jackfruit). The genus Artocarpus and Ficus are related, and therefore categorized in the same plant family Moraceae [19].
The latex of Artocarpus heterophyllus is commonly used by traditional and ethnic groups to glue ceramics, clays, and pots as well as to seal boats and trap birds [20]. The fruits, which are the largest ones in the world, are popular in tropical regions and commonly consumed fresh, canned, or processed as fruit juice or dried chips [21]. Unfortunately, only 35% of the fruit consists of edible flesh, and the latex in the fruit causes difficulties for preparation. In jackfruit processing factories, the inedible parts of the fruits are used either as animal feed or discarded, which implies that there is a possibility to increase the value of those by-products, for example by using the natural rubber in the latex for industrial purposes [21].
In [22], the latex was obtained by cutting the fruit into pieces and collecting the liquid with a spoon. The physical properties of the latex were analyzed via FTIR, 1H NMR, and GC-MS (gas chromatography coupled with mass spectrometry), as well as measuring other properties with DSC (differential scanning calorimetry) and TGA (thermogravimetric analysis).
The DSC was performed in three cycles between -110°C and 120°C at a heating rate of 10°C/min under helium with a flow rate of 20mL/min. According to their results, the latex forms crystals at a temperature lower than 41°C, and the melting temperature of the crystals is at 58°C. However, the authors could not identify the glass transition temperature Tg of the jackfruit natural rubber [22].
In comparison, [23] carried out a DSC of the latex of Hevea brasiliensis. The sample was heated in a nitrogen atmosphere from -100 to 300°C at a rate of 10°C/min. The authors found that the glass transition temperature Tg is -65°C [23].
In both the analyses of the latex of Hevea brasiliensis and the latex of Artocarpus heterophyllus, the natural rubber was not extracted prior to the DSC analysis.
Additionally, [22] measured the molecular weight of the polymer chain with gel permeation chromatography. The dried sample was dissolved in tetrahydrofuran (used as the mobile phase) with a concentration of 2mg/mL. It was passed through the column at 1mL/min at 35°C and then detected using a triple detector. The GPC results showed an Mw of 10,916g/mol, and Mn of 5,254g/mol and a polydispersity Mw/Mn of 2.078 [22].
According to [22], the polymer chains of Artocarpus heterophyllus are much shorter than the chains of Hevea brasiliensis, and therefore it cannot be a replacement for the commonly used natural rubber. However, the authors also created rubber compounds with the natural rubber obtained from the jackfruit and analyzed the characteristics. They concluded that in some applications, especially in tire production, it could be advantageous to use the rubber from Artocarpus heterophyllus [22].
In the latex, not only natural rubber and water can be found, but also many other components. As a result, in most research, an extraction is carried out prior to the analysis.
Other components in the latex are for example terpenoids, polyterpenes, proteins, alkaloids, carbohydrates, and resins. These resins can be extracted with acetone [20].
Analyzed different physical parameters of Artocarpus heterophyllus and related Artocarpus species. They did not use the fruits for their analysis but rather extracted the latex by tapping the tree trunks.
Durio zibethinus
A fruit often confused with a jackfruit at first sight is the durian (Durio zibethinus). This fruit is called the king of fruits, because of the distinctive taste, which can be described as fruity, sweet, creamy, and foul with a smell of gasoline [24]. Although the outer appearance of the fruit can be compared with jackfruit, the size is much smaller, the inner composition varies greatly, and they are of a different plant order. The durian tree, Durio zibethinus, can only grow in a warm, humid climate and is therefore mainly grown in South East Asia (the top 3 producers of durian fruit are Thailand, Malaysia, and Indonesia). The fruit is mostly eaten in South East Asia and East Asia (for example China and Taiwan). Unfortunately, only 30% of the fruit is edible, and the residues are often burned or sent to landfills [25]. Therefore, a lot of research is focusing on finding ways to utilize durian waste.
So far, it has not been described that there is any latex in durian fruits or trees; however, the fruit pulp has a specific stickiness that arises the suspicion that there might be latex or similar polymers present. The main components of the fruit are shown in the following Table 1.
Water
65g
Protein
1.47g
Lipids
5.33g
Carbohydrates
27.09g
Dietary Fiber
3.8g
Table 1: Summary of the main components found in frozen or raw durian [26].
Additionally, several vitamins and minerals can be found in the edible part of the fruit [26].
Durian, as well as jackfruit, are mostly inedible; therefore finding additional ways to utilize the residues is very important. Many papers focus on utilizing the durian seeds. Depending on the breed, the number of seeds inside the pulp varies. These seeds contain hydrocolloids, which are hydrophile polymers consisting of polysaccharides or proteins. Dispersed in water, they form viscous colloid gels, solutions, suspensions, or foams. They are hydrophilic and are often used as food additives. Examples of hydrocolloids found in plants would be modified starch, cellulose, pectin, gum arabic, and gum karaya [27].
In traditional Malay cuisine, the thickening properties of the seeds are known and used in various dishes [28]. However, if the hydrocolloids are extracted from the seeds, they could be used in the food industry [28]
Extracted the hydrocolloids by milling dried seeds into flour. After defatting and decolorizing the flour with petroleum ether and ethanol, 1% acetic acid was added, and the crude gum was collected as precipitate after centrifugation. Additionally, the gum was chemically purified [28].
