
Research Article
J Blood Disord. 2014;1(3): 5.
Observational Study of Transfusion Support and Hemorrhagic Tendencies in Newly Diagnosed Patients of AML Undergoing Chemotherapy
Chaurasia R1*, Elhence P2, Nityanand S3, Verma A2 and Zaman S
1Department of Transfusion Medicine, All India Institute of Medical Sciences, India
2Department of Transfusion Medicine, Sanjay Gandhi Postgraduate Institute of Medical Sciences, India
3Department of Hematology, Sanjay Gandhi Postgraduate Institute of Medical Sciences, India
*Corresponding author: Rahul Chaurasia, Department Of Transfusion Medicine, All India Institute of Medical Sciences, New Delhi, India.
Received: October 08, 2014; Accepted: November 06, 2014; Published: November 07, 2014
Abstract
Background: Transfusion support is an important and integral aspect for management of as substantial amount of blood may be required per patient during course of chemotherapy. We undertook this study to evaluate the quantitative aspect of blood component support in newly diagnosed AML patients undergoing chemotherapy. We also documented bleeding outcomes during the course of treatment.
Methods: Newly diagnosed 19 patients who opted for chemotherapy were included in the study. Quantitative transfusion details throughout the chemotherapy were recorded. Presence of fever, bleeding, infection/sepsis and splenomegaly, use of medications such as chemotherapeutic agents, AMB, G-CSF and antifibrinolytics before every transfusion were also recorded.
Results: Mean requirement of Leucopoor Red Blood Cells (LPRBC), Random Donor Platelets (RDP) and Fresh Frozen Plasma (FFP) was 14, 81.6 and 10 units respectively. Transfusion needs were more during induction as compared to consolidation phase. Bleeding was noted on 19.7 % patient days. Modified WHO grade 1 bleeding was observed on 115 days (8.8%) followed by grade 2 (6.6%) and grade 3 (4.2%). the most common site for bleeding was gastrointestinal tract (28.5%) followed by oropharynx (19.1%) patient days. No bleeding related mortality was noted.
Conclusion: The study provided insights into the complex issues of transfusion requirement of AML patients. We emphasize individualizing transfusion management of these patients as clinical factors are the major determinants of transfusion requirements. We also recommend that bleeding grades should be used as an outcome measure when determining the efficacy of platelet transfusions.
Keywords: Acute myeloid leukemia; Transfusion; Red blood cells; Platelets; Chemotherapy; Bleeding
Introduction
Over the past three decades, chemotherapy regimens and supportive care have been extensively improved and major advances have been made in the diagnosis and management of acute myeloid leukaemia (AML) [1]. Patients with acute leukemia usually present with features of bone marrow failure and symptoms and signs related to anemia, thrombocytopenia and neutropenia. Hemostatic abnormalities are common occurrences in these patients in as much as 50% of patients have platelet counts < 50x109/L [2]. Clinically thrombocytopenia in these patients manifests as easy bruising, petechie, epistaxis, gingival bleeding, conjunctival hemorrhages, and prolonged bleeding from skin injuries. Recent studies have elucidated coexistent thrombocytopathies in form of defective platelet aggregation, inadequate 5-hydroxytryptamine release and poor granulations [3]. In addition occasional patients may also experience rapid destruction of red cells due to an unknown mechanism (milieu hemolysis) [4]. During the course of treatment, chemotherapy causes myelosupression which further deranges the already disturbed haemostatic parameters. Laboratory studies almost constantly show marked decreases in red cells and platelets. Therefore, Red cell transfusions are given to maintain hemoglobin level greater than 8.0 g/ dl. Platelet transfusions used for hemorrhagic manifestations related to thrombocytopenia and prophylactically if necessary to maintain the platelet count above 10 x109/L [5]. Clotting abnormalities are managed by transfusion of fresh frozen plasma and cryoprecipitate. Hence, Transfusion support is integral to patient care and substantial amount of blood may be required per patient. However, there is little information about the transfusion requirements of these patients. We undertook this study to evaluate the quantitative aspect of blood component support in newly diagnosed AML patients undergoing chemotherapy. We also documented bleeding outcomes during the course of treatment.
Material and Methods
The study was carried at the Department of Transfusion Medicine in conjunction with Department of Hematology, Sanjay Gandhi Postgraduate Institute of Medical Sciences, Lucknow, over a period of 18 months. The study was approved by Institutional ethical committee. A total of 175 new patients were diagnosed as AML during the study period, 54 opted for chemotherapy. Only 19 of these patients were enrolled in study. Patients were excluded from the study if they had acute promyelocytic leukemia, secondary AML, if they had received a blood transfusion before the diagnosis of AML, or if they declined to participate.
Patient details
Patient particulars noted included registration number, age, gender, height, weight, ABO and Rh blood group, diagnosis including FAB subtype and phase of chemotherapy. Clinical details such as presence of fever, infection/sepsis, splenomegaly and medications including chemotherapeutic agents (AMB, G-CSF, tranexamic acid) were also noted. Complete blood counts (CBC) were done every morning.
Treatment regimen
Standard treatment regimen during induction included Daunorubicin (60-90mg /m2) for 3 days and cytarabine (100- 200mg/m2) for 7 days. High dose cytarabine (1.5-3g/m2) was used for consolidation which was given every 12 hourly for day 1, 3 and 5. All patients who received treatment were followed up till complete remission, relapse, and resistance to chemotherapy or death whichever was earlier.
Transfusion support
Transfusion support given to patients was in form of Leuco Poor Red Blood Cells (LPRBC), Random Donor Platelets (RDP) prepared from whole blood (by Buffy-coat method) or Single Donor Platelets (SDP) and Fresh Frozen Plasma (FFP). RBC transfusions were given for Hb levels ≤8 g/dl or for symptomatic anemia. RDP/SDPs were transfused therapeutically in the presence of thrombocytopenic bleed and prophylactically when platelet count was ≤10x109/L or 10-20x109/L in the presence of risk factors such as fever, chemotherapy etc. Transfusion details recorded included component being administered, indication, trigger and number/dose of components transfused. Post transfusion samples were collected for determination of Hb and platelet counts. For monitoring efficacy of platelet transfusion, the post transfusion platelet counts were done both at 1 and 24 hours based on which Corrected Count Increment (CCI) was calculated.
Bleeding data
Patients were assessed daily for bleeding events by patient interview and physical examination. Results were reported using a modified WHO scale for bleeding [6]. Grade 0: no bleeding. Grade 1: Mild petechiae, ecchymosis and epistaxis/oral bleeding or occult blood loss. Grade 2: Moderate bleeds not requiring RBC transfusion e.g., large petechiae, ecchymosis, epistaxis, vaginal bleeding, mild hematemesis, melena, mild hematuria. Grade 3: Hemorrhage resulting in rapid decrease of HCT, necessitating RBC transfusions or failure to obtain a post transfusion increment. Grade 4: Hemorrhage resulting in severe hemodynamic compromise or bleeding into a vital organ (e.g. intracranial hemorrhage).
Statistical analysis and data management was done using SPSS statistical computer software (version 13.0, USA). Comparison between two groups were made using χ2 test for proportions. A 2-sided p value < 0.05 was considered significant.
Results
All study patients were followed for 32 chemotherapy cycles which included 19 inductions, 3 reinductions and 10 consolidation chemotherapy. The patient details are summarized in the Table 1. Mean duration of stay per chemotherapy cycle was 23.7(range 3-53) days while for entire treatment the mean duration of hospitalization was 68.5 (range 22-146) days.
No. of patients = 19
Male
15 (79%)
Female
4 (21%)
Median age (range)
33 (1 – 58) years
FAB Subtype
M1
2
10.5%
M2
13
68.4%
M4
3
15.8%
MPAL
1
5.3%
Baseline variables
Hb (g/dl)
6.8 ± 1.43
4.0-8.9
TLC (x103/µL)
54.8 ± 51.1
1.5-166
Platelet count (x103/µL)
35.1 ± 14.5
12-61
Blood group distribution of patients
O (%)
6 (31.6)
A (%)
7 (36.8)
B (%)
6 (31.6)
Table 1: Patient characteristics.
Blood/component usage
During the period of hospitalization, overall usages of all components are shown in Table 2.
No of units
No of transfusion episodes
Mean per patient
Range
LPRBC
266
204
14
3-25
RDP
1550
426
81.6
20-172
SDP
5
5
0.3
0-3
FFP
189
44
10
0-68
Cryoprecipitate
11
2
0.6
0-7
Table 2: Blood components transfused during the study period.