Another way of obtaining the gum from the ground durian seeds is through extraction with water [29]. Applied this method under changing conditions. The research focused on the coagulation properties of the gum.
If there were also hydrocolloids in the edible durian pulp, they would be part of the identified dietary fiber.
Materials and Methods
The objective was to extract polymers from Ficus carica, Durio zibethinus and Artocarpus heterophyllus and then perform GPC and DSC analyses.
The experimental procedure for the extraction and the polymer analysis was the same for all samples to be able to compare the results.
Extraction
Materials:
Cyclohexane: ROTISOLV ≥99.9%, GC Ultra Grade
Acetone: ROTISOLV ≥99.9%, GC Ultra Grade
Method: The plant components were extracted partially with acetone or cyclohexane. For the extraction, the components were cut up into small pieces and filled in a flask. For each part of the plants/ fruits, two approaches were selected. One was filling the flask with acetone and letting the components extract for five days followed by removing the acetone and extracting the pieces with cyclohexane afterwards for four days before filtering the components out and keeping the cyclohexane extracts for further analysis. The other was filling the flask with cyclohexane and extracting the natural rubber for nine days before filtering out the fruit components.
Jackfruit: There was some dried latex on the stalk from when it had been cut from the tree; this latex was collected for the analysis. Then, the jackfruit was cut open with a knife. Immediately, sticky latex threads adjunct to the knife, which were also collected as a sample. The jackfruit consists of several parts: the seeds, which are encased in edible pulp surrounded with fibers, and the core. The latex was mainly excreted by the core and the strands of fiber around the pulp.
The fruit was separated into its components and samples were taken from the pulp, the fibers, and the core. Pictures of the sample fruit can be seen in Figure 1. The seeds were discarded.
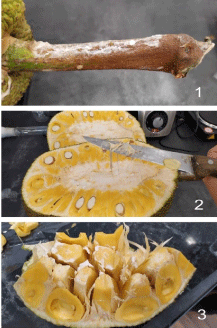
Figure 1: 1) Dried latex on the stalk; 2) Latex sticking to knife when cutting
the fruit open; 3) A piece of fruit with visible pulp, white latex, and fibers.
Fig tree: Samples of branches, fruits, and leaves were taken from two trees. One tree was small, with green branches and leaves because of being an indoor pot plant, the other tree was hibernating outdoors and already much older. When the branches were cut off, milky white latex flowed out, which then dried. The fruits had fallen off the tree previously because of the season; they were unripe and partially dried. Four different plant components were cut into pieces for further analysis: old branches, young branches, unripe fruits, and leaves, which can be seen in Figure 2.
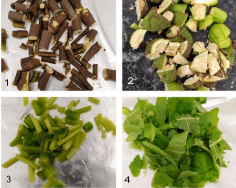
Figure 2: 1) Chopped branches of the old fig tree; 2) Chopped unripe figs;
3) Chopped branch of the young tree; 4) Chopped leaves of the young tree.
Durian: The durian fruit has a very hard outer shell with stout spines and five from the outside partially visible oval compartments. Opening it requires precision, force, and thick gloves to retrieve the custard-like pulp in the compartments. Embedded in this pulp, a few seeds the size of a chestnut can be found. For the experiments, the pulp, as well as the fibers around it, were analyzed. The pictures of the fruit can be seen in Figure 3.

Figure 3: 1) Durian used in the experiment; 2) Durian cut open, visible pulp
and fibers.
Polymer analysis
Materials:
Rotary evaporator
Tetrahydrofuran (THF) by Honeywell, 99.9% purity, for HPLC
Columns: 3 Phenomenex Phenogel columns: 10 000 Å, 500 Å, 50 Å
RI-Detector: PerkinElmer Series 200 Refractive Index Detector
Pump: Jasco PU-980 Intelligent HPLC Pump
Standard: Polystyrene, molecular weights: 162g/mol, 945g/mol, 3,090g/mol, 6,660g/mol, 12,980g/mol, 27,060g/mol, 67,600g/mol, 90,000g/mol
DSC3+ by Mettler Toledo
Purging gas: Nitrogen
Methods:
Gel permeation chromatography: In order to analyze the extracts with GPC, the extraction solvent cyclohexane had to be evaporated. For this, a rotary evaporator was used. The cyclohexane was evaporated at 200 mbar and a temperature of 40°C. Afterwards, the extract was dissolved in THF with a concentration of around 10mg/mL. Insoluble components were removed through a syringe filter and the samples were then analyzed with the mentioned GPCsetup.
The experimental procedure was the following: The autosampler injected 100μL into the column with a flow rate of 0.35mL/min. The analysis took 47 minutes per sample at 43°C.
With the polystyrene standards, a standard curve to identify the molecular weight of the unknown samples based on their retention volume could be calculated.
In order to analyze the data, a baseline correction had to be implemented. Afterwards, for each value of the retention volume, the molecular mass was calculated.
Then, the areas A of the measured segments were calculated with the following formula (1):
A_i=((H_i+H_(i+1) )*0.5)/(|V_i-V_(i+1) |) (1)
Hi is the peak height at the point i. The numerator describes the mean value of two selected peak heights. Vi describes the retention volume at the point i. The denominator describes the volume difference between the two selected points of interest.
For this analysis, the peak areas between all the measured points were calculated. Then, the molecular weight distribution can be identified with the values Mw (weight average Molecular weight), Mn (number average Molecular weight), and Polydispersity d (Mw/Mn).
These values can be calculated with the peak areas and the respective molecular weight determined with the standard curve with the following formula (2) for Mn and (3) for Mw.
(M_n ) −=(S▒A_i )/(S▒A_i /M_i ) (2)
(M_w ) −=(S▒A_i *M_i)/(S▒A_i ) (3)
Differential scanning calorimetry: Around 2-4 mg of the extracted sample was heated and cooled at a rate of 10°C/min. There were two heating/cooling cycles: first from -30°C to 100°C and then from 100°C to -30°C. The nitrogen flow rate was 50mL/min.
Results
When dissolving the samples in THF for the GPC analysis, in 3 cases, precipitation was observed after several hours. These were the latex samples directly obtained from the jackfruit without any extraction; therefore, it is not surprising that not all components were soluble in THF. However, the chromatograms of the supernatant could still be used for the analysis; the natural rubber did not precipitate.
Generally, the chromatograms showed various issues. Firstly, the proportion of natural rubber was very low compared to the other components. Not only that, but the peaks were also so low that it can be assumed that the results are not fully accurate. Secondly, the components of lower molecular weight were not separated by the baseline; therefore, their molecular weight distribution cannot be analyzed. Thirdly, only in some cases, it was possible to evaluate the peak of the natural rubber. Additionally, a full baseline separation was never achieved, so the peaks had to be cut off at some point. For the molecular weight analysis, it was decided to start the peak analysis at a retention volume of 5mL and then analyze the values until the beginning of the next peak.
Durio zibethinus polymer
The chromatogram of the durian extract indicated the existence of a polymer of lower molecular weight. In the fruit extracts, there was a small peak visible before the major peak of low molecular weight (some unknown impurity). In the fiber extract, it was difficult to tell whether there is a polymer as well because of the lack of baseline separation from the other peaks of low molecular weight. Both the pulp chromatograms and the fibers chromatograms show similarities to one another but are very different from the other plant sample (Figure 4).
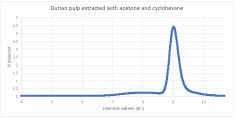
Figure 4: Chromatogram of the durian pulp extracted with acetone and
cyclohexane.
The first small peak of the pulp samples was further evaluated. Although small compared to the main peak of the component of lower molecular weight, the peak height was considerably larger than of the other plant samples (fig and jackfruit). Additionally, the polymer peak of the sample extracted with cyclohexane only was higher in comparison to the other peak.
The results for the average molecular weight were very similar to one another.
It can be said with great certainty that there is a polymer in the durian pulp, also potentially in the fibers. To determine the identity of this polymer, more analyses are necessary.
The DSC-analysis of the extracted sample of the durian pulp did not show conclusive results.
Artocarpus heterophyllus latex
In the pulp chromatograms, the difference in extraction methods was visible. When extracted with cyclohexane only, the polymer peak was very small relative to the other peaks of lower molecular weight. With the second extraction method, the polymer peak was bigger relative to the other peaks. The absolute peak height of the polymer was almost equal for both extraction methods. The chromatograms of the pulp had an appearance similar to the latex chromatograms. Considering the number of peaks and peak height relative to one another, the samples extracted with cyclohexane appear similar to one another, as well as similar to the chromatograms of the unextracted latex. The chromatograms of the samples extracted with cyclohexane and acetone are also partially similar (Figure 5).
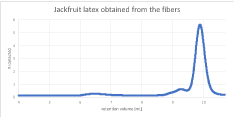
Figure 5: Chromatogram of the jackfruit latex with small polymer peak.
Although the chromatogram of the extracted fibers does not show a separate peak at the polymer molecular weight, this is caused by the lack of baseline separation, since there is a polymer in the unextracted latex from the fibers. It can also be assumed that there is polymer present in the core, though there is no separate peak. In the following Table 3, the results of the molecular weight evaluation are summarized.
There is a considerable deviation, which can be explained by the low peak height. As a result, it is difficult to tell whether there is a difference in molecular weight of different parts of the jackfruit.
It can be assumed that the latex obtained while cutting open the fruit should have the same molecular weight as the latex obtained from the fibers, which is around 200kDa. Therefore, it can be said that the average molecular weight is probably higher than the durian polymer (Table 2) and the fig natural rubber (Table 4). The latex obtained from the stalk shows a different value compared to the other results which are at around 180-220 kDa.
Pulp Extract (Cyclohexane)
Pulp Extract (Acetone + Cyclohexane)
Mn [g/mol]
7500
7500
Mw [g/mol]
15600
15000
Mw/Mn [g/mol]
2.07
1.99
Table 2: Average molecular weight and polydispersity of the durian pulp extract.
Pulp Extract (Cyclohexane)
Pulp Extract (Acetone + Cyclohexane)
Latex Obtained While Cutting Open the Fruit
Latex Obtained from the Stalk
Latex Obtained from the Fibers
Mn [g/mol]
85300
68900
60400
47400
56500
Mw [g/mol]
181000
194000
203000
131000
216000
Mw/Mn [g/mol]
2.13
2.82
3.35
2.78
3.83
Table 3: Average molecular weight and polydispersity of the jackfruit samples.