Red cell transfusions
70% (186) of the LPRBC were transfused during induction. Mean units transfused during induction was 9.8 vs. 8 units during consolidation (as 19 patients underwent induction whereas only 10 received consolidation chemotherapy cycles). Majority (51.9%) of the LPRBCs used were for management of hemorrhage.
Platelet transfusions
Total of 1050 RDP and 4 SDP were transfused during induction and 500 RDP and 1 SDP were transfused for consolidation chemotherapy. The mean dose of the platelet transfused was similar in both phases of chemotherapy (1.6x1011). A total 711 (45.8%) units of RDP and 4 SDP were transfused prophylactically on 205 occasions, with a mean dosage of 1.5 X 1011 platelets per episode. The mean pre transfusion platelet count at which platelet transfusion was given was 13.0x103/μl. Response to platelet transfusion was inadequate (CCI <4500 at 24 hours) in 255 (60%) episodes. All 19 patients were found to be refractory (24 hours CCI < 4500 for 2 or more consecutive transfusions) at some point of time during the treatment. Factors associated with inadequate CCI were analyzed by employing Pearson's chi square (χ2) test .Presence of fever, infection/sepsis, splenomegaly, use of Daunorubicin, Cytarabine and Amphotericin B were found to significantly associated with poor platelet recovery at 1 and 24 hours (p <0.05 in all factors) (table 3).
Variables
(total days of exposure)
CCI at 1 hour(n=426)
CCI at 24 hours (n=426)
CCI <7500
at 1 hr
CCI >7500
at 1 hr
P
value
CCI < 4500 at 24 hr
CCI > 4500 at 24 hr
P
value
Fever (n=138)
92
46
0.00
111
27
0.00
Infection/sepsis (n=134)
76
58
0.00
105
29
0.00
Splenomegaly (n=73)
48
25
0.00
60
13
0.00
Daunorubicin (n=19)
17
2
0.00
18
1
0.00
Cytarabine (n=62)
49
13
0.00
56
6
0.00
Amphotericin B (n=291)
189
102
0.00
229
62
0.00
Table 3: Factors associated with inadequate CCI.
FFP transfusion
Of the total 189 units of FFP transfused during the treatment, 175 units were transfused during induction chemotherapy for 40 episodes, while during consolidation chemotherapy 14 units were transfused for 4 episodes. Mean FFP transfused per patient was 8.42 during induction and 1.4 units during consolidation.
Bleeding data
Bleeding was noted on 256 (19.7%) patient days of total 1302 patient days, which included grade 1 bleeding on 115 (8.8%) days, grade 2 on 86 (6.6%) days and grade 3 on 55(4.2%) days. No grade 4 or fatal bleed was noted. Gastro-intestinal and oropharyngeal were most common bleeding sites Table 4. Morning platelet counts on bleeding days are shown in figure 1. Most (133 patient days, 52%) of the bleeding were observed when platelet count was between 10-20 x109/L. As evident from table 5 presence of fever, infection/sepsis and use of Amphotericin B and Tranexamic acid were found to be significantly associated with bleeding (p<0.05).
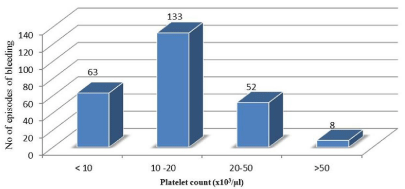
Figure 1: Platelet count on bleeding days.
Site of bleeding
Bleeding days (n=256)
%
Gastro-intestinal
73
28.5
Oropharyngeal
49
19.1
Epistaxis
44
17.2
Mucocutaeous
42
16.4
Genitourinary
37
14.5
Invasive site
4
1.6
Others
7
2.7
Table 4: Sites of bleeding.
Variables
(total days of exposure)
Days of transfusion
with Bleeding
Days of transfusion
without bleeding
P value
Fever (n=166)
104
62
0.00
Infection/sepsis (n=151)
85
66
0.048
Splenomegaly (n=76)
37
39
0.89
Daunorubicin (n=24)
14
10
0.37
Cytarabine (n=82)
39
43
0.69
Amphotericin B (n=307)
183
124
0.00
G-CSF (n=316)
154
162
0.69
Tranexamic acid (n=343)
193
150
0.00
Table 5: Factors associated with risk of bleeding.