Fig Fruit Extract (Acetone + Cyclohexane)
Fig Branch Extract, Old Tree (Cyclohexane)
Fig Leaf Extract (Acetone + Cyclohexane)
Mn [g/mol]
33600
70800
61100
Mw [g/mol]
96800
182000
184000
Mw/Mn [g/mol]
2.88
2.57
3.01
Table 4: Average molecular weight and polydispersity of the fig tree samples.
The DSC analysis of 2.7mg of extracted jackfruit natural rubber provided the following curve (Figure 6).
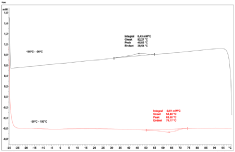
Figure 6: DSC analysis of the natural rubber extract of Artocarpus
heterophyllus fibers (extracted with acetone and cyclohexane).
At around 46°C, the cooling cycle showed an exothermic peak. This can be the crystallization of the polymer. In the heating cycle, an endothermic peak at around 64°C can be seen, which can correspond to the melting of the crystalline structure. There is no glass transition visible in the DSC curve.
Ficus carica latex
No pattern in the chromatograms could be seen regarding the extraction method. The peak height of some chromatograms was quite low, not only for the polymer but also for the other components (Figure 7).
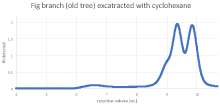
Figure 7: Chromatogram of the latex obtained from a branch of the old tree
The following Table 4 summarizes the results of the molecular weight evaluation.
The average molecular weight of the natural rubber obtained from the branch of the old fig tree appears to be similar to the rubber obtained from the leaves of the young tree. The values of Mw are between 180-190 kDa, as mentioned in [6,7]. However, the results imply that the molecular weight of the rubber in the fruit might be considerably lower.
The DSC curve of 2.2mg of the sample can be seen in the following Figure 8.
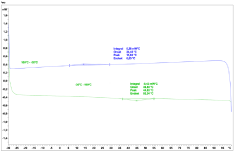
Figure 8: DSC analysis of the natural rubber extract of Ficus carica fruits
(extracted with cyclohexane).
The endothermic peak at 45°C in the heating cycle could correlate to the melting of the polymer. The exothermic peak at 14°C could be the crystallization of the polymer.
There is no glass transition visible in the DSC curve.
Discussion
Sample collection
In the experiments, only two samples from each plant part were taken, each extracted with a different method. This was done to create an overview to be able to determine where further experiments could be interesting. In order to confirm the accuracy of the results, several samples should be taken from each plant part. This way, mean values can be collected and comparisons of various parts of the plant can be made. Additionally, it could be of interest to also take samples of the seeds of durian. The hydrocolloids in the seeds could be used for industrial applications, and the results could be used for comparisons with the polymers found in the pulp and other parts of the fruit.
Extraction
Following the same extraction procedure with all samples gives comparable results. However, because of an incomplete extraction caused by the experimental setup, the percentage of natural rubber in the plant latex could not be analyzed and compared.
Adding an extraction step with acetone before extracting the natural rubber with cyclohexane did not show any influence on the molecular weight of the polymer found. However, the additional extraction step of the impurities with acetone seemed to have increased the proportion of natural rubber in relation to the other peaks in the chromatograms. This would imply that adding more extraction steps like in [7] could be beneficial. First, acetone would be added to the sample to remove resin and other impurities, and the supernatant would be discarded after centrifugation. These extraction steps would be repeated at least two times. Afterwards, cyclohexane would be added to extract the natural rubber. After homogenization and centrifugation, the supernatant would be collected, and the extraction step would be repeated at least two times. To use the sample for further analyses, the solvent would be removed with a rotary vacuum evaporator.
This modified extraction method could provide better results in the GPC analysis. A baseline separation might be achieved, as well as bigger polymer peaks relative to the peaks of lower molecular weight.
Determination of natural rubber content in jackfruit latex
If the extraction is modified, reliable results about the natural rubber content in the latex can be obtained [7]. Already analyzed this with latex from various parts of the fig tree by weighing the latex before the extraction and then comparing it with the weight of the extracted rubber. However, the natural rubber content of the jackfruit latex has not been determined yet. It could be interesting to verify the results of [7] as well as analyzing the rubber content in various parts of the jackfruit.
Gel permeation chromatography
Because of the difference in cyclohexane content in the samples (in some cases, the rotary evaporator had been used, in other cases, the solvent evaporated during storage), as well as the stickiness of the extract because of the natural rubber content making it hard to take an exact amount of extract out of the flask, a comparison between the amounts of natural rubber in different plant parts could not be made.
The chromatograms showed a low resolution, as most peaks did not have a baseline separation. Additionally, the peaks were also broad and the peak heights relative to one another varied greatly. Especially the polymer content compared to the other components was very low. This makes it difficult to obtain reliable results. It would be necessary to carry out additional experiments. Several adjustments should be made: firstly, the concentration of the sample in the THF could be increased. Instead of around 10 mg/mL, more than twice the amount could be used. However, the peaks of the other components could be cut off that way.