Discussion
Understanding transfusion needs of AML patients is crucial for the appropriate management and planning of health care strategies. Our study sheds light on transfusion requirements for newly diagnosed AML patients undergoing chemotherapy. The mean LRBC usage per patient was 14 units (range 3-25) throughout the hospital stay. Favre et al. evaluated transfusion requirements in patients undergoing initial induction consolidation chemotherapy for newly diagnosed AML. The group reported a median usage of 18 RBC units (range 3-44) during the course of chemotherapy [7]. In the present study the mean RBC requirement during the induction phase was higher than during the subsequent consolidation phase (9.8 vs. 8). The same pattern of RBC usage was also found in studies by Dawson et al. and Favre et al. who reported that the mean RBC requirement was higher during initial phase of induction chemotherapy [7,8].
During the chemotherapy, mean no of RDP units (55.3 units during induction vs. 50 during consolidation) and thus the dose of transfused platelets was not significantly different during the induction and consolidation phases. The usage pattern as reported by Dawson et al. [8] reported, 12 platelet units being transfused during induction 7 and 9 platelet units during subsequent consolidation phases for 111 AML patients undergoing chemotherapy while Favre et al. [7] reported median usage of 6 platelet units during first induction and 4 platelet units in the second induction and during consolidation the median usage was 2 and 4 platelet units in subsequent consolidation phase of chemotherapy. In current study mean dosage of 3.6 RDP units (1.6 x 1011 platelets) per transfusion episode was administered, while in studies by Dawson et al.[8] and Favre et al.[7] dosage of the platelet being used was 2 x1011 platelets per transfusion derived form 4-5 pools of whole blood derived platelets or a single apheresis units.
The mean platelet dose transfused in our study was (~1.6 x 1011 platelets) which was equivalent to the low dose category as reported by Slichter et al. [9] (1.1 x 1011 platelets/m2) and Tinmouth et al [10] (3 whole blood derived platelets, WBDP). Tinmouth et al. studied the safety and effectiveness of low dose (3 WBDP) vs. high dose platelets (WBDP) in acute leukemics and recipients of autologous stem cell transplant. He reported that the patients who were transfused with low dose prophylactic platelets received 25% fewer platelets thus, favoring the use low dose platelet transfusion which appeared to be safe and effective [10]. Slichter et al. compared three different platelet doses in hypoproliferative thrombocytopenia and reported that the use of low dose (1.1 x 1011 platelets/m2) platelet transfusion led to decreased usage of platelets. The low dose platelet transfusions were also found to be effective without any adverse events [9]. Recently another large randomized multicentre trial comparing the traditional prophylactic platelet transfusion strategy with a therapeutic transfusion strategy in patients with acute myeloid leukaemia or autologous haematopoietic stem-cell transplantation investigators recommended restrictive platelet transfusion strategy than is presently used worldwide in non-bleeding and clinically stable patients despite a morning platelet count of less than 10x109/L [11].
The response to platelet transfusion was inadequate in 60% episodes. Friedberg et al. [12] evaluated clinical and
blood bank factors or management of platelet refractoriness and alloimmunization in haemato-oncology patients. They reported CCI <5000 in 50.2% of platelet transfusions. In the current study, presence of fever, infection/sepsis, splenomegaly, and use of Daunorubicin, cytarabine and Amphotericin B were significantly associated with poor CCI both at 1 hour and 24 hours. Bishop et al. [13] has reported significant correlation between splenomegaly, administration of AMB and poor CCI at both 1 hour and 24 hour, with other factors marginally affecting the CCI. Similar findings have also been reported by Friedmann et al. [14] where he found clinical factors such as fever, bleeding, DIC, sepsis, splenomegaly, neutropenia to affect the response to platelet transfusions. Slichter et al. [15] has also reported that the presence of palpable spleen, bleeding, fever, infection and use of amphotericin B negatively affects the platelet increment at 1 hour and 24 hour.
All 19 patients in our study were refractory to platelet transfusions at some point of time during the treatment, the increased rate of refractoriness in our study could be largely due to the use of ABO incompatible and non leukofiltered platelets. Fever, infection/sepsis, splenomegaly, and use of Daunorubicin, cytarabine and Amphotericin B also have contributed to refractoriness. Unfortunately, the humoral causes of platelet refractoriness e.g. HLA or HPA antibodies were not evaluated.
Mean FFP units transfused per patient was 10 (range 0-68). Majority of which were (92.6%) transfused during the induction phase while cryoprecipitate was required only on two instances. We could not find any study quantifying FFP/Cryoprecipitate usage in patients of AML (excluding APML).