Secondly, a change of the GPC setup (for example columns, flow rate) could be applied. Thirdly, a modified extraction could probably solve most of the issues. If an extraction similar to [7] is applied, the polymer peaks would be more prevalent, and the peaks of lower molecular weight might be less in number and separated by baseline.
Additionally, to account for a normal deviation of the experimental results, several samples of each plant and extraction type should be analyzed, to calculate a mean average molecular weight. This way, differences in the various parts of the fruits or plant could be detected and confirmed. The difference in the average molecular weight of jackfruit parts and comparing the molecular weight of the rubber in fig fruits and other parts of the fig would be especially of interest.
Differential scanning calorimetry
To identify artifacts in the DSC curve and to interpret the results correctly, several analyses of each sample, as well as comparisons to other samples are required. Because of the low number of analyzed samples, it cannot be guaranteed that the artifacts in the DSC curve, as well as the results, are interpreted correctly.
The selected temperature range for these experiments was -30°C to 100°C. In this range, the crystallization and melting temperatures of the natural rubber can be determined. However, the glass transition temperature Tg of the latex of Hevea brasiliensis is at -65°C, according to [23]. Therefore, it is likely that the glass transition temperature of the natural rubber in jackfruit and fig tree cannot be determined in the selected temperature range.
Additionally, it is difficult to compare the melting and crystallization temperature of the fig tree and jackfruit latex with the latex of Hevea brasiliensis, since [23] did not mention these temperatures in their results. Also, they did not perform a cooling cycle, which means that the crystallization temperature cannot be determined. In their DSC curve, a small dent at around 60-70°C could indicate the melting of the latex, which would be a similar melting temperature as the latex of Artocarpus heterophyllus.
It could be interesting to compare the DSC results of latex prior and after natural rubber extraction. This could confirm the results of [22], who did not extract the jackfruit natural rubber before the analyses. Also, it could be of great interest to compare the results to the natural rubber (or unextracted latex) of Hevea brasiliensis. By including lower temperatures (up to -100°C) in the analyzes, the glass transition temperature of the natural rubber of jackfruit and fig tree latex could be defined as well, if it is similar to the low Tg of Hevea brasiliensis as found in [23]. Additionally, the crystallization and melting temperature of the latex of Hevea brasiliensis could be determined and compared to the latex of the fruit trees.
Durio zibethinus polymer
The chromatograms showed that there might be a relation of the extraction method to the polymer present in the sample. In the samples extracted with acetone, the polymer peaks seemed to be smaller compared to the other peaks. This might indicate that the polymer is soluble in acetone.
It was not possible to tell whether the fibers also contained any polymer of similar molecular weight. There could be one small polymer peak in one of the chromatograms; however, it is hardly visible and halfway overlapping with another big peak of lower molecular weight.
Although the average molecular weight of the extracted polymer can be estimated to be around 15kDa, it is not possible to identify whether that polymer is natural rubber. Also, the DSC curves did not show any characteristic polymer peaks such as melting temperature or crystallization temperature.
As previously mentioned, another polymer that can be found in durian seed is gum. Additionally, there might also be resins and other components present.
In order to determine whether the polymer in the durian is natural rubber, FTIR and NMR analyses can be made. These results can be compared with natural rubber obtained from other sources, for example H. brasiliensis, Artocarpus heterophyllus, or Ficus. Additionally, samples with unsuccessful extraction could already be excluded at this step.
If the suspicion of the durian polymer being gum is reinforced, it can be tried to follow the experimental procedure of [28]. By hydrolyzing the gum, the sugar components can be determined with HPLC (high performance liquid chromatography). For this, 2mL of sulfuric acid (1mol/L) is added to 10mg of the gum, and it is heated to 80°C for 24 hours. Then the acid is evaporated with a rotary vacuum evaporator and the residues are dissolved in deionized water. After degassing, the sample can be injected into the column. This column needs to be usable for carbohydrate analyses. As a mobile phase, a mixture of acetonitrile and water (4:1) can be used. To compare the results, sugar standards are used. The results would show whether the polymer is gum and whether it is similar to the results of previous research.
In order to clarify whether the polymer found in the durian is gum, the gum in the durian seeds can also be extracted and compared with the results of the polymer analysis.
In conclusion, it would be very interesting to adapt the extraction method and carry out experiments to identify this polymer. Several samples should be taken, not only from the pulp and the fibers but also from the seeds, to compare the polymers found and identify in which parts of the fruit they are commonly found.
Artocarpus heterophyllus latex
The natural rubber obtained from the stalk showed a lower molecular weight compared to the other samples. This latex was already dried and subjected to outside conditions for several weeks since the latex poured out when the fruit was cut off the tree, which was several weeks before the analysis took place. In the meantime, the fruit was packaged and flown across the ocean under unknown conditions. Therefore, the natural rubber on the stalk might have been broken down, for example by microorganisms [30]. Summarized the organisms, which can cause a microbial degradation of natural rubber. In order to confirm this, further experiments under known conditions would need to be carried out.
It would also be interesting to determine which part of the jackfruit contains the most natural rubber. Depending on these results, industrial applications could be developed, and the reduction of waste could be achieved.
The results of the average molecular weight Mn, Mw, and the polydispersity vary greatly compared to the source [22]. In this experiment, the result for Mw was around 200kDa compared to 11kDa in [22]. This would be a very low molecular weight for natural rubber, as natural rubbers from plant latex mostly have a Mw of more than 100kDa [15]. Another important factor to consider is that the latex was directly used for analysis, without prior extraction. It can be seen in these experiments that if there is no prior extraction, the polymer peak is very low.
The results of the DSC seem to be similar to the results of [22]. They found the crystallization peak temperature to be at 58°C and the melting peak temperature at 41°C [22]. This is around 4°C lower than the results of the DSC measurements of these experiments.
Ficus carica latex
The analysis showed a Mw between 180-190 kDa for the leaf and the branch extract [6,7]. Also claim that the natural rubber obtained from the fig tree has an average molecular weight Mw of 180-190 kDa. The molecular weight of the polymer in the fruit appears to be lower than the others though, with an Mw of only around 100kDa. This could be a false result because of the previously discussed limitations of the experiments; however, there could also be natural reasons for a smaller polymer in the fruits. When the fruit ripens, the natural rubber is broken down. Although the fruits were small and green, this process might have started already. Additionally, the fruits had fallen off the tree days or weeks before being analyzed; therefore, some processes of degradation might have started too.
If the DSC results were interpreted correctly, it would mean that the melting and crystallization temperatures of the natural rubber in the fig tree latex vary greatly from the temperatures of the jackfruit natural rubber. This could indicate a lower molecular weight or a different structure of the polymer [31]. However, the results of the GPC indicate that in this experiment, the molecular weight of the natural rubber in the fruits is lower compared to the other parts. The result of the DSC would affirm that. However, to verify the results, more analyzes would be necessary.
In general, for an industrial application, it would not be advisable to use the natural rubber inside the unripe fruits if there are other sources available, especially since [7] determined that most of the natural rubber could be found in the latex obtained from branches or the trunk. Additionally, it could be interesting to consider whether it is possible to obtain not only the natural rubber from the latex but also other components at the same time. One example would be the previously mentioned enzyme ficin.
Conclusion
The experiments showed that the polymer found in the jackfruit and fig tree latex is indeed natural rubber. The extraction was successful, although not very efficient. The natural rubber obtained from the fig tree showed a similar average molecular weight Mw as mentioned in the literature. The jackfruit natural rubber seemed to have a similar Mw compared to the fig tree, which contradicts the results described in the literature. It still needs to be determined which parts of the jackfruit contain the most latex and how it could best be extracted in order to reduce the waste of inedible fruit components.
In the durian pulp, there was also a polymer present, which could not be identified. Because of the durian not being related to the jackfruit and the presence of latex or natural rubber not mentioned in the literature, it cannot be assumed that the polymer is natural rubber.
Industrial Applications
Ficus carica latex
The protease ficin could be used in industrial applications, not only in the field of food biotechnology but also in pharmaceutical applications [32]. There is already a lot of research analyzing the possible application of the enzyme. It could be of great interest to combine the enzyme extraction with the natural rubber extraction, to use the latex as best as possible. Ficin is water-soluble, whereas natural rubber is soluble in organic solvents (like benzene or cyclohexane). One way to extract the ficin is described by [12]. Petroleum ether was added, which extracted the rubber content. After centrifugation, there were two phases: the petroleum ether on the top and the aqueous layer on the bottom. The enzyme was in the aqueous phase and the natural rubber can be found in the organic phase. Several extraction steps could be added to increase the yield [12]. In another source, sodium acetate buffer was added to the latex and after centrifugation, insoluble material (which could be the natural rubber) was removed and the supernatant was used as the ficin extract [33]. In this case, additional purification steps would also be necessary.
Overall, it should be possible to combine the extraction of ficin with the extraction of natural rubber, since, in all research experiments; the natural rubber was removed and discarded at the beginning of the analysis of the enzyme. However, many experiments regarding the ficin were based on the green unripe fruits. This would not be advisable in an industrial application. Therefore, it needs to be analyzed whether the latex in the branches, which should be cut every spring, can be used for both ficin and natural rubber extraction with a considerable yield.
Artocarpus heterophyllus fruit
Most of the inedible parts of the jackfruit (like the peel and the fibers) are discarded, or at best used as animal feed [21]. Jackfruit seeds are used in traditional cuisine; they can be roasted or boiled. However, mostly they are discarded, especially when the fruit is processed into dried chips or other forms [21]. Nevertheless, the seeds can also be of interest to the food industry in the future, for example when milled into flour. This seed flour has good water and oil absorption abilities and therefore can be used as a gluten-free alternative to wheat flour [34]. The seeds contain at least 5% of protein [21]. Additionally, the native starch in the seeds could also be used as an alternative to modified starches, which could be a great benefit since modified starches are classified as food additives in the EU [35].
One popular jackfruit product, which is also exported to the EU, is fried jackfruit chips. Because of the low labor costs in the countries where jackfruit trees are cultivated, most of the processing steps are manual. The peeling could be automated with machines similar to automatic pineapple peelers; however, because of the stickiness from the latex, it would be difficult to automate the removal of the fruit pulp from the fibers.
There has not been any method mentioned of how the latex could be best retrieved from the fruit in order to obtain a high yield of both edible pulp and natural rubber. However, in a manual process of separating the pulp and seeds from the fibers after peeling, the latter could be then cut into small pieces and the natural rubber could be extracted on a large scale. The seeds then could be used for milling it into flour or other purposes. Additionally, since the fruits are manually harvested, the latex could also be obtained when cutting off the fruit from the tree.