We observed clinically significant bleeding (grade >2) on 141 days (10.8%) and severe bleeding on 55 days (4.2%). Webert et al. has also reported total of 743 (10.13%) days of bleeding over 7335 patientdays. They found 560 days (75.4%) days with grade 1, 108 (14.5%) days with grade 2, 51 (6.9%) with grade 3 and 24 (3.2%) with grade 4 bleeding severity [16]. Heddle et al. [17] reported clinically significant bleeding on 12.1% patient days and fatal bleed on 0.5 % days of observation with low dose prophylactic transfusion (corresponding to a dose of 1.5-3x1011 platelet per transfusion). Similarly in an another multicentre trial, Heddle et al. [18] observed 6% days with clinically significant bleed (> Grade 2) of the total patient days observed when platelets were transfused prophylactically at a trigger of 10 x109/L (centre: Italy). Thus, severity of the bleeding observed in our study was concordant to other studies. Bleeding from gastrointestinal tract was most frequent in our study present on 28.5% of bleeding days. This was closely followed by oropharyngeal bleeding which was seen on 19.1% bleeding days. Rebulla et al evaluated the sites of major of major bleeding (≥WHO grade 2) and observed that most common site of bleeding was gastrointestinal tract (23.6%) [19].
Sixty-three (24.6%) bleeding episodes were observed with platelet count <10 x109/L and 133 (52%) when the platelet count was 10- 20 x109/L while 52 (20.3 %) episodes of bleeding occurred when platelet count was 20- 50x103/μl, and 8 (3.1 %) when platelet count was >50 x109/L. We noted that majority of bleeding episodes (76.6%) occurred when the platelet count was less than 20 x109/L. This is in corroboration of study by Lawrence et al. [20] who while studying relationship between platelet count and presence of major or minor bleed, observed close correlation between lower platelet counts (<10x109/L) and minor bleeding. A similar relation was also observed for major bleeding. However, majority of their bleeding episodes (50%) were reported when the counts were <5 x109/L. This is in contrast to our study where majority of bleeding episodes occurred at 10-20x109/L (52%) This could be attributed to the fact that our patients received prophylactic platelet transfusions at counts ≤10 x109/L. The difference in the platelet counts at which bleeding occurred was also influenced by the presence of other associated risk factors that led to bleeding. In our study, presence of fever, infection/sepsis and use of Amphotericin B and Tranexamic acid were found to be significantly associated with bleeding. Presence of various factors associated with bleeding has also been investigated in a study by Friedmann et al. [14] where he found that neither the first morning platelet count nor the lowest platelet count of the day were significantly related to the risk of bleeding. He also reported a correlation between bleeding and presence of fever (OR-1.21), bacteraemia (OR-1.06) and use of Amphotericin B (OR-1.62). Tranexamic acid is mainly used as a supportive therapy in bleeding patients thus; its use was associated with presence of bleeding [21].
Blood transfusion support remains an indispensable part of the patient undergoing chemotherapy as substantial number of RBC, platelets and FFP are required during course of treatment. Our findings indicate that the threshold of 10x109/L for prophylactic platelet transfusion is safe for AML patients who are not actively bleeding or not high risk. In cases where additional risk factors are present the threshold should be increased up to 20 x109/L and in the presence of major bleeding complications the platelets count should be maintained greater than 20x109/L. In conclusion the study provided insights into the complex issues of transfusion requirement of AML patients. We emphasize individualizing transfusion management of these patients as clinical factors are the major determinants of transfusion requirements. We also recommend that bleeding grades should be used as an outcome measure when determining the efficacy of platelet transfusions.
References
- British Committee for Standards in Haematology1, Milligan DW, Grimwade D, Cullis JO, Bond L, Swirsky D, Craddock C. Guidelines on the management of acute myeloid leukaemia in adults. Br J Haematol. 2006; 135: 450-474.
- O'Donnell MR, Abboud CN, Altman J, Appelbaum FR, Arber DA, Attar E, et al. Acute myeloid leukemia, NCCN Clinical practice Guidelines in Oncology. 2011.
- Woodcock BE, Cooper PC, Brown PR, Pickering C, Winfield DA, Preston FE. The platelet defect in acute myeloid leukaemia. J Clin Pathol. 1984; 37: 1339-1342.
- Kaushansky K, Lichtman M, Kipps T, et al. Acute myeloid leukemia. In: Marshall A, Lichtman TJK, Uri Seligsohn, Kenneth Kaushansky, editors. Williams hematology, MC-Graw Hill. 2010; 1559-1560.
- O'Donnell MR, Abboud CN, Altman J, Appelbaum FR, Arber DA, Attar E, et al. Acute myeloid leukemia. J Natl Compr Canc Netw. 2012; 10: 984-1021.
- Miller AB, Hoogstraten B, Staquet M, Winkler A. Reporting results of cancer treatment. Cancer. 1981; 47: 207-214.
- Favre G, Fopp M, Gmür J, Tichelli A, Fey MF, Tobler A, Schatzmann E. Factors associated with transfusion requirements during treatment for acute myelogenous leukemia. Ann Hematol. 1993; 67: 153-160.
- Dawson MA, Avery S, McQuilten ZK, Bailey MJ, Shortt J, Polizzotto MN, et al. Blood transfusion requirements for patients undergoing chemotherapy for acute myeloid leukemia how much is enough? Haematologica. 2007; 92: 996-997.
- aSlichter SJ, Kaufman RM, Assmann SF, McCullough J, Triulzi DJ, Strauss RG, et al. Dose of prophylactic platelet transfusions and prevention of hemorrhage. N Engl J Med. 2010; 362: 600-613.
- Tinmouth A, Tannock IF, Crump M, Tomlinson G, Brandwein J, Minden M, et al. Low-dose prophylactic platelet transfusions in recipients of an autologous peripheral blood progenitor cell transplant and patients with acute leukemia: a randomized controlled trial with a sequential Bayesian design. Transfusion. 2004; 44: 1711-1719.
- Wandt H, Schaefer-Eckart K, Wendelin K, Pilz B, Wilhelm M, Thalheimer M, et al. Therapeutic platelet transfusion versus routine prophylactic transfusion in patients with haematological malignancies: an open-label, multicentre, randomized study. Lancet. 2012; 380: 1309-1316.
- Friedberg RC, Donnelly SF, Boyd JC, Gray LS, Mintz PD. Clinical and blood bank factors in the management of platelet refractoriness and alloimmunization. Blood. 1993; 81: 3428-3434.
- Bishop JF, Matthews JP, McGrath K, Yuen K, Wolf MM, Szer J. Factors influencing 20-hour increments after platelet transfusion. Transfusion. 1991; 31: 392-396.
- Friedmann AM, Sengul H, Lehmann H, Schwartz C, Goodman S. Do basic laboratory tests or clinical observations predict bleeding in thrombocytopenic oncology patients? A reevaluation of prophylactic platelet transfusions. Transfus Med Rev. 2002; 16: 34-45.
- Slichter SJ, Davis K, Enright H, Braine H, Gernsheimer T, Kao KJ, et al. Factors affecting post transfusion platelet increments, platelet refractoriness, and platelet transfusion intervals in thrombocytopenic patients. Blood. 2005; 105: 4106-4114.
- Webert K, Cook RJ, Sigouin CS, Rebulla P, Heddle NM. The risk of bleeding in thrombocytopenic patients with acute myeloid leukemia. Haematologica. 2006; 91: 1530-1537.
- Heddle NM, Cook RJ, Tinmouth A, Kouroukis CT, Hervig T, Klapper E, et al. A randomized controlled trial comparing standard- and low-dose strategies for transfusion of platelets (Stop) to patients with thrombocytopenia. Blood. 2009; 113: 1564-1573.
- Heddle NM, Cook RJ, Sigouin C, Slichter SJ, Murphy M, Rebulla P; BEST Collaborative (Biomedical Excellence for Safer Transfusion). A descriptive analysis of international transfusion practice and bleeding outcomes in patients with acute leukemia. Transfusion. 2006; 46: 903-911.
- Rebulla P, Finazzi G, Marangoni F, Avvisati G, Gugliotta L, Tognoni G, et al. The threshold for prophylactic platelet transfusions in adults with acute myeloid leukemia. Gruppo italiano malattie ematologiche maligne dell'adulto. The New England journal of medicine. 1997; 337: 1870-1875.
- Lawrence JB, Yomtovian RA, Dillman C, Masarik SR, Chongkolwatana V, Creger RJ, et al. Reliability of automated platelet counts: comparison with manual method and utility for prediction of clinical bleeding. Am J Hematol. 1995; 48: 244-250.
- Verstraete M. Clinical application of inhibitors of fibrinolysis. Drugs. 1985; 29: 236-261.