If any jackfruit processing steps were automated, the latex recovery and extraction would depend on the automation mechanisms [22], concluded that the properties of the jackfruit latex would be beneficial for certain applications, such as tire production. After vulcanizing the latex, the compound of the jackfruit latex showed potential for having a higher wet, skid resistance in tires compared to a compound made with conventional natural rubber. Additionally, the rolling resistance, an important factor relating to fuel consumption, was the same for both the compounds of Hevea brasiliensis natural rubber and Artocarpus heterophyllus natural rubber. Therefore, using the natural rubber in jackfruit latex for tire production could be more beneficial than using the natural rubber of Hevea brasiliensis [22]. If these findings were verified, it could also be relevant for the Ficus carica latex since it seems to have a similar average molecular weight.
Durio zibethinus fruit
Most importantly, the polymer found in the pulp has to be identified, possibly with the previously mentioned experimental procedure. Additionally, it would be important to verify which part of the durian contains the most component of interest. Then, industrial processes to gain and use the polymer can be implemented.
Durian is often eaten as fresh fruit in South East Asia, but there are also many different sweets and other processed foods that contain durian, which are popular in all of Asia. Therefore, it can be assumed that parts of the processes are automated, although the separation of the different parts of the fruits is mostly done manually as well [36]. In that case, the seeds and the pulp can be easily separated from the fibers and shells, and the parts can be individually used for further processing. Much research has focused on the durian seeds; not only hydrocolloids can be found in the seeds, but also enzymes that could be useful in the food industry, such as β-galactosidase (used in dairy products) [25]. Additionally, the seeds contain starch, which could be used in various applications. The starch in the durian seeds has similar properties compared to mung beans, whereas the jackfruit seed starch shows different properties [37]. There are also various possible applications of using other residues of the fruit, such as the shells, which are summarized in [25].
Biographical Note
Isabella Wagner studied Food Science and Biotechnology at the University of Natural Resources and Life Sciences, Vienna. Because of a growing interest in technology, she started to study International Business and Engineering at the University of Applied Sciences Technikum Wien while completing her master’s degree in Food Science and Technology. Her research interest lies in polymers from renewable resources and recycling.
Maximilian Lackner studied Technical Chemistry at Vienna University of Technology and earned his habilitation in Chemical Engineering. His main research interests include biobased polymers, bioprocess development and in-situ brownfield remediation. He is study programme Director at the University of Applied Sciences Technikum Wien, Vienna.
Acknowledgements
The authors want to thank Prof. Dr. Christian Paulik and DI Klara Saller from Johannes Kepler University, Institute of Organic Chemistry, 4020 Linz, Austria, for support in the polymer analysis.
References
- JT Sakdapipanich and P Rojruthai. “Natural Rubber: Biosynthesis, Structure, Properties and Application,” in Natural Rubber Materials Volume 1: Blends and IPNs, Cambridge. The Royal Society of Chemistry. 2014: 28-46.
- Statista. “Natural rubber production worldwide from 2000 to 2019 (in 1,000 metric tons)”. Malaysian Rubber Board. 2020.
- Statista. “Leading natural rubber producing countries worldwide in 2018 and 2019 (in 1,000 metric tons)”. DBS Group Research. 2020.
- JB van Beilen and Y Poirier. “Establishment of new crops for the production of natural rubber”. Trends in biotechnology. 2007; 11: 522-529.
- A Gent. “Rubber”. Encyclopædia Britannica. 2020.
- H Kang, YS Kim and GC Chung. “Characterization of natural rubber biosynthesis in Ficus benghalensis”. Plant physiology and biochemistry. 2000; 38: 979-987.
- H Kang, MY Kang and K-H Han. “Identification of Natural Rubber and Characterization of Rubber Biosynthetic Activity in Fig Tree”. Plant physiology (Bethesda). 2000; 123: 1133-1142.
- Food and Agriculture Organization of the United Nations (FAO), “Fig Production Quantity worldwide in 2018”. 2020.
- BG Raskovic and ND Polovic. “Collegenase activity in fig latex could contribute to its efficacy in ethnomedicinal preparations”. Journal of Herbal Medicine. 2016; 6: 73-78.
- M Elsayed, UM Hegazy, MG Hegazy, SS Abdel-Ghany, WH Salama, AM Salem, et al. “Purification and biochemical characterization of peroxidase isoenzymes from Ficus carica latex”. Biocatalysis and Agricultural Biotechnology. 2018; 16: 1-9.
- K Raafat and M Wurglics. “Phytochemical analysis of Ficus carica L. active compounds possessing anticonvulsant activity”. Journal of Traditional and Complementary Medicine. 2010; 9: 263-270.
- MB Hamed, MO El-Badry, EI Kandil, IH Borai and AS Fahmy. “A contradictory action of procoagulant ficin by a fibrinolytic serine protease from Egyptian Ficus carica latex”. Biotechnology Reports. 2020; 27: e00492.
- M Gagaoua, N Boucherba, A Bouanane-Darenfed, F Ziane, S Nait-Rabah, K Hafid, et al. “Three-phase partitioning as an efficient method for the purification and recovery of ficin from Mediterranean fig (Ficus carica L.) latex”. Separation and Purification Technology. 2014; 132: 461-467.
- BC Hill, CA Hanna, J Adamski, HP Pham, MB Marques and LA Williams. “Ficin-Treated Red Cells Help Identify Clinically Significant Alloantibodies Masked as Reactions of Undetermined Specificity in Gel Microtubes”. Laboratory Medicine. 2016; 48: 24-28.
- CL Swanson, RA Buchanan and FH Otey. “Molecular Weights of Natural Rubbers from Selected Temperate Zone Plants”. Journal of Applied Polymer Science. 1979; 23: 743-748.
- W Li, R Li, C Li, Z-R Chen and L Zhang. “Mechanical Properties of Surface- Modified Ultra-High Molecular Weight Polyethylene Fiber Reinforced Natural Rubber Composites”. Polymer composites. 2017; 38: 1215-1220.
- RD Stipanovic, DH O’Brien, CE Rogers and KD Hanlon. “Natural Rubber from Sunflower”. Journal of agricultural and food chemistry. 1980; 28: 1322-1323.
- W Ji, CR Benedict and MA Foster. “Seasonal Variations in Rubber Biosynthesis, 3-Hydroxy-3-Methylglutaryl-Coenzyme A Reductase, and Rubber Transferase Activities in Parthenium argentatum in the Chihuahuan Desert”. Plant Physiology. 1993; 103: 535-542.
- JH Langenheim. Plant Resins: Chemistry, Evolution, Ecology, Ethnobotany, Portland, Or: Timber Press. 2003.
- MRJ Nepacina, VC Linis and JIB Janairo. “Physical Characterization of Latex from Artocarpus heterophyllus Lam. (Jackfruit) and Four Related Artocarpus spp.”. Key Engineering Materials. 2020; 833: 107-117.
- RASN Ranasinghe, SDT Maduwanthi and RAUJ Marapana. “Nutritional and Health Benefits of Jackfruit (Artocarpus heterophyllus Lam.): A Review”. International Journal of Food Science. 2019; 2019.
- S Bhadra, N Mohan, G Parikh and S Nair. “Possibility of Artocarpus heterophyllus latex as an alternative source for natural rubber”. Polymer Testing. 2019; 79: 106066.
- V Rao and J Johns. “Thermal behavior of chitosan/natural rubber latex blends TG and DSC analysis”. Journal of Thermal Analysis and Calorimetry. 2008; 92: 801-806.
- The Editors of Encyclopedia Britannica. “Durian tree and fruit”. Britannica. 2020.
- KY Foo and BH Hameed. “Transformation of durian biomass into a highly valuable end commodity: Trends and opportunities”. Biomass and Bioenergy. 2011; 35: 2470-2478.
- US department of agriculture. “Durian, raw or frozen”. Food Data Central. 2019.
- D Saha and S Bhattacharya. “Hydrocolloids as thickening and gelling agents in food: a critical review”. Journal of Food Science and Technology. 2010; 47: 587-597.
- M Amin, AS Ahmad, YY Yin, N Yahya and N Ibrahim. “Extraction, purification and characterization of durian (Durio zibethinus) seed gum”. Food Hydrocolloids. 2007; 21: 273-279.
- T Amid and H Mirhosseini. “Optimisation of aqueous extraction of gum from durian (Durio zibethinus) seed: A potential, low cost source of hydrocolloid”. Food Chemistry. 2012; 132: 1258-1268.
- K Rose and A Steinbüchel. “Biodegradation of Natural Rubber and Related Compounds: Recent Insights into a Hardly Understood Catabolic Capability of Microorganisms”. Applied and Environmental Microbiology. 2005; 71: 2803-2812.
- K Balani, V Verma, A Agarwal and R Narayan. “Physical, Thermal, and Mechanical Properties of Polymers”. In Biosurfaces: A Materials Science and Engineering Perspective. John Wiley & Sons, Inc. 2014: 329-344.
- R Morellon-Sterling, H El-Siar, OL Tavano, Á Berenguer-Murcia and R Fernández-Lafuente. “Ficin: A protease extract with relevance in biotechnology and biocatalysis”. International Journal of Biological Macromolecules. 2020; 162: 394-404.
- D Baeyens-Volant, A Matagne, R El Mahyaoui, R Wattiez and M Azarkan. “A novel form of ficin from Ficus carica latex: Purification and characterization”. Phytochemistry. 2015; 117: 154-167.
- J Eke- Ejiofor, EA Beleya and NI Onyenorah. “The effect of processing methods on the functional and compositional properties of jackfruit seed flour”. International Journal of Nutrition and Food Sciences. 2014; 3: 166-173.
- Mukprasirt and K Sajjaanantakul. “Physico-chemical properties of flour and starch from jackfruit seeds (Artocarpus heterophyllus Lam.) compared with modified starches”. International Journal of Food Science and Technology. 2004; 39: 271-276.
- L Gasik. “Inside A Durian Factory Making Freeze Dried Durian”. Year of the Durian. 2017.
- T Tongdang. “Some Properties of Starch Extracted from Three Thai Aromatic Fruit Seeds”. Starch - Stärke. 2008; 60: 199-207